Abstract
Field experiments were conducted at the edge of the Horqin Desert to study the impact of biochar on peanut nutrient accumulation and soil nitrogen and phosphorus in a desert soil. The study utilized a split-plot design with drip irrigation (D) (CK) and mulched drip irrigation (M) as the main plot factors. The sub-plots received three maize straw biochar application rates: B0 (0 t/ha), B1 (24 t/ha), and B2 (48 t/ha). In 2020 and 2021, the podnitrogen accumulation(NA) of MB1 was 52.3 and 32.6% higher than MB0 at the pod-filling stage, respectively. In 2020, the phosphorus accumulation of MB0 and MB1 was 107.37 and 50.79% higher than MB2 at the pod filling stage, respectively. The correlation coefficient between soil total N and protein was 0.73, and that between soil pH and peanut oil was 0.83. Compared with MB2 and DB0, MB1 increased yield by 62.5 and 38.3%, respectively. Biochar combined with M increased the nitrate-Ncontent of soil, so that it could be fixed in the root area. Nevertheless, this intervention exhibited no discernible impact on the content of soil available phosphorus, and most of the soil phosphorus is concentrated in soil 40–60 cm. In conclusion, applying biochar (B1) to a peanut cultivation system in a semi-arid windy desert area increased peanut NA, and improved soil properties related to peanut yield and quality, but no effect on soil phosphorus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Peanut holds a prominent position as a vital oil crop and essential raw material for food processing in China, contributing significantly to both the national economy and the assurance of edible oil safety (He et al. 2021). Beyond the inherent genetic traits of cultivars (Bhogireddy et al. 2020), the yield and quality of peanut seed kernel protein and fatty acid constituents are influenced by diverse factors including meteorological conditions (Ruane et al. 2014), cultivation methodologies (Zerbato et al. 2015; Hou et al. 2014), and soil conditions (Rathore et al. 2021; Basu et al. 2008). Kang et al. (2017) demonstrated that drought stress markedly diminished the seed kernel oleic acid: linoleic acid ratio by 16.83%. Adequate water in conjunction with appropriate nitrogen fertilization can lead to a 10% rise in peanut oil yield to the soil (Rathore et al. 2021). Further investigations have underscored the substantial enhancement of peanut quality through heightened soil organic matter content (Xu et al. 2015). Thus, the augmentation of soil water and nutrient status is pivotal in elevating both peanut yield and quality.
Mulched drip irrigation serves as a primary method for augmenting soil moisture levels in semi-arid, wind-prone desert regions (Qi et al. 2018). Previous research has demonstrated the capacity of mulched drip irrigation to enhance oil and oleic acid contents within peanut seed kernels, alongside elevating the ratio of oleic acid to linoleic acid (Ding et al. 2020). The rationale behind this lies in mulched drip irrigation’s enhancement of soil hydrothermal capacity, which optimizes soil nitrogen mineralization, thus boosting nitrogen accumulation within peanut plants and ultimately elevating peanut quality (Xia et al. 2021). However, the mineralization process of organic nitrogen necessitates ample soil organic matter (with an approximate C:N ratio of 25:1), yet it consumes minimal amounts of soil organic matter (only 1.7 g kg−1), leading to a continual decline in soil nitrogen supply capacity and fertility (Rasul et al. 2022; Xia et al. 2021). Given these challenges, enhancing peanut yield and quality in semi-arid, windy desert regions through traditional peanut-water-nitrogen coupling planting remains challenging. Biochar soil improvement technology introduces a transformative approach by modifying the soil carbon pool, boosting carbon stability, and thereby augmenting soil organic matter content (Joseph et al. 2020). Notably, biochar, with its porous structure, high specific surface area, and diverse surface functional groups, proves beneficial for enhancing soil water conservation abilities as a soil conditioner (Razzaghi et al. 2020). Some studies have found that biochar supplemented nitrogen and phosphorus for phosphorus deficient soil, promote the nitrogen fixation of peanut root nodules, and then improve the yield and quality of peanut (Xu et al. 2015).In conclusion, biochar holds the potential to enhance peanut yield and quality, yet further investigation is warranted, particularly within semi-arid windy desert areas.
Nitrogen is as a fundamental element in all living organisms, serving as a critical constituent of cellular proteins and nucleic acids for synthesizing diverse compounds that limit terrestrial ecosystem productivity, particularly in arid regions, where nitrogen is regarded as the second most crucial factor influencing ecosystem stability, following water (Badr et al. 2012). Available phosphorus is one of the indispensable elements in plant growth and development. It is involved in the regulation of key biological processes such as energy transfer, enzyme activity and DNA synthesis (Xu et al. 2013). Therefore, nitrogen and phosphorus play a pivotal role in enhancing crop yield and quality (Pluer et al. 2020; Truffault et al. 2019). In a three-year field experiment on alkaline soil, Xiao et al. (2017) revealed that increasing the biochar application rate (10, 20, and 30 t ha−1) significantly increased nitrogen and phosphorus accumulation in grains, with the enhancement proportionate to the biochar application rate. Investigating the impact of biochar incorporation on nitrogen and phosphorus content in barley plants across two sites, Agegnehu et al. (2016) observed a 1.1-fold increase in nitrogen concentration in barley plants compared to the control. Sun et al. (2019) demonstrated that biochar could increase wheat amino acid content by improving nitrogen use efficiency in saline soil conditions. Minhas et al. (2020) found that the application of biochar under the condition of phosphorus reduction increased soil phosphorus content and thus improved the utilization efficiency of phosphorus fertilizer. Prakongkep et al. (2020) found that the application of biochar to sandy soil for more than 3 years significantly improved the soil and increased the available phosphorus content of the soil. Thus, biochar holds promise for enhancing the utilization rate of nitrogen and phosphorus, thereby potentially elevating peanut yield and quality under mulched drip irrigation. Nonetheless, further investigation is needed to elucidate the intricate mechanisms through which soil nutrients modulate peanut quality by influencing the nitrogen accumulation process and soil properties.
This study aimed to investigate the impact of combining irrigation methods with biochar on soil properties, nitrogen accumulation, phosphorus accumulation, yield, and quality in a semi-arid dryland. The primary objectives included (1) assessing the influence of biochar on soil nitrogen, phosphorus and peanut nutrient accumulation and (2) elucidating the relationships among soil nutrients, yield and quality.
Materials and methods
Site conditions
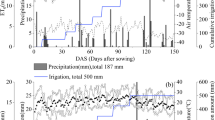
Field experiments were conducted in Aerxiang, Fuxin, situated at the edge of the Horqin Desert in Northwest Liaoning, China (Fig. 1) from May 2020 to October 2021. This region falls within the north-temperate zone and experiences a semi-arid continental monsoon climate. The average annual precipitation is 307 mm. Fig. S1 illustrates the daily precipitation and temperature at the experimental site over the two-year study period. Table S1 presents the physical and chemical properties of the topsoil (0–30 cm) in the experimental plots.
Experimental design
Field experiments were conducted using a split-plot design, which included two factors: irrigation methods and biochar application rates. These factors encompassed two irrigation methods and three biochar application rates, resulting in six treatment combinations that were replicated three times. The main plot comprised drip irrigation (D) (CK) and mulched drip irrigation (M), with irrigation initiated when soil moisture content dropped below 55% of the field capacity (FC), and a maximum irrigation limit set at 90% FC. Biochar application rates included 0 t ha−1 (B0), 24 t ha−1 (B1), and 48 t ha−1 (B2), corresponding to 0, 1, and 2% of the soil mass, respectively.
The locally cultivated peanut variety, Baisha, was planted with a spacing of 15 cm between plants. Each plot, covering an area of 10 m2, was surrounded by a 0.5-m wide isolation zone. Mechanical seeding was used in the experiment. Seeds were sown at a depth of 5 cm, with two seeds per hole, resulting in a planting density of 180,000 hills ha−1. All plots received compound fertilizer (600 kg ha−1, N:P2O5:K2O is 13:6:6) as basal fertilizer according to local standards. Biochar, sourced from Longtai Biological Engineering Co., Ltd., Shenyang City, Liaoning Province, was applied to puddled plots and incorporated into the soil to a depth of 15 cm using a rake. Biochar application was limited to the first year.
Sample measurements
Precipitation and temperature were measured by the TRM-ZS2 Weather Station (YangGuang Weather Co. Ltd., Jinzhou City, China). Soil water content of 0–60 cm was measured every 5 days via an automatic SM1 soil moisture monitor (Adcon Telemetry GmbH, Germany). The plants were divided into roots, leaves, stems and pods. Samples were oven-dried at 105 ℃ for 30 min and then subsequently dried at 75 ℃ until constant weigh (Zhang et al 2023). The dried samples were weighed, crushed with a grinder and sift through a 2 mm sieve to be measured.. The micro-Kjeldahl method and Molybdenum-scandium colorimetry method were used to determine the N and P concentration in plant tissues. The N and P accumulation (kg ha−1) in plant tissues was calculated by multiplying tissue N/P concentration (%) and tissue dry weights (kg ha−1). In 2020, the dry weights sampling days were as follows: 2nd July (seedling stage), 1st August (flower-pegging stage), 31st August (pod-setting stage), and 1st October (pod filling-stage). In 2021, the sampling days were: 9th July (seedling stage), 30th July (flower-pegging stage), 23rd August (pod-setting stage), and 22nd September (pod-filling stage). The peanut quality was determined using Foss Tecator Infratec 1241 Grain Analyzer (FOSS, Hillerød, Denmark). Soil pH (water:soil = 2.5:1) was measured by pH potentiometric titration (pH838) (SMART SENSOR, Dongguan, China). Soil organic matter (SOM) was measured by the potassium dichromate volume-external heating method. Soil cation exchange capacity (CEC) was determined by the ammonium acetate method. Soil samples were taken from a depth of 0–15 cm at the beginning (17th June 2020, 15th June 2021) and the end (2nd October 2020, 25th September 2021) period of growth. Soil core drilling method was used to collect soil (inner diameter of 5 cm, drill bit length of 20 cm, drill body length of 100 cm) after harvesting peanuts. Soil samples from the test plot were collected at intervals of 20 cm within 0 cm (directly below the drip irrigation belt), 17.5 cm (peanut side), and 25 cm (film edge). The content of nitrate nitrogen and available phosphorus was determined using a flow Analytical Autoanalyzer 3 (Germany). At the peanut harvest, seeds were air-dried outdoors for 10–13 days and peanut yields were calculated based on 14% moisture. The water consumption by the peanut crop was calculated as follows (Zhang et al 2021):
Where ET is the water demand (mm); W0 and Wt are the water storage in the planned wet layer of soil at the beginning and end of a growth period (mm); WT is the water increase due to the increase of soil wet layer (mm), WT = (H2 − H1)γ \(\overline{\theta }\), \(\overline{\theta }\) is the average moisture content (water retention by weight), H1 is the planned depth of the wet layer at the beginning of the calculation period, H2 is the planned depth of the wet layer at the end of the calculation period, γ is soil bulk density; P0 is the effective precipitation (mm), P0 = σ × P, P is the precipitation (mm), σ is the effective utilization coefficient of precipitation (P ≤ 5 mm, σ = 0; When P = 5–50 mm, σ = 1; When P ≥ 50 mm, σ = 0.75); K is the groundwater replenishment (mm), and the depth of groundwater is 8–13 m, so K = 0; M is the irrigation water (mm). The water use efficiency of the peanut plants was estimated from Eq. (2):
Where: WUE is water use efficiency (kg m−3); Y is peanut yield (kg); ET is water consumption (m3).
A logistic growth equation (Eq. (3)) was used to describe nitrogen accumulation:
where W is the N accumulation, t is the days after planting, a is the maximum accumulation, and b and c are environment parameters.
Taking the first derivative of Eq. (3) yields the rate equation:
Taking the second derivative of Eq. (3) yields the rate at which V changes with time, t equation:
Take the third derivative of Eq. (3):
The N accumulation equation has two inflection points. When Eq. (5) = 0, we obtain two critical points with coordinates at t1 and t2: t1 = (a − 1.317)/b; t2 = (a + 1.317)/b. Total nitrogen accumulation time: T = t3 = (a + 4.59512)/b. Thus, three stages can be determined: gradually increasing stage T1, fast-increasing period T2, retarding period T3. The duration of a gradual increase of nitrogen accumulation T1 = t1, the duration of a fast increase of nitrogen accumulation T2 = t2−t1, the duration of slow increase of nitrogen accumulation T3 = t3−t2. The N accumulation in each stage is represented by Y1, Y2, and Y3, respectively. V1, V2, and V3 represent the nitrogen accumulation rate at each stage, calculated as follows: V1 = Y1/T1, V2 = Y2/T2, and V3 = Y3/T3.
Accumulation amount of N accumulation during gradually increasing period:
Accumulation amount of N accumulation during the fast increasing period:
Accumulation amount of N accumulation during the retarding period;
Statistical analysis
Data for each year were analyzed separately using the SAS GLM procedure (SAS ver. 9.3) in a split-plot design, with irrigation method as the main factor and biochar application as the sub-factor. Data from each sampling date were analyzed separately. All the data obtained were checked for normality and homogeneity of variance. Mean comparisons among treatments were conducted using Tukey’s HSD test at the significance level of 5%.
Result
Nutrient accumulation
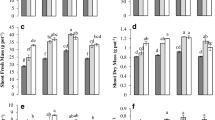
Biochar application rates and irrigation method had a significant interaction effect on total peanut nitrogen accumulation (NA) (Table S1). Overall, peanut nitrogen accumulation (NA) increased gradually, peaked at the pod setting stage, and then stabilized during the pod filling stage (Fig. 2). In 2020 and 2021, the pod nitrogen accumulation (NA) of MB1 increased by 52.3 and 32.6%, respectively, compared to MB0 at the pod filling stage. The pod NA of mulched drip irrigation (M) was significantly higher than that of drip irrigation (D), and the pod NA of MB1 was 32.1% higher than that of DB1 in 2020. Compared with DB0, MB0 increased NA by 5.2% (P > 0.05) and 45.4% (P < 0.05), respectively, in 2020 and 2021. To calculate the amount and rate of nitrogen uptake at each stage, the Logistic growth function was applied to fit peanut nitrogen accumulation (NA), providing theoretical support for subsequent analysis of the relationship between NA process parameters and yield. The Logistic equation effectively simulated peanut nitrogen accumulation (Table 1), with fitting curve R2 values above 0.97 for each treatment. The values of Y1, Y2, and Y3 for B1 were higher than those in other treatments, indicating that B1 promoted higher nitrogen accumulation (NA) at each stage and had a faster accumulation rate at each stage. Mulched drip irrigation (M)accellerated nitrogen accumulation rate. Biochar application rates and irrigation method had a significant interaction effect on total peanut phosphorus accumulation (PA) in 2020. The PA of MB1 and MB0 treatment was significantly increased by 107.37 and 50.79% compared with MB2 treatment, respectively.
Nitrogen (N) and phosphorus (P) accumulation in different growth stages for two growing seasons. I and B represent irrigation regime and biochar application, respectively. M and D represent mulched drip irrigation and drip irrigation, respectively. B0, B1, and B2 represent biochar application rates at 0, 24 t ha−1, and 48 t ha−1, respectively. Means (columns) with different letters are significantly different at the P < 0.05 significance level
Peanut quality
The interaction effect of irrigation method and biochar application on peanut quality was not significant (Table S3). Biochar and irrigation method had no significant effect on peanut protein content in two years (Fig. 3). The O/L of M was 3.7% higher than that of D. The oil content of B2 was 4.5 and 9.0% higher than that of B0, in 2020 and 2021, respectively. The O/L of B2 was 18.3 and 16.8% higher than that of B0, in 2020 and 2021, respectively. The eicosanic acid of B2 was 47.37 and 42.5% higher than that of B0, in 2020 and 2021, respectively (see Table 2).
Effects of different treatments on peanut quality in 2020 and 2021. I and B represent irrigation regime and biochar application, respectively. M and D represent mulched drip irrigation and drip irrigation, respectively. B0, B1, and B2 represent biochar application rates at 0, 24 t ha−1, and 48 t ha−1, respectively. *significant at p < 0.05; **significant at p < 0.01, ns insignificant. O/L, Oleic to linoleic acid
The vectors in the Fig. 4 illustrate the relationship between each indicator and the first two principal components, with the cosine of the included angle representing the correlation between the indicators B1 was significantly correlated with protein content, and Y1 and V1 were significantly correlated with protein content, suggesting that B1 increased peanut protein content because it increased nitrogen accumulation in the first stage. Similarly, B0 increased the linoleic acid content and reduced O/L by increasing T1 and T2, which also verified that B0 was negatively correlated with O/L and was not conducive to peanut O/L. B2 was positively correlated with oleic, oil, O/L, and negatively correlated with T. The correlation between MB2, DB2 and eicosanoic was positive, but the correlation between MB2 and DB2 was greater than that of DB2.
Nitrogen accumulation parameters, quality and its principal component analysis. T (d) represents the total N accumulation time, T1 (d) represents the gradually increasing stage, T2 (d) represents the fast increasing period. Y1 (kg/hm2) represents the N accumulation at the gradually increasing stage. V1 (kg/hm2/d) and V2 (kg/hm2/d) represent the nitrogen accumulation rate at each stage, respectively. I and B represent irrigation regime and biochar application, respectively. M and D represent mulched drip irrigation and drip irrigation, respectively. B0, B1, and B2 represent biochar application rates at 0, 24 t ha−1, and 48 t ha−1, respectively
Soil properties
Biochar application rates and irrigation methods had no significant interaction effect on soil properties (Table S4). The sum of bands at each stage represents the overall condition of the soil (Fig. 5). Soil organic matter (SOM), total nitrogen (N), cation exchange capacity (CEC), and pH were not significantly reduced, indicating a sustained positive effect of biochar application on improving these parameters over the two-year period. In 2020, B2 demonstrated a significant increase in SOM, with its content being 109.7% higher than that of B0 at the end of the growth period. The effect of biochar on soil total N was not significant at the end period of growth in 2020, and B2 increased by 5.0% (P > 0.05) compared with B0. The total N content of B1 was 28.8 and 19.8% higher than that of B0 and B2, respectively, at the end of growth in 2021. B2 increased CEC by 75.9% compared with B0, with no significant difference between B1 and B2 at the beginning of the growth period. B2 increased soil pH by 15.2% compared with B0 in 2020. The correlation heat map illustrates a significant positive correlation between SOM and CEC (Fig. 6). The correlation coefficient between soil total N and protein content was 0.73 (P < 0.05). Soil total nitrogen and CEC showed positive correlations with eicosanoic acid, while soil pH exhibited positive correlations with oil and O/L. The synergistic utilization of biochar and mulched drip irrigation effectively retards the downward leaching of soil nitrate nitrogen, resulting in its predominant accumulation within the rhizosphere. However, biochar exhibited limited efficacy in retaining soil available phosphorus, leading to its predominant distribution within the soil layer spanning 40–60 cm (Fig. 7).
Effects of different treatments on soil properties. SOM (soil organic matter), Total N (total nitrogen). CEC (cation exchange capacity). B0, B1, and B2 represent biochar application rates at 0, 24 t ha−1, and 48 t ha−1, respectively. “Beginning” and “end” represent the period before planting and the period after harvesting, respectively. The width of the ties in the chord diagram represents the numerical size
Yield and water use efficiency
There was a significant I × B interaction effect on peanut yield and water use efficiency (WUE) in 2020 (Table 4). MB1 increased yield by 62.5 and 38.3% compared with MB2 and DB0, respectively. MB1 exhibited the highest WUE among all treatments, being 42.7% higher than DB0. Mulched drip irrigation significantly increased peanut yield, 28.8% higher than traditional drip irrigation (D). A significant linear correlation was found between peanut yield and nitrogen accumulation (R2 = 0.80, P < 0.05 in 2020; R2 = 0.69, P < 0.05 in 2021). A significant linear correlation was found between peanut yield and phosphorus accumulation (R2 = 0.61, P < 0.05 in 2020; R2 = 0.54, P < 0.05 in 2021) (Fig. 8).
Discussion
Soil properties and peanut quality
Peanuts are drought-tolerant, but a soil nutrient deficit will reduce the quality of peanuts (Zhang et al. 2021). Traditional irrigation methods often solve the adverse effects of water deficit on peanut quality, but peanut quality frequently fails to improve because soil nutrient deficiencies remain the growth-limiting factor (Cao et al. 2022). Tan and Kang (2009) found that drip irrigation can lead to a reduction in soil pH in the top 40 cm, while also reducing organic matter (2.02 g kg−1) content in the top 5 cm. This suggests that drip irrigation has a risk of reducing the soil’s nutrient status. Utilizing maize straw biochar to enhance soil nitrogen represents a significant approach within the context of irrigation methodology (Wang et al. 2022). This is attributed to the dual function of biochar: nitrogen release and mitigation of inorganic nitrogen leaching (Borchard et al. 2019). Previous research has demonstrated a significant rise in protein and oil contents of peanuts with increased nitrogen application (Xia et al. 2021). Our findings align with this trend, as evidenced by the positive correlation between peanut seed kernel protein content and soil total N, with biochar (B1) inducing a notable 28.8% augmentation in soil total N. Across a 16-year investigation, consistent supplementation of soil organic matter (SOM) was found to enhance soil trace elements (available N and P), consequently leading to substantial improvements in peanut seed kernel quality (Wang et al. 2020). Mahrous et al. (2015) highlighted that SOM supplementation increases microbial community activity within saline sandy soil, thereby fostering increased peanut protein content; an implication that increased SOM levels confer advantages to peanut quality. Nevertheless, in this study only a limited correlation between SOM and peanut quality was observed. This outcome can be attributed mainly to the application of biochar for SOM enhancement in our research, resulting in an improved retention of soil moisture. This heightened moisture level, however, does not foster the conducive conditions requisite for peanut protein or oil synthesis (Xia et al. 2021). This might constitute the primary rationale for the absence of a statistically significant correlation amongst SOM, protein, and oil within the scope of this investigation. The substantial ash content of biochar, encompassing calcium, magnesium, potassium, sodium, and other essential cations, contributes to increased saturation of soil base cations. This is achieved through a reduction in soil hydrogen ion and exchangeable aluminum ion levels, that increases soil cation exchange capacity (CEC) Zwieten et al. (2010). CEC embodies the soil’s capacity for adsorbing and exchanging cations (such as Ca2+, Mg2+, K+, Na+, and NH4+), directly mirroring soil fertilizer retention, supply, and buffering capabilities. An elevated CEC ensures ample nutrient provisioning and enhances soil quality, as validated by the positive correlation between CEC and eicosanic acid content (Fig. 6). Consequently, the application of biochar serves to heighten eicosanoic acid content through the increase of CEC. Biochar is composed of different functional organic groups (–COO–, –COOH, –O–, and –OH), which are responsible for reducing exchangeable aluminum while absorbing soil protons and increasing soil pH (Hossain et al. 2020). Research dating back to the 1980s has already substantiated the advantages of enhancing soil pH for augmenting peanut quality. An optimal pH nurtures the symbiotic alliance between soil bacteria and fungi, thereby fostering the advancement of peanut quality (Blamey and Chapman 1982; Chen et al. 2019). In our investigation, soil pH (< 7, weakly acidic) exhibited a positive correlation with peanut oil and O/L, implying that biochar sustained peanut storage longevity and oil content through pH elevation. Xu et al. (2015) reported that the application of peanut shell biochar to a red Ferrosol substantially enhanced the quality of peanut seed kernels. Addition of rice husk biochar and wood vinegar increased soil nutrient accessibility and improved blueberry quality (Zhang et al. 2020). In summation, biochar facilitated enhancements in SOM, total N, CEC, and pH, along with peanut seed kernel protein, oil, O/L, and sundry other quality metrics. This substantiates biochar’s role in augmenting peanut quality by enhancing soil attributes.
Nutrient accumulation, peanut quality and yield
Biochar improved crop quality by promoting root absorption of nutrients (Rogovska et al. 2014), promoting nitrogen synthesis (Almaroai and Eissa 2020), inhibiting toxic substances(Schmidt et al. 2014).Our study’s outcomes are consistent with these findings, illustrating that the utilization of biochar in a sandy soil environment enhances nitrogen accumulation. In line with the sink-source theory, nitrogen accumulation in peanuts is a pivotal factor influencing kernel quality alongside genetics, with early-stage accumulation being particularly crucial Demotes-Mainard and Jeuffroy (2004). Our study findings align with this, showing a strong correlation between nitrogen accumulation during the initial stage (Y1) and protein content. Nikolic et al. (2012) suggested a notable correlation between nitrogen content in plant shoots and grain quality, with a nitrogen contribution rate of 0.46–0.67 per pod nitrogen. These findings highlight the potential for enhancing peanut quality through optimized nitrogen distribution in plants, aligning with our study outcomes. Film mulching is an effective strategy to mitigate water evaporation and enhance soil temperature. The desertification-prone region of northwest Liaoning Province experiences significant diurnal temperature variations. The peanut seedling stage falls in June, coinciding with low soil temperatures. Our investigation revealed that film mulching substantially bolstered nitrogen accumulation during this phase, consequently enhancing peanut quality. This outcome can be primarily attributed to the favorable soil temperature maintained by the film mulching practice (Liu et al. 2022). In the study area, soil nutrients are limited and desertification is prevalent. Sole application of film mulching technology fails to mitigate issues of soil water and nutrient leakage. Introducing biochar into the peanut drip irrigation system not only enhanced soil nutrient content but also bolstered soil adsorption capacity. The synergistic impact of biochar in conjunction with drip irrigation showed the potential for augmenting peanut nitrogen accumulation (Wang et al. 2020). Our findings revealed that employing a substantial proportion of biochar within the mulched drip irrigation system (MB2) led to decreased overall peanut nitrogen accumulation. This outcome primarily stems from the alkaline nature of biochar; excessive application of biochar can induce a lime effect, influencing plant growth (Li et al. 2018). Additionally, under drip irrigation, excessive biochar application contributes to elevated soil moisture content, detrimental to dry matter accumulation and nitrogen accumulation. Optimal biochar levels can enhance peanut nitrogen accumulation under both irrigation methods (MB1; DB1), aligning with findings from Khan’s et al. (2021). Furthermore, an appropriate biochar dose (B1) demonstrates a significant improvement in peanut yield, consistent with Schmidt’s et al. (2021). The substantial linear correlation between grain yield and N accumulation (Fig. 8) underscores how biochar enhances yield by facilitating plant N uptake. Moreover, the increased yield attributed to biochar could be attributed to improved soil properties, as documented by Xu et al. (2015). In conclusion, the utilization of biochar (B1) in combination with mulched drip irrigation fosters nitrogen accumulation, leading to advancements in peanut quality and yield.
Soil N and P
Nitrogen and phosphorus are the most critical elements for crop growth and development in agricultural systems (Ettema and Wardle 2022), and studies have shown that mulched drip irrigation (MDI) helps distribute water evenly in soil, reducing nitrogen and phosphorus leaching and loss risks (Tan et al. 2023).However, this study found that MDI did not mitigate phosphorus leaching downward in the soil. Biochar application may affect soil P dynamics by altering soil pH (Wang et al. 2024), coordinating adsorption equilibrium (Soinne et al. 2014), and modifying enzyme activity Pokharel et al. (2020). This study found no significant influence of biochar and MDI on soil available phosphorus content. The concentrated rainfall during the experimental year might have caused phosphorus leaching to depths of 40–60 cm, suggesting that biochar’s retention effect on soil available phosphorus is insignificant. In contrast, biochar addition to soil can have varied effects on soil inorganic N availability, influenced by abiotic processes (adsorption, desorption) and N transformation (mineralization, immobilization, nitrification) (Ahmad et al. 2021; Song et al. 2020). This study concludes that biochar has a certain retention effect on nitrate nitrogen at different depths, which can concentrate nitrate nitrogen in the peanut root zone. This is consistent with the findings of Chen et al. (2020), who observed a reduction in nitrate loss of 13.6 to 59.8% under simulated rainfall conditions in the wind-sand areas of the Loess Plateau. Overall, the enhancement effect of biochar on nitrate –N is more pronounced. However, the effect of biochar on soil available phosphorus in various soil profiles is not significant in our study, possibly due to factors such as soil type, acidity and alkalinity, and rainfall. Further research is needed to elucidate its underlying mechanisms. MDI aids in uniform water distribution throughout the soil, thus reducing the risk of nitrogen and phosphorus leaching and loss (Tan et al. 2023). By precisely regulating water supply, MDI systems can more effectively cater to the specific needs of plants, thereby minimizing unnecessary losses of nitrogen and phosphorus. However, this study revealed that MDI did not mitigate phosphorus leaching into deeper soil layers.
Conclusions
The application of biochar and irrigation method exerted significant influence on peanut nitrogen accumulation (NA). Mulched drip irrigation augmented NA in the seedling stage. B1 application (24 t/ha) led to increased peanut NA across the entire growth period, whereas B2 application (48 t/ha) suppressed peanut NA. Biochar incorporation elevated peanut O/L, eicosanoic, and linoleic acids. NA during the initial stage (Y1) exhibited a notable correlation with peanut protein content. The MB1 treatment yielded the highest harvest, with NA exhibiting a positive association with yield. Biochar amendment in semi-arid windy desert area enhanced soil quality and yield by elevating SOM, total N, CEC, and pH levels. Biochar and mulched drip irrigation increased the content of nitrate-Nin the soil of the peanut rooting area, but the effect of available phosphorus fixation was not obvious, the soil available phosphorus was concentrated at 40–60 cm.
References
Agegnehu G, Bass AM, Nelson PN (2016) Benefits of biochar, compost and biochar-compost for soil quality, maize yield and greenhouse gas emissions in a tropical agricultural soil. Sci Total Environ 543:295–306
Ahmad Z, Mosa A, Zhan L, Gao B (2021) Biochar modulates mineral nitrogen dynamics in soil and terrestrial ecosystems: a critical review. Chemosphere 278:130378
Almaroai YA, Eissa MA (2020) Effect of biochar on yield and quality of tomato grown on a metal-contaminated soil. Sci Hortic Amst 265:109210
Badr MA, El-Tohamy WA, Zaghloul AM (2012) Yield and water use efficiency of potato grown under different irrigation and nitrogen levels in an arid region. Agric Water Manag 110:9–15
Basu M, Bhadoria PBS, Mahapatra SC (2008) Growth, nitrogen fixation, yield and kernel quality of peanut in response to lime, organic and inorganic fertilizer levels. Bioresour Technol 99(11):4675–4683
Bhogireddy S, Xavier A, Garg V (2020) Genome-wide transcriptome and physiological analyses provide new insights into peanut drought response mechanisms. Sci Rep UK 10(1):1–16
Blamey FPC, Chapman J (1982) Soil amelioration effects on peanut growth, yield and quality. Plant Soil 65(3):319–334
Borchard N, Schirrmann M, Cayuela ML (2019) Biochar, soil and land-use interactions that reduce nitrate leaching and N2O emissions: a meta-analysis. Sci Total Environ 651:2354–2364
Cao Y, Cai H, Sun S (2022) Effects of drip irrigation methods on yield and water productivity of maize in Northwest China. Agric Water Manag 259:107227
Chen L, Jiang Y, Liang C, Luo Y, Xu Q, Han C, Sun B (2019) Competitive interaction with keystone taxa induced negative priming under biochar amendments. Microbiome 7(1):1–18
Chen L, Liu M, Ali A, Zhou Q, Zhan S, Chen Y, Zeng Y (2020) Effects of biochar on paddy soil fertility under different water management modes. J Soil Sci Plant Nut 20:1810–1818
Demotes-Mainard S, Jeuffroy MH (2004) Effects of nitrogen and radiation on dry matter and nitrogen accumulation in the spike of winter wheat. Field Crop Res 87(2–3):221–233
Ding H, Zhang G, Shi C, Xu Y, Ci DW, Yuang G, Qin FF, Jiang CS, Zhang ZM (2020) Effect of topdressing under mulch drip irrigation on growth photosynthetic characteristics and yield of peanut. J Peanut Sci 49(03):46–51 ((in Chinese with English abstract))
Ettema CH, Wardle DA (2002) Spatial soil ecology. Trends Ecol Evol 17(4):177–183
He S, Chen Y, Xiang W (2021) Carbon and nitrogen footprints accounting of peanut and peanut oil production in China. J Clean Prod 291:125964
Hilty J, Muller B, Pantin F (2021) Plant growth: the what, the how, and the why. New Phytol 232(1):25–41
Hossain MZ, Bahar MM, Sarkar B (2020) Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2:379–420
Hou J, Dong Y, Fan Z (2014) Effects of coated urea amended with biological inhibitors on physiological characteristics, yield, and quality of peanut. Commun Soil Sci Plan 45(7):896–911
Jones DL, Oburger E (2011) Solubilization of phosphorus by soil microorganisms. Phosphorus Action Biol Process Soil Phosphorus Cycl 26:169–198
Joseph S, Pow D, Dawson K, Rust J, Munroe P, Taherymoosavi S, Solaiman ZM (2020) Biochar increases soil organic carbon, avocado yields and economic return over 4 years of cultivation. Sci Total Environ 724:138153
Kang T, Dai LX, Li WJ, Li HD, Zhang YY, Ding H, Cheng JS, Zhang LM, Ren ZH (2017) Effect of drought stress on root physiological charateristics, yield and quality of different peanut varieties. J Peanut Sci 46(1):53–58 ((in Chinese with English abstract))
Khan Z, Nauman Khan M, Luo T, Zhang K, Zhu K, Rana MS, Jiang Y (2021) Compensation of high nitrogen toxicity and nitrogen deficiency with biochar amendment through enhancement of soil fertility and nitrogen use efficiency promoted rice growth and yield. Gcb Bioenergy 13(11):1765–1784
Li Y, Hu S, Chen J, Müller K, Li Y, Fu W, Wang H (2018) Effects of biochar application in forest ecosystems on soil properties and greenhouse gas emissions: a review. J Soil Sediment 18(2):546–563
Liu Z, Wang B, Li Z, Huang F, Zhao C, Zhang P, Jia Z (2022) Plastic film mulch combined with adding biochar improved soil carbon budget, carbon footprint, and maize yield in a rainfed region. Field Crop Res 284:108574
Mahrous NM, Safina SA, Abo-Taleb HH, El-Sayed El-Behlak SM (2015) Integrated use of organic, inorganic and bio fertilizers on yield and quality of two peanut (Arachis hypogaea L.) cultivars grown in a sandy saline soil. Am-Euras. J Agric Environ Sci 15(6):1067–1074
Minhas WA, Hussain M, Mehboob N, Nawaz A, UL-Allah S, Rizwan MS, Hassan Z (2020) Synergetic use of biochar and synthetic nitrogen and phosphorus fertilizers to improves maize productivity and nutrient retention in loamy soil. J Plant Nutr 43(9):1356–1368
Nikolic O, Zivanovic T, Jelic M, Djalovic I (2012) Interrelationships between grain nitrogen content and other indicators of nitrogen accumulation and utilization efficiency in wheat plants. Chil J Agric Res 72(1):111
Pluer EGM, Schneider RL, Pluer WT (2020) Returning degraded soils to productivity: water and nitrogen cycling in degraded soils amended with coarse woody material. Ecol Eng 157:105986
Pokharel P, Ma Z, Chang SX (2020) Biochar increases soil microbial biomass with changes in extra-and intracellular enzyme activities: a global meta-analysis. Biochar 2:65–79
Prakongkep N, Gilkes R, Wisawapipat W, Leksungnoen P, Kerdchana C, Inboonchuay T, Hammecker C (2020) Effects of biochar on properties of tropical sandy soils under organic agriculture. J Agric Sci 13(1):1–17
Qi Z, Feng H, Zhao Y (2018) Spatial distribution and simulation of soil moisture and salinity under mulched drip irrigation combined with tillage in an arid saline irrigation district, northwest China. Agric Water Manag 201:219–231
Rasul M, Cho J, Shin HS, Hur J (2022) Biochar-induced priming effects in soil via modifying the status of soil organic matter and microflora: a review. Sci Total Environ 805:150304
Rathore VS, Nathawat NS, Bhardwaj S (2021) Optimization of deficit irrigation and nitrogen fertilizer management for peanut production in an arid region. Sci Rep UK 11(1):1–14
Razzaghi F, Obour PB, Arthur E (2020) Does biochar improve soil water retention? a systematic review and meta-analysis. Geoderma 361:114055
Rogovska N, Laird DA, Rathke SJ, Karlen DL (2014) Biochar impact on midwestern mollisols and maize nutrient availability. Geoderma 230:340–347
Ruane AC, McDermid S, Rosenzweig C (2014) Carbon-temperature-water change analysis for peanut production under climate change: a prototype for the AgMIP coordinated climate-crop modeling project (C3 MP). Global Chang Biol 20(2):394–407
Schmidt HP, Kammann C, Niggli C, Evangelou MW, Mackie KA, Abiven S (2014) Biochar and biochar-compost as soil amendments to a vineyard soil: influences on plant growth, nutrient uptake, plant health and grape quality. AGR Ecosyst Environ 191:117–123
Schmidt HP, Kammann C, Hagemann N, Leifeld J, Bucheli TD, Sánchez Monedero MA, Cayuela ML (2021) Biochar in agriculture–a systematic review of 26 global meta-analyses. GCB Bioenergy 13(11):1708–1730
Soinne H, Hovi J, Tammeorg P, Turtola E (2014) Effect of biochar on phosphorus sorption and clay soil aggregate stability. Geoderma 219:162–167
Song X, Razavi BS, Ludwig B, Zamanian K, Zang H, Kuzyakov Y, Gunina A (2020) Combined biochar and nitrogen application stimulates enzyme activity and root plasticity. Sci Total Environ 735:139393
Sun H, Shi W, Zhou M, Ma X, Zhang H (2019) Effect of biochar on nitrogen use efficiency, grain yield and amino acid content of wheat cultivated on saline soil. Plant Soil Environ 65(2):83–89
Tan JL, Kang YH (2009) Changes in soil properties under the influences of cropping and drip irrigation during the reclamation of severe salt-affected soils. AGR Sci China 10:1228–1237
Tan M, Zong R, Lin H, Dhital YP, Ayantobo OO, Chen P, Wang Z (2023) Responses of soil nutrient and enzyme activities to long-term mulched drip irrigation (MDI) after the conversion of wasteland to cropland. Appl Soil Ecol 190:104976
Truffault V, Ristorto M, Brajeul E (2019) To stop nitrogen overdose in soilless tomato crop: a way to promote fruit quality without affecting fruit yield. Agronomy 9(2):80
Van Zwieten L, Kimber S, Morris S, Chan KY, Downie A, Rust J, Cowie A (2010) Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 327(1):235–246
Wang XB, Liu WX, Li ZG, Teng Y, Christie P, Luo YM (2020) Effects of long-term fertilizer applications on peanut yield and quality and plant and soil heavy metal accumulation. Pedosphere 30(4):555–562
Wang S, Xia G, Zheng J, Wang Y, Chen T, Chi D, Ok YS (2022) Mulched drip irrigation and biochar application reduce gaseous nitrogen emissions, but increase nitrogen uptake and peanut yield. Sci Total Environ 830:154753
Wang X, Ding J, Han L, Tan J, Ge X, Nan Q (2024) Biochar addition reduces salinity in salt-affected soils with no impact on soil pH: a meta-analysis. Geoderma 443:116845
Xia G, Wang Y, Hu J, Wang S, Zhang Y, Wu Q, Chi D (2021) Effects of supplemental irrigation on water and nitrogen use, yield, and kernel quality of peanut under nitrogen-supplied conditions. AGR Water Manag 243:106518
Xiao Q, Zhu LX, Tang L (2017) Responses of crop nitrogen partitioning, translocation and soil nitrogen residue to biochar addition in a temperate dryland agricultural soil. Plant Soil 418(1–2):405–421
Xu X, Thornton PE, Post WM (2013) A global analysis of soil microbial biomass carbon, nitrogen and phosphorus in terrestrial ecosystems. Glob Ecol Biogeogr 22(6):737–749
Xu CY, Bai SH, Hao Y, Rachaputi RC, Xu Z, Wallace HM (2015) Peanut shell biochar improves soil properties and peanut kernel quality on a red ferrosol. J Soil Sediment 15(11):2220–2231
Zerbato C, Furlani CEA, Almeida RF (2015) Quality of peanut mechanical sowing in function of seeding density and size of the seeds. Eng AGR Jaboticabal 35:711–720
Zhang P, Yang F, Zhang H, Liu L, Liu X, Chen J, Li C (2020) Beneficial effects of biochar-based organic fertilizer on nitrogen assimilation, antioxidant capacities, and photosynthesis of sugar beet (Beta vulgaris L.) under saline-alkaline stress. Agronomy 10(10):1562
Zhang J, Wang Q, Xia G, Wu Q, Chi D (2021) Continuous regulated deficit irrigation enhances peanut water use efficiency and drought resistance. Agric Water Manag 255:106997
Zhang J, Liu X, Wu Q (2023) Mulched drip irrigation and maize straw biochar increase peanut yield by regulating soil nitrogen, photosynthesis and root in arid regions. Agric Water Manag 289:108565
Funding
National Natural Science Foundation of China, 52279039, National Nature Foundation Regional Innovation Development Joint fund,U21A20217, National key research and development plan, 2022YFD1500701
Author information
Authors and Affiliations
Contributions
Junxiao Zhang: Conceptualization, Experiment design, Investigation, Data analysis, Writing-original draft. Xiaowei Liu: Investigation. Qi Wu:Writing-review & editing. Guimin Xia: Conceptualization, Supervision, Funding acquisition, Writing-review & editing. Resources, Supervision. Daocai Chi: Resources, Supervision, Funding acquisition. Emmanuel Arthur: Writing-review & editing
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, J., Sun, Y., Liu, X. et al. Maize straw biochar improved soil nitrogen, but no effect on phosphorus in desert under mulched drip irrigation. Irrig Sci (2024). https://doi.org/10.1007/s00271-024-00948-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00271-024-00948-0