Abstract
Research on field water consumption is critical for optimizing crop growth and policy-making, in which the computer models play an increasingly important role. As a water-driven crop model, the AquaCrop model has been used in a large number of studies since its launch in 2009. However, how the model performs in predicting the ecohydrological process of farmland under film-mulched drip irrigation is still unclear, especially its application on partitioning crop evapotranspiration is very rarely reported. To make up for the above insufficiency, maize experiments were conducted under full mulch drip irrigation with observation instruments of eddy covariance systems, heat balance stem-flow gauges, micro-lysimeters and other tools, during seasons of 2014–2018. The AquaCrop model was first calibrated using measured data in 2014, and subsequently validated with data in 2015–2018. Results indicate that the parameterized model could precisely simulate the canopy cover (\({R}^{2}\) = 0.97), biomass (\({R}^{2}\) = 0.99) and grain yield (standard deviation was 4.13%), as well as reflect the patterns of daily variation in transpiration and evapotranspiration with satisfactory \({R}^{2}\) of 0.91 and 0.87, respectively. Nevertheless, the \({R}^{2}\) values of soil water content and evaporation were not good, ranging between 0.23 and 0.45, and 0.26 and 0.75, respectively. The AquaCrop model adopts canopy cover instead of leaf area index to describe the growing process of crops; this is an important innovation for model extension and application but also may lead to some inaccuracies in water balance simulation. Summarizing, this study shows that the AquaCrop model is appropriate for supporting crop production but not for predicting the soil moisture content and evaporation variation for maize under film-mulching drip irrigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As the newest international water-driven crop model, the AquaCrop model has notable features including few input parameters, wide application range, simple user interface and high simulation accuracy (Raes et al. 2009b; Steduto et al. 2009; Vanuytrecht et al. 2014; Abdalhi et al. 2018). Early in model development, Steduto et al. (2009) illustrated the framework systematically, Raes et al. (2009a) introduced the main algorithms, and Hsiao et al. (2009) used 6-year maize data to validate and verify the model. Since then, the AquaCrop model has shown advantages in studying relations between crops and water, with increasing attraction from researchers all over the world.
Currently, studies of the AquaCrop model are mainly divided into three categories: (1) the model’s validation and evaluation (Zeleke et al. 2011; Abrha et al. 2012; Katerji et al. 2013; Jin et al. 2014); (2) comparison with other crop models such as WOFOST (Todorovic et al. 2009), CropSyst (Saab et al. 2015), CERES-maize (Babel et al. 2019), and CERES-wheat (Castañeda et al. 2015), and applicability analysis; (3) extended application, such as coupling with climate patterns (Mainuddin et al. 2011; Vanuytrecht et al. 2011; Deb et al. 2015), economic models (García-Vila et al. 2012) or GIS (Lorite et al. 2013). Calibration and verification of the model is the premise of the latter two studies, although many research results have shown that the AquaCrop model performed well in simulating canopy cover, biomass, yield and harvest index, while for different climate regions (García-Vila et al. 2009; Wang et al. 2013; Kumar et al. 2015), different irrigating treatments (Heng et al. 2009; Abrha et al. 2012) and different kinds of crops (Geerts et al. 2009; Todorovic et al. 2009), more researches are needed. The validation of model needs to be conducted because it was developed under specific edaphic and climatic conditions that do not necessarily prevail in other regions of the world (Belay and Patil 2017). Moreover, most studies focused on the model performances on simulating crop growth but neglected the water-related indexes, like partitioned evapotranspiration and soil water content, which are critical for irrigation scheduling.
Considering the broad application prospects of the AquaCrop model, we conducted this study for several reasons: First, since the AquaCrop model is a water-driven crop model, almost all calculations are based on accurate evapotranspiration \((\mathrm{ET})\) separation. Results of Heng et al. (2009) and Farahani et al. (2009) demonstrated the successful performance of AquaCrop in simulating \(\mathrm{ET}\) during the whole growth period, whereas Paredes et al. (2014, 2015) and Pereira et al. (2015) concluded that the accuracy of \(\mathrm{ET}\) separation decreased because the AquaCrop model abandoned the classical dual crop coefficient approach but adopted the empirical coefficient method based on canopy cover; hence, further verification of the model’s accuracy on partitioning \(\mathrm{ET}\) is necessary. Second, although a soil mulching module is contained in the AquaCrop model, it only considers the impact of mulch on \(\mathrm{ET}\), while mulched soil can also influence the growth and development of crops; besides, the soil mulching proportion can also change with time. There are only a few reports on the simulation of soil moisture dynamics and crop growth under mulching conditions (Yang et al. 2015; Zhao et al. 2018). Finally, compared with other countries, studies and application cases of the AquaCrop model are not much shown in China, with most subjects of wheat (Du et al. 2011; Jin et al. 2014) and maize (Ran et al. 2017; Zhao et al. 2018), so parameter localization needs to be enhanced here. In addition, previous model verification mainly focused on uniform irrigation methods such as border irrigation, while for localized irrigation approaches like drip irrigation, relevant studies are weak. The aim of this study was to, therefore, assess the accuracy of AquaCrop model on the maize with film-mulching drip irrigation; also, the applicability of the \(\mathrm{ET}\) separation module was verified to provide a scientific basis for the optimization and improvement of the model.
Materials and methods
Site and climate
Maize experiments were conducted in Shiyanghe Basin in northwest China (37°52′N, 102°50′E, and 1581 m altitude) and performed from 2014 to 2018, with sowing by late April and harvesting in early September (Table 1). The seeding maize was planted with a pattern of ‘one mulch-two drip tapes-four maize rows’, and irrigated with adequate water and nutrients during the whole growing period; row and plant spacings were 0.25 and 0.22 m, respectively (see details in He et al. 2018).
The climate in the experiment site is temperate continental arid type, with over 3000 annual sunshine hours and a mean evaporation of ~ 2000 mm, but multiyear average precipitation of only 164 mm and average precipitation during the maize growing season (April to September) of ~ 135.4 mm. In the 0–100 cm soil layer, the soil texture is sandy loam, mean dry bulk density is 1.4 g cm−3, mean saturated water content is 0.41 cm3 cm−3, mean field capacity is 0.30 cm3 cm−3, and permanent wilting point is 0.10 cm3 cm−3 (Ran et al. 2017). The geographical location and climate type of the experimental area are typical and representative of northwest China.
Measurements
Evapotranspiration
Open-path eddy covariance systems (1.5 m height above the canopy) were installed in the maize field with an area of 2000 m \(\times\) 1000 m during 2014–2015 and 400 m \(\times\) 200 m during 2016–2018, which can provide adequate fetch. The eddy covariance (EC) system consisted a CO2/H2O open-path gas analyzer (model EC150), three temperature and humidity probes (model HMP155A), a radiation meter (model CNR4), two soil heat flux plates (model HFP01), a set of water content reflectometers (model CS616), a set of soil thermocouple probes (model TCAV), and an infrared radiometer (model S1-111). All the sensors were connected to a data logger (model CR3000, Campbell Scientific Inc., USA) and the 10-min statistics were computed. The EC system requires homogeneous underlying and calm winds, while some errors may occur for the interference of external factors such as uncontrollable weather. Hence, the flux data needed to be disposed with Eddy Pro 4.0 software, specific handling process included: detection and elimination of raw peaks; the double coordinate rotation method (Finnigan et al. 2003; Paw et al. 2000); the frequency loss correction; and air density correction (Webb et al. 1980). Besides, for data which the footprint extended out of the experimental area, they should be deleted and remaining missing data were interpolated with linear method when < 4 observations missed, or the mean diurnal variation method when five or more missed (Falge et al. 2001). After data correction and gap filling, the measured energy budget components of daytime EC-based data were forced to close using “Bowen-ratio closure” method, and the “residual-λET closure” method was adopted for nighttime periods (Twine et al. 2000). Finally, combining these calibrated data, evapotranspiration (\(\mathrm{ET}\)) of maize within the EC flux source area was calculated.
Soil evaporation
Six micro-lysimeters were installed in line between the rows (bare soil) and plants (under mulch) with three replications. The micro-lysimeters composed of PVC inner and outer tubes with the diameter of 10 cm and 11 cm, respectively, and the height of both tubes was 20 cm. Each micro-lysimeter was weighted before dawn (7:00) and after sunset (19:00) every day, by an electronic scale (Mettler Toledo, PL6001-L, USA) with accuracy of 0.1 g, then the soil evaporation was obtained by calculating the difference between the weights. Furthermore, to make sure that the soil in the tubes was almost the same as the surrounding soil, it was replaced every week, while if irrigation or heavy precipitation events happened, the soil in micro-lysimeters should also be replaced and soil evaporation was not observed during these periods. Soil evaporation measured by micro-lysimeters was calculated using the equation:
where \({E}_{\mathrm{s}}\) is soil water evaporation (mm day−1); \({\Delta M}_{i}\) is the change in weight of the micro-lysimeters (g day−1); and \({A}_{\mathrm{e}}\) is the transverse area of the micro-lysimeters (cm2).
Maize transpiration
Estimating sap flow by the heat balance method, where the entering and leaving energy in system are measured to quantify the heat transported by sap stream, is one of the important methods used to measure real-time plants transpiration (Sakuratani 1981). Packaged stem flow gauges (Flow32-1K, Dynamax Co. USA) described by Ham and Heilman (1990) were used in this study. Each thermal balance system had eight probes and each probe was installed 20 cm above the ground stem part on eight plants randomly, which was often processed during the shooting stage. Before gauges installation, two or three bottom leaves were required to be removed to facilitate the sensors occupancy. Axial temperature gradients were measured with two pairs of thermocouple sensors positioned above and below the heater, wrapped with foam ‘O’ rings. The entire gauge was encapsulated in aluminum foil to seal it from temperature fluctuations. All the probes were connected to a CR1000 data logger with a sampling frequency of 20 Hz, and sampling interval of 30 min. Transpiration of each maize plant was calculated as (Ding et al. 2013):
where \(T\) is maize transpiration (mm day−1); \(n\) is sampling number; \({Q}_{i}\) is sap flow density of the plant (L day−1); \({A}_{i}\) is the leaf area through which water transpires in the plant (m2); and \(\mathrm{LAI}\) is leaf area index (m2 m−2).
Since the observations of stem-flow often began in the shooting stage, the early growth stage data were lost and interpolation of the maize transpiration was required by analyzing the nonlinear regression equations of the daily transpiration variation with \(\mathrm{LAI}\), surface temperature, vapor pressure difference, net radiation and average soil moisture in the 0–80-cm soil layer (Qin et al. 2019). Within the observation period, the mean values of other observational days were used for interpolation when the maize transpiration or soil evaporation data were missing owing to rainfall or irrigation.
Soil water content
Real-time soil moisture was measured by five probes (CS616, USA) buried at soil depths of 0.2 m, 0.4 m, 0.6 m, 0.8 m and 1.0 m under the ground, respectively. The apparatus recorded data every 30 min and then stored in CR3000. Meanwhile, soil of the five layers was also sampled every week during the whole growing period using soil-drilling method. The sampling frequency was increased when irrigation or heavy rainfall events occurred. By weighing the fresh soil samplings and soil after drying in an oven with temperature of 105 °C for 24 h, the soil water content was measured gravimetrically, and subsequently converted to a volume basis using the bulk density profile values which were measured at the end of the experiment with cutting ring method. Measurements from oven drying method were used for calibrating the data observed by CS616 instrument.
Plant parameters
-
(a)
Maize leaf area was measured manually, that is, using tape to measure the length and maximum leaf width of all leaves on the same plant, multiplying each length and width with a factor of 0.7 and summing them up. This method was derived from the linear regression (\({R}^{2}\) = 0.998) of the calculated and actual values measured by the AM300 leaf area meter (ADC BioScientific Ltd., UK). The leaf area index (\(\mathrm{LAI}\)) was calculated as leaf area divided by the mean land area of each maize plant. \(\mathrm{LAI}\) was measured every 7 days throughout the growing season by sampling eight random plants every time. In this study, maize canopy cover (\(\mathrm{CC}\)) was calculated with \(\mathrm{LAI}\) according to the formula proposed by Hsiao et al. (2009):
$$\mathrm{CC}=1.005\times {\left[1-\mathrm{exp}(-0.6\times \mathrm{LAI})\right]}^{1.2}.$$(3) -
(b)
Measurement of aboveground biomass (\(B\)) was synchronized with \(\mathrm{LAI}\). Eight maize plants were randomly selected to take to the laboratory, then every plant was partitioned by stem, leaf and cob. After bagging and numbering, they were processed in an oven at 105 °C for 30 min (preconditioning) and next at 80 °C for 72 h until the dry weight was unchanged.
-
(c)
During harvest, thirty maize cobs were measured, including the ear number, seeds per ear, hundred-grain weight and grain moisture content. Random sampling method was adopted to eliminate the marginal effect. Mean of the 30 results represented maize yield of the experimental field.
Climate data
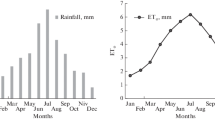
For each day of the simulating period, the AquaCrop model required minimum and maximum air temperatures (\({T}_{\mathrm{min}}\) and \({T}_{\mathrm{max}}\)) to calculate the growing degree day (\(\mathrm{GDD}\)); solar radiation (\({R}_{\mathrm{S}}\)), wind speed (\({u}_{2}\)), relative humidity (\(\mathrm{RH}\)), and air temperature (\({T}_{\mathrm{a}}\)), which were used to calculate the reference evapotranspiration (\({\mathrm{ET}}_{0}\)) (Allen et al. 1998); besides, the precipitation (\(P\)) was also needed. In this study, all climate data were measured by an automatic meteorological station (H21001, Onset Computer Crop., Cape Cod, MA, USA) with recording data every 15 min. Daily temperature and \({\mathrm{ET}}_{0}\) are shown in Fig. 1.
The AquaCrop model
Model description
-
(a)
The soil profile (\(Z\)) and timeline (\(T\)) were divided into small segments in the AquaCrop model (\(\Delta Z\) was set as 12 mm and \(\Delta T\) as 1 day), so the soil water content at every node was calculated as:
$${\theta }_{i,j}={\theta }_{i,j-1}+\Delta {\theta }_{i,\Delta t}.$$(4)where \({\theta }_{i,j}\) is the water content at soil depth of \({z}_{i}\) and time of \({t}_{j}\) (m3 m−3); \({\theta }_{i,j-1}\) is the water content at node (\({z}_{i},{t}_{j-1}\)) (m3 m−3); \(\Delta {\theta }_{i,\Delta t}\) is the change in water content over \(\Delta t\) (m3 m−3).
-
(b)
For describing crop growth process, canopy cover in model was expressed as
$$\left\{\begin{array}{ll}{\rm CC}={\mathrm{CC}}_{0}\times {e}^{t\mathrm{CGC}}&\quad {\rm for} \,{\rm CC}\le \frac{{\mathrm{CC}}_{x}}{2}\\ {\rm CC}={\mathrm{CC}}_{x}-0.25\frac{{\left({\mathrm{CC}}_{x}\right)}^{2}}{{\rm CC}_{0}}{e}^{-t\mathrm{CGC}} & \quad {\rm for\; CC} >\frac{{\mathrm{CC}}_{x}}{2}\\ {\rm CC}={\mathrm{CC}}_{x}\left[1-0.05\left({e}^{\frac{\mathrm{CDC}}{{\rm CC}_{x}}t}-1\right)\right]&\quad {\rm for\, CC\,declining}\end{array},\right.$$(5)where \({\mathrm{CC}}_{0}\) is the initial canopy cover at 90% emergence; \({\mathrm{CC}}_{x}\) is the maximum canopy cover; \(\mathrm{CGC}\) is the canopy growth coefficient (day−1); \(\mathrm{CDC}\) is the canopy decline coefficient (day−1); \(t\) is the time (d). The stress of water, temperature, salinity and fertility are also considered to modify \(\mathrm{CGC}\) and \(\mathrm{CDC}\).
-
(c)
Considering that the small-scale convection between different rows of crops contributed to the transpiration, \(\mathrm{CC}\) was adjusted before being used:
$${\mathrm{CC}}^{*}=1.72\times \mathrm{CC}-{\mathrm{CC}}^{2}+0.30\times {\mathrm{CC}}^{3},$$(6)$${T}_{\mathrm{r}}={K}_{\mathrm{s}}\times {\mathrm{CC}}^{*}\times {K}_{{c}_{\mathrm{tr},\mathrm{adj}}}\times {\mathrm{ET}}_{0},$$(7)$${K}_{{c}_{\mathrm{tr},\mathrm{adj}}}=\left\{\begin{array}{ll}{K}_{{c}_{\mathrm{tr},x}} & t < {t}_{1}\\ {f}_{\mathrm{age}}\times {K}_{{c}_{\mathrm{tr},x}} & {t}_{1}\le t < {t}_{2}\\ {f}_{\mathrm{sen}}\times {f}_{\mathrm{age}}\times {K}_{{c}_{\mathrm{tr},x}} & t\ge {t}_{2}\end{array},\right.$$(8)where \({K}_{\mathrm{s}}\) is the stress coefficient for stomatal closure (\({K}_{{s}_{\mathrm{sto}}}\)) or for water logging (\({K}_{{s}_{\mathrm{aer}}}\)); \({K}_{{c}_{\mathrm{tr},x}}\) is the coefficient for maximum crop transpiration; \({t}_{1}\) is the time required to reach \({\mathrm{CC}}_{x}\); \({t}_{2}\) is the time before senescence; \({f}_{\mathrm{age}}\) is the adjustment factor that decreases \({K}_{{c}_{\mathrm{tr},x}}\) by a constant fraction (default value is 0.3%); \({f}_{\mathrm{sen}}\) is the adjustment factor which declines from 1 (\(\mathrm{CC}={\mathrm{CC}}_{x}\)) to 0 (\(\mathrm{CC}=0\)).
As for soil evaporation, the calculation was divided into three stages, considering the influences of film mulch and drip irrigation:
$$E=\left\{\begin{array}{ll}{K}_{r}\times {K}_{{C}_{e, \mathrm{wet}}}\times {\mathrm{ET}}_{0} &\quad {\rm for\,bare\,soil}\\ \left(1-{\mathrm{CC}}^{*}\right)\times {K}_{{C}_{e, \mathrm{wet}}}\times {\mathrm{ET}}_{0} & \quad {\rm for\,stage\,I}\\ \left(1-{\mathrm{CC}}^{*}\right)\times \left(1-{f}_{\mathrm{CC}}\times {\mathrm{CC}}_{\mathrm{top}}\right)\times {K}_{{C}_{e, \mathrm{wet}}}\times {\mathrm{ET}}_{0} &\quad {\rm for\,stage\,II}\end{array},\right.$$(9)$$E_{{{\text{adj}}}} = \left( {1 - f_{{\text{r mulch}}} } \right) \times f_{{\text{w}}} \times E,$$(10)where \(K_{{\text{r}}}\) is the dimensionless evaporation reduction coefficient; \(K_{{C_{{e, {\text{wet}}}} }}\) is the evaporation coefficient for fully wet and unshaded soil surface (default value is 1.10); \(f_{{{\text{CC}}}}\) is the adjustment factor expressing the shelter effect of the dead canopy cover (default value is 6.0); \({\text{CC}}_{{{\text{top}}}}\) is the canopy cover before the senescence; \(f_{{\text{r mulch}}}\) is the fraction of soil mulched by plastic film (set as 0.65 in this study); \(f_{{\text{w}}}\) is the fraction of the soil surface wetted by irrigation (set as 0.4 in this study); stage I is the time when soil is covered by crop canopy and stage II is when CC declines (induced by phenology or water stress).
-
(d)
To reduce the effect of different climatic conditions on crop water productivity, the AquaCrop model adopted standardized crop water productivity to simulate biomass:
$$B = K_{{s_{{\text{b}}} }} \times f_{{{\text{WP}}}} \times {\text{WP}}^{*} \times \frac{{T_{{\text{r}}} }}{{{\text{ET}}_{0} }},$$(11)where \(K_{{s_{{\text{b}}} }}\) is the coefficient of temperature stress; \(f_{{{\text{WP}}}}\) is the adjustment factor to account for differences in chemical composition of the vegetative biomass and harvestable organs; \({\text{WP}}^{*}\) is the crop water productivity normalized for \({\text{ET}}_{0}\) and \({\text{CO}}_{2}\). Crop yield was directly obtained from the calculated biomass:
$$Y = f_{{{\text{HI}}}} \times {\text{HI}}_{0} \times B$$(12)where \(f_{{{\text{HI}}}}\) is the adjustment coefficient which can reflect the effects of various stresses on the crop yield; \({\text{HI}}_{0}\) is the reference harvest index (%).
Model calibration and validation
Although there are some recommended values for the maize parameters provided in the AquaCrop manual (Raes et al. 2012), some of them are not universal and change with geographic location, management method, crop variety and so on. Thus, calibration and validation of the model are necessary. All the data from the experiment carried out in 2014 were selected for calibration and after that, the model was validated using the measured data from 2015–2018, as shown in Table 2. The outputs of AquaCrop were assessed with following indicators:
-
(a)
A linear regression through the origin having as indicator the regression coefficient, which is computed as (Pereira et al. 2015):
$$b_{0} = \frac{{\mathop \sum \nolimits_{i = 1}^{n} M_{i} \times S_{i} }}{{\mathop \sum \nolimits_{i = 1}^{n} M_{i}^{2} }}.$$(13) -
(b)
An ordinary least first squares regression between measured and simulated values, using the determination coefficient as indicator (Eisenhauer et al. 2003):
$$R^{2} = \left\{ {\frac{{\mathop \sum \nolimits_{i = 1}^{n} \left( {M_{i} - \overline{M}} \right) \times \left( {S_{i} - \overline{S}} \right)}}{{\left[ {\mathop \sum \nolimits_{i = 1}^{n} \left( {M_{i} - \overline{M}} \right)^{2} } \right]^{0.5} \times \left[ {\mathop \sum \nolimits_{i = 1}^{n} \left( {S_{i} - \overline{S}} \right)^{2} } \right]^{0.5} }}} \right\}^{2} .$$(14) -
(c)
The normalized root mean square error (NRMSE) (Bannayan et al. 2009):
$${\text{NRMSE}} = \frac{100}{{\overline{M}}} \times \left[ {\frac{{\mathop \sum \nolimits_{i = 1}^{n} \left( {M_{i} - S_{i} } \right)^{2} }}{n}} \right]^{0.5} .$$(15) -
(d)
The modeling efficiency (EF) proposed by Nash and Sutcliffe (1970) was used to determine the relative magnitude of the residual variance compared to the measured data variance, which is defined as
$${\text{EF}} = 1 - \frac{{\mathop \sum \nolimits_{i = 1}^{n} \left( {M_{i} - S_{i} } \right)^{2} }}{{\mathop \sum \nolimits_{i = 1}^{n} \left( {M_{i} - \overline{M}} \right)^{2} }}.$$(16) -
(e)
Willmott’s index of agreement was also used (Willmott 1984):
The five goodness-of-fit indicators were computed from the pairs of measured and simulated values, \(M_{i}\) and \(S_{i}\) with means \(\overline{M}\) and \(\overline{S}\), respectively. For \(b_{0}\), values close to 1.0 indicate that model performance is very good; for \(R^{2}\), values greater than 0.5 indicate than the simulation results are acceptable; for \({\text{NRMSE}}\), values less than 10%, between 10 and 20%, between 20 and 30%, and > 30%, correspond to the perfect, good, acceptable and poor simulation results, respectively; values of \({\text{EF}}\) range from negative infinity to 1, when \({\text{EF}}\) is close to 0 or negative this means that there is no gain in using the model; As for \(d\), the target value also is also 1.0.
Results and discussion
Evaluation of the AquaCrop model for estimating maize canopy cover, biomass and yield
The most logical pathway for a systematic calibration of AquaCrop is to ensure a sound prediction of the canopy cover firstly due to that model’s ability to predict ET, \(E\) and \(T\) depends on the simulated \({\text{CC}}\) curve (Farahani et al. 2009). Whereas the field management would influence \({\text{CC}}_{x}\), \({\text{CGC}}\) and \({\text{CDC}}\), it was required to perform distinct parameterizations of the \({\text{CC}}\) curves for different years (Table 2). The canopy coverage increased rapidly at seedling and shooting stages, then all the leaves expanded during heading period to reach the peak until senescence occurred with the gradual decrease of plant cover degree, as shown in Fig. 2. A good match was found between the simulated \({\text{CC}}\) and those measured for maize under film-mulching drip irrigation, with \(b_{0}\) = 1.00, \(R^{2}\) = 0.97, \({\text{NRMSE}}\) = 6.97%, \({\text{EF}}\) = 0.93 and \(d\) = 0.99 (Table 3), this was similar to the results of previous studies, like Ran et al. (2018) (\(R^{2}\) = 0.93), Zhao et al. (2018) (\(R^{2}\) = 0.97) and Shen et al. (2019) (\(R^{2}\) = 1.00).
Similar to \({\text{CC}}\), high \(b_{0}\) (1.05) and \(R^{2}\) (0.99), and low \({\text{NRMSE}}\) (11.98%) demonstrated acceptable simulations of aboveground biomass (Fig. 3; Table 3), consistent with the previous studies on the AquaCrop performance for maize in Davis, CA (Hsiao et al. 2009) and in Bushland, TX, Gainesville, FL and Zaragoza, Spain (Heng et al. 2009). As seen from Fig. 3, the AquaCrop model can well simulate the biomass of maize during early and middle growing seasons, but for later in the season, an overestimation existed, which may have been caused by the same \({\text{WP}}^{*}\) used in the overall stage. The aboveground biomass should gradually stabilize later in the period, and according to formula (11), if different values of \({\text{WP}}^{*}\) are adopted in different stages, the simulating effect would be improved; simulation of biomass was also influenced by the accuracy of calculated transpiration, which would be discussed in Sect. 3.2.
Based on formula (12), the simulation results of biomass and setting value of \({\text{HI}}_{0}\) would directly affect the accuracy of the yield simulation, as shown in Table 4. For 2018, the maize yield of this year was lower than normal value, which was because of the continuous rainfall during pollination. Weather belonged to uncontrollable natural conditions and had great impacts on field experiment, so the measured yield value of this year could be neglected; analyzing data of 2014–2017, the standard deviation (D) between simulated and measured values ranged from − 8.98% to 15.39%, with mean value of 4.13%. Araya et al. (2010) did testing for the applicability of AquaCrop on barley; the results indicated that unifying the harvest indexes (HI) of different cultivars into the same value would reduce the simulating precision of yield. Studies of Ran et al. (2018) have also shown the importance of the HI module in AquaCrop model for simulating yield.
Overall, the AquaCrop model can well simulate the growth process of maize, but the simulation results of the final biomass and yield were not as good as those of the canopy cover; for this section, improving the settings of \({\text{WP}}^{*}\) and HI are critical to enhance the accuracy.
Evaluation of the AquaCrop model for estimating maize evapotranspiration
The AquaCrop model was able to reflect the trend of daily changes in \({\text{ET}}\) well (Fig. 4), with 5-year mean \(b_{0}\), \(R^{2}\), \({\text{NRMSE}}\), \({\text{EF}}\) and \(d\) of 0.89, 0.87, 22.87%, 0.81 and 0.95, respectively. There was underestimation, especially to the middle of the season; this is consistent with the ET simulation results for wheat by Toumi et al. (2016) and maize by Katerji et al. (2013).
The respective ratios of \(E\) and \(T\) in \({\text{ET}}\) determine the water use efficiency of crops, and the separated simulation of \(E\) and \(T\) is also one of the difficulties in model construction. In AquaCrop, the simulation of \({\text{ET}}\) was based principally on two parameters: the maximum soil evaporation coefficient (\(K_{{C_{{e,{\text{ wet}}}} }}\)) and maximum crop transpiration coefficient (\(K_{{c_{{{\text{tr}},x}} }}\)). The value of \(K_{{C_{{e, {\text{wet}}}} }}\) was 1.10 (default value) in formula (9), adjusted by soil water content before emergency, canopy cover after germination and mulched soil fraction. As shown in Fig. 5, the AquaCrop model could only reflect the soil evaporation at the seedling stage, in other words, formulas (9–10) were applicable while the canopy cover was very low, but after the early season, the \({\text{CC}}\) value increased and \(\left( {1 - {\text{CC}}^{*} } \right)\) decreased. Generally, \({\text{CC}}\) reached the maximum value (91–98%) on the 65th day after sowing (at shooting stage), but \(\left( {1 - {\text{CC}}^{*} } \right)\) was directly used to represent the effect of crop canopy coverage on soil evaporation, hence causing the simulated values of \(E\) to be far less than the measured values. From 2014 to 2018, the mean b0 was only 0.48, \({\text{NRMSE}}\) was 84.25% and \({\text{EF}}\) was − 0.20. In addition, the simulation effect of the model on the diurnal variation trend of \(E\) was also not good, with \(R^{2}\) of only 0.48 and d of 0.71. Abandoning the FAO dual \(K_{{\text{c}}}\) approach is likely the main reason causing this limitation. According to the comparison results from Paredes et al. (2015) and Wei et al. (2015), the SIMDualKc, a model based on dual \(K_{{\text{c}}}\) approach, could perform better in partitioning \({\text{ET}}\), and it computed soil evaporation coefficient \(K_{{\text{e}}}\) by performing a daily soil water balance of the evaporable layer (Allen et al. 2005; Rosa et al. 2012), which was totally different from the AquaCrop which connects \({\text{ET}}\) components directly to the simulated crop canopy cover \({\text{CC}}^{*}\). This modeling approach used with \(K_{{\text{e}}}\) was very likely to cause a poor reaction to the precipitation or irrigation events occurring in mid- and late-season when the maximum \(f_{{\text{c}}}\) or \({\text{CC}}\) was attained, thus, the model underestimated \(E\). Moreover, the micro-lysimeter evaporation was not affected by root water uptake while those roots did exist in the soil, so \(E\) measured by micro-lysimeters was expected to be higher which could also explain the model trend to underestimate micro-lysimeters’ observation data. Similar results have been reported for soybean (Wei et al. 2015) and maize (Zhao et al. 2013).
The AquaCrop model nearly abandons the FAO “\(K_{{\text{c}}} *{\text{ET}}_{0}\)” approach and adopts \(K_{{c_{{\text{tr,adj}}} }}\), a coefficient following a curvilinear curve proportional to the \({\text{CC}}\) curve and varying with other internal adjustment factors. The calibrated \(K_{{c_{{{\text{tr,}}x}} }}\) was 1.20 (Table 2). In this study, as shown in Fig. 6, the model also underestimated crop transpiration especially during mid-season, but the simulating performance of daily transpiration was evidently better than that of evaporation, with \(b_{0}\) of 0.97, \(R^{2}\) of 0.91, \({\text{NRMSE}}\) of 22.57%, \({\text{EF}}\) of 0.90 and \(d\) of 0.97 (Table 3). One reason is that the values of \(T\) were higher, so the simulation errors would be relatively small when compared with those for \(E\); another is that although \({\text{CC}}\) cannot accurately reflect the degree of variation in \(E\), it can directly represent the impact of crop growth on \(T\). In other words, there was a closer relationship between \({\text{CC}}\) and \(T.\) Some scholars have assessed the performance of \({\text{ET}}\) partitioning with AquaCrop: Ran et al. (2017) considered that the AquaCrop model took into account the plant itself (\({\text{CC}}\)) affected by changed soil water content, so \(T\) would be calculated with higher accuracy, while results of Paredes et al. (2014) showed the opposite, in which the adjusted crop coefficient linked with \({\text{CC}}\) was the reason for simulation error. Pereira et al. (2015) thought \(K_{{c_{{{\text{tr}},x}} }}\) was difficult to be calibrated because it was assumed for = \({\text{CC}}\)100%, when the actual < \({\text{CC}}\)100%, it required internal adjustments, as shown in formula (8). In this study, the AquaCrop model simulated daily \(T\) with a constant value (\(Kc_{{{\text{Tr}},x}}\)=1.20) during 5 years, and the simulation errors were acceptable, suggesting the feasibility of calculating \(T\) based on \({\text{CC}}\); besides, this method can not only avoid measurement of many parameters such as physiological factors in response to water stress, but also benefit application at a regional scale.
Evaluation of the AquaCrop model for estimating soil water content
The AquaCrop model was also used to simulate total water content in a meter of soil (Fig. 7). It is obvious that the model could effectively reflect the effect of irrigation and heavy rainfall events, but the downtrend proved faster than the actual situation. The simulation area had a depth of 1.0 m, so the degree of fluctuation would be reduced after averaging the measured soil water content values of each soil layer. Besides, although error indicators, such as \(b_{0}\), \({\rm NRMSE}\), and \(d\), showed high accuracy, with values of 1.01, 6.25% and 0.68, respectively, the \(R^{2}\) was low (0.33) and the \({\text{EF}}\) was even negative (− 0.81), indicating that the simulated soil moisture variation were not consistent well with the measurements. As a crop model, AquaCrop can simulate the dynamic balance among transpiration, rainfall and irrigation well, but there were some shortages for the soil, such as the underestimation of \(E\) as found in Sect. 3.2, which was also one of the reasons for errors in simulating soil water content. Coupling the crop model and soil models can be considered in the future to achieve a higher accuracy of farmland water balance simulation.
Conclusions
In this paper, the AquaCrop model was applied to maize under film-mulching drip irrigation in northwest China and a large amount of experimental data gathered during the years of 2014–2018 were used for model assessment. Although the simulation results of canopy cover, biomass and grain yield were good, \({\text{WP}}^{*}\) and \({\text{HI}}\), as the nonconservative parameters in model, directly affected the calculation precision, setting segmented values for the two parameters according to growth stages can be considered for enhancing the simulating accuracy. For the simulation of diurnal variation of \({\text{ET}}\) and \(T\), AquaCrop model performed well during the entire growing period; however, the simulation effect for \(E\) was not good. Model adopted canopy cover to calculate \(E\), but in our investigation, the canopy cover could exceed 90% after 65 days of sowing, causing simulated values of \(E\) to be far less than actual values. In fact, the canopy only intercepts a portion of the rainfall and reduces \(E\) by a small amount. Apparently, the AquaCrop model overestimated the influence of canopy cover on the soil evaporation. The model revealed the crop-water response mechanism by analyzing the relationship between the effective use of water in soil and the crop yield, so it could well simulate values of soil water content (\(b_{0}\) = 1.01) but not the dynamic variation process (\(R^{2}\) = 0.33).
At present, only a few studies have verified the accuracy of the AquaCrop model using measured \({\text{ET}}\), \(E\) and \(T\). Results obtained in this paper suggest that the formula used in the model to calculate \(E\) has some defects, leading to a large deviation between the simulated and measured values. How to consider the influence of film-mulching drip irrigation, as well as improve the existing soil moisture and evaporation calculation modules, would be of great significance to enhance the applicability of the AquaCrop model to a heterogeneous surface. In the near future, further studies will be conducted to provide impetus for the development of the crop model.
References
Abdalhi MAM, Jia ZH (2018) Crop yield and water saving potential for AquaCrop model under full and deficit irrigation managements. Ital J Agron 13:267–278
Abrha B, Delbecque N, Raes D, Tsegay A, Todorovic M, Heng LK, Vanutrecht E, Geerts S, Garciavila M, Deckers S (2012) Sowing strategies for barley (Hordeum vulgare L.) based on modelled yield response to water with AquaCrop. Exp Agr 48:252–271
Allen RG, Pereira LS, Raes D, Smith M (1998) Crop evapotranspiration—guidelines for computing crop water requirements. FAO irrigation and Drainage Paper 56, Rome
Allen RG, Pereira LS, Smith M, Raes D, Wright JL (2005) FAO-56 Dual crop coefficient method for estimating evaporation from soil and application extensions. J Irrig Drain 131:2–13
Araya A, Habtu S, Hadgu KM, Kebede A, Dejene T (2010) Test of AquaCrop model in simulating biomass and yield of water deficient and irrigated barley (Hordeum vulgare). Agr Water Manage 97:1838–1846
Babel MS, Deb P, Soni P (2019) Performance evaluation of AquaCrop and DSSAT-CERES for maize under different irrigation and manure application rates in the Himalayan region of India. Agr Res 8:207–217
Bannayan M, Hoogenboom G (2009) Using pattern recognition for estimating cultivar coefficients of a crop simulation model. Field Crops Res 111:290–302
Belay AT, Patil RH (2017) Evaluation of DSSAT-CERES maize model for northern transitional zone of Karnataka. J Agric Res Technol 42:036–043
Castañeda A, Leffelaar PA, Álvaro-Fuentes J, Cantero-Martínez C, Minguez MI (2015) Selecting crop models for decision making in wheat insurance. Eur J Agron 68:97–116
Deb P, Shrestha S, Babel MS (2015) Forecasting climate change impacts and evaluation of adaptation options for maize cropping in the hilly terrain of Himalayas: Sikkim, India. Theor Appl Climatol 121:649–667
Ding RS, Kang SZ, Zhang YQ, Hao XM, Tong L, Du TS (2013) Partitioning evapotranspiration into soil evaporation and transpiration using a modified dual crop coefficient model in irrigated maize field with ground-mulching. Agr Water Manage 127:85–96
Du WY, He XK, Shamaila Z, Hu ZF, Zeng AJ, Muller J (2011) Yield and biomass prediction testing of AquaCrop model for winter wheat. TCSAM 42:174–178 (in Chinese)
Eisenhauer JG (2003) Regression through the origin. Teach Stat 25:76–80
Falge E, Baldocchi DD, Olson R, Anthoni P, Aubinet M, Bernhofer C, Burba G, Ceulemans R, Clement R, Dolman H, Granier A, Gross P, Grunwald T, Hollinger D, Jensen NO, Katul G, Keronen P, Kowalski A, Ta Lai C, Law BE, Meyers T, Moncrieff J, Moors E, Munger JW, Pilegaard K, Rannik U, Rebmann C, Suyker A, Tenhunen J, Tu K, Verma S, Vesala T, Wilson K, Wofsy S (2001) Gap filling strategies for long term energy flux data sets. Agric For Meteorol 107:71–77
Farahani HJ, Izzi G, Oweis TY (2009) Parameterization and evaluation of the AquaCrop model for full and deficit irrigated cotton. Agron J 101:469–476
Finnigan JJ, Clement R, Malhi Y, Leuning R, Cleugh HA (2003) Re-evaluation of long-term flux measurement techniques. Part I: averaging and coordinate rotation. Bound Layer Meteorol 107:1–48
García-Vila M, Fereres E (2012) Combining the simulation crop model AquaCrop with an economic model for the optimization of irrigation management at farm level. Eur J Agron 36:21–31
García-Vila M, Fereres E, Mateos L, Orgaz F, Steduto P (2009) Deficit irrigation optimization of cotton with AquaCrop. Agron J 101:477–487
Geerts S, Raes D, Garcia M, Taboada C, Miranda R, Cusicanqui J, Mhizha T, Vacher J (2009) Modeling the potential for closing quinoa yield gaps under varying water availability in the Bolivian Altiplano. Agr Water Manage 96:1652–1658
Ham JM, Heilman JL (1990) Dynamics of a heat balance stem flow gauge during high flow. Agron J 82:147–152
He QS, Li SE, Kang SZ, Yang HB, Qin SJ (2018) Simulation of water balance in a maize field under film-mulching drip irrigation. Agr Water Manage 210:252–260
Heng LK, Hsiao T, Evett S, Howell TA, Steduto P (2009) Validating the FAO AquaCrop model for irrigated and water deficient field maize. Agron J 101:488–498
Hsiao TC, Heng LK, Steduto P, Rojas-Lara B, Raes D, Fereres E (2009) AquaCrop-the FAO crop model to simulate yield response to water: III. Parameterization and testing for maize. Agron J 101:448–459
Jin XL, Feng HK, Zhu XK, Li ZH, Song SN, Song XY, Yang GJ, Xu XG, Guo WS (2014) Assessment of the AquaCrop model for use in simulation of irrigated winter wheat canopy cover, biomass, and grain yield in the North China Plain. PLoS ONE 9:e86938
Katerji N, Campi P, Mastrorilli M (2013) Productivity, evapotranspiration, and water use efficiency of corn and tomato crops simulated by AquaCrop under contrasting water stress conditions in the Mediterranean region. Agr Water Manage 130:14–26
Kumar P, Sarangi A, Singh DK, Parihar SS (2015) Evaluation of AquaCrop model in predicting wheat yield and water productivity under irrigated saline regimes. Irrig Drain 63:474–487
Lorite IJ, García-Vila M, Santos C, Ruiz-Ramosc M, Fereresb E (2013) AquaData and AquaGIS: two computer utilities for temporal and spatial simulations of water-limited yield with AquaCrop. Comput Electron Agr 96:227–237
Mainuddin M, Kirby M, Hoanh CT (2011) Adaptation to climate change for food security in the lower Mekong Basin. Food Security 3:433–450
Nash JE, Sutcliffe JV (1970) River flow forecasting through conceptual models part I—a discussion of principles. J Hydrol 10:282–290
Paredes P, Melo-Abreu JPD, Alves I, Pereira LS (2014) Assessing the performance of the FAO AquaCrop model to estimate maize yields and water use under full and deficit irrigation with focus on model parameterization. Agr Water Manage 144:81–97
Paredes P, Wei Z, Liu Y, Xu D, Xin Y, Zhang B, Pereira LS (2015) Performance assessment of the FAO AquaCrop model for soil water, soil evaporation, biomass and yield of soybeans in North China Plain. Agr Water Manage 152:57–71
Paw KTU, Baldocchi DD, Meyers TP, Wilson KB (2000) Correction of eddy-covariance measurements incorporating both advective effects and density fluxes. Bound Layer Meteorol 97:487–511
Pereira LS, Paredes P, Rodrigues GC, Neves MM (2015) Modeling malt barley water use and evapotranspiration partitioning in two contrasting rainfall years. Assessing AquaCrop and SIMDualKc models. Agr Water Manage 159:239–254
Qin SJ, Li SE, Kang SZ, Du TS, Tong L, Ding RS, Wang YH, Guo H (2019) Transpiration of female and male parents of seed maize in northwest China. Agr Water Manage 213:397–409
Raes D, Steduto P, Hsiao TC, Fereres E (2009a) AquaCrop—the FAO crop model to simulate yield response to water: II. Main algorithms and software description. Agron J 101:438–447
Raes D, Steduto P, Hsiao TC, Fereres E (2009b) AquaCrop—the FAO crop model to simulate yield response to water: reference manual annexes. Available from: https://www.fao.org/nr/water/aquacrop.html
Raes D, Steduto P, Hsiao T, Fereres E (2012) AquaCrop Version 4.0. ReferenceManual. FAO, Land and Water Division, Rome, Italy
Ran H, Kang SZ, Li FS, Tong L, Ding RS, Du TS, Li SE, Zhang XT (2017) Performance of AquaCrop and SIMDualKc models in evapotranspiration partitioning on full and deficit irrigated maize for seed production under plastic film-mulch in an arid region of China. Agr Syst 151:20–32
Ran H, Kang SZ, Li FS, Du TS, Tong L, Li SE, Ding RS (2018) Parameterization of the AquaCrop model for full and deficit irrigated maize for seed production in arid Northwest China. Agr Water Manage 203:438–450
Rosa RD, Paredes P, Rodrigues GC, Alves I, Fernando RM, Pereira LS, Allen RG (2012) Implementing the dual crop coefficient approach in interactive software: 1. Background and computational strategy. Agric Water Manage 103:8–24
Saab MTA, Todorovic M, Albrizio R (2015) Comparing AquaCrop and CropSyst models in simulating barley growth and yield under different water and nitrogen regimes. Does calibration year influence the performance of crop growth models? Agr Water Manage 147:21–33
Sakuratani T (1981) A heat balance method for measuring water flux in the stem of intact plants. J Agric Meteorol 37:9–17
Shen QX, Ding RS, Du TS, Tong L, Li SE (2019) Water use effectiveness is enhanced using film mulch through increasing transpiration and decreasing evapotranspiration. Water 11:1153
Steduto P, Hsiao TC, Raes D, Fereres E (2009) AquaCrop—The FAO crop model to simulate yield response to water: I. Concepts and underlying principles. Agron J 101:426–437
Todorovic M, Albrizio R, Zivotic L, Saab MTA, Stockle C, Steduto P (2009) Assessment of AquaCrop, CropSyst, and WOFOST models in the simulation of sunflower growth under different water regimes. Agron J 101:509–521
Toumi J, Er-Raki S, Ezzahar J, Khabba S, Jarlan L, Chehbouni A (2016) Performance assessment of AquaCrop model for estimating evapotranspiration, soil water content and grain yield of winter wheat in Tensift Al Haouz (Morocco): application to irrigation management. Agr Water Manage 163:219–235
Twine TE, Kustas WP, Norman JM, Cook DR, Houser PR, Meyers TP, Prueger JH, Starks PJ, Wesely ML (2000) Correcting eddy-covariance flux underestimates over a grassland. Agric For Meteorol 103:279–300
Vanuytrecht E, Raes D, Willems P (2011) Considering sink strength to model crop production under elevated atmospheric CO2. Agric For Meteorol 151:1753–1762
Vanuytrecht E, Raes D, Steduto P, Hsiao TC, Fereres E, Heng LK, Vila MG, Moreno PM (2014) AquaCrop: FAO’s crop water productivity and yield response model. Environ Model Softw 62:351–360
Wang XX, Wang QJ, Fan J, Fu QP (2013) Evaluation of the AquaCrop model for simulating the impact of water deficits and different irrigation regimes on the biomass and yield of winter wheat grown on China’s Loess Plateau. Agr Water Manage 129:95–104
Webb EK, Pearman GI, Leuning R (1980) Correction of flux measurements for density effects due to heat and water vapor transfer. Q J R Meteor Soc 106:85–100
Wei Z, Paredes P, Liu Y, Chi WW, Pereira LS (2015) Modelling transpiration, soil evaporation and yield prediction of soybean in North China Plain. Agr Water Manage 147:43–53
Willmott CJ (1984) On the evaluation of model performance in physical geography. In: Gaile GL, Willmott CJ (eds) Spatial statistics and models, vol 40. Springer, Dordrecht, pp 443–460
Yang N, Sun ZC, Zhang LZ, Zheng JN, Feng L, Li K, Zhang Z, Feng C (2015) Simulation of water use process by film mulched cultivated maize based on improved AquaCrop model and its verification. TCSAE 31:122–132 (in Chinese)
Zeleke KT, Luckett D, Cowley R (2011) Calibration and testing of the FAO AquaCrop model for Canola. Agron J 103:1610–1618
Zhao NN, Yu L, Cai JB, Paredes P, Rosa RD, Pereira LS (2013) Dual crop coefficient modelling applied to the winter wheat-summer maize crop sequence in North China Plain: basal crop coefficients and soil evaporation component. Agr Water Manage 117:93–105
Zhao Y, Mao XM, Bo LY (2018) Simulation of soil moisture dynamics and seed-maize growth under different mulching and irrigation conditions. TCSAE 49:195–204 (in Chinese)
Acknowledgements
We greatly appreciate the careful and precise reviews by the anonymous reviewers. They paid great efforts on improving the manuscript and study. This work was financially supported by the National Key Research and Development Program of China (2016YFC0400201), and Chinese National Natural Science Fund (51622907, 51879262).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
He, Q., Li, S., Hu, D. et al. Performance assessment of the AquaCrop model for film-mulched maize with full drip irrigation in Northwest China. Irrig Sci 39, 277–292 (2021). https://doi.org/10.1007/s00271-020-00705-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00271-020-00705-z