Abstract
Purpose
This study aimed to present the institutional experience and algorithm for performing biliary interventions in liver transplant patients using the modified Hutson loop access (MHLA) and the impact of percutaneous endoscopy via the MHLA on these procedures.
Methods
Over 13 years, 201 MHLA procedures were attempted on 52 patients (45 liver transplants; 24 living and 21 deceased donors) for diagnostic (e.g., cholangiography) and therapeutic (e.g., stent/drain insertion and cholangioplasty) purposes. The most common indications for MHLA were biliary strictures (60%) and bile leaks (23%). Percutaneous endoscopy was used to directly visualize the biliary-enteric anastomosis, diagnose pathology (e.g., ischemic cholangiopathy), and help in biliary hygiene (removing debris/casts/stones/stents) in 138/201 (69%) procedures. Technical success was defined as cannulating the biliary-enteric anastomosis and performing diagnostic/therapeutic procedure via the MHLA.
Results
The technical success rate was 95% (190/201). The failure rate among procedures performed with and without endoscopy was 2% (3/138) versus 13% (8/63) (P = 0.0024), and the need for new transhepatic access (to aid the procedure) was 12% (16/138) versus 30% (19/63) (P = 0.001). Despite endoscopy, failure in 2% of the cases resulted from inflamed/friable anastomosis (1/3) and high-grade stricture (2/3) obstructing retrograde cannulation of biliary-enteric anastomosis. Major adverse events (bowel perforation and injury) occurred in 1% of the procedures, with no procedure-related mortality.
Conclusions
MHLA-based percutaneous biliary intervention is a safe and effective alternative to managing complications after liver transplant. Percutaneous endoscopy via the MHLA improves success rates and may reduce the need for new transhepatic access.
Level of Evidence Level 4
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biliary interventions address various complications like strictures, bile leaks. [1, 2] Endoscopic retrograde cholangiopancreatography (ERCP) is the initial approach, with percutaneous transhepatic cholangiography (PTC) reserved for ERCP failures or patients with complex (post-surgical) gastrointestinal anatomy [3, 4]. Liver transplant and major hepatobiliary surgery patients often need repeat biliary procedures because of recurrent issues such as strictures. A systematic review of 14,359 liver transplants reported a 13% incidence of posttransplant anastomotic strictures. Notably, patients with strictures frequently have nondilated intrahepatic biliary ducts, posing challenges for PTC due to the risk of injuring the portal vein and hepatic artery in transplanted livers [5,6,7,8,9,10,11].
Initially introduced by Hutson et al. in 1984, the concept aimed to create a stoma in the anterior abdominal wall to access the Roux limb of the Roux-en-Y hepaticojejunostomy, providing easier access for repeat biliary interventions in patients with a biliary-enteric anastomosis [12]. This technique evolved to the modified Hutson Loop (MHL), replacing the stoma with surgical affixation (jejunopexy) of the Roux limb to the anterior abdominal wall, resulting in decreased infection and leakage risks, attributed to the absence of an external stoma [4, 5, 13]. MHL access (MHLA) has been described previously by the authors' group [4]. The safety and effectiveness of percutaneous transjejunal access interventions without surgically-affixed Roux limb has also been described [5, 6].
Compared to PTC, MHLA offers easier access and eliminates the risk of hepatic injury by avoiding transhepatic needle insertion. It allows repeated interventions upon numerous biliary segments and anastomoses from single transjejunal access instead of requiring multiple access sites and long-term percutaneous drain [4, 5]. Despite these benefits, MHLA has yet to gain traction. Furthermore, the introduction of endoscopy in interventional radiology (IR) has improved the ability to perform diagnostic and therapeutic procedures using MHLA [14].
This study aims to expand upon the previously published study with larger and more comprehensive sample size and present the institutional algorithm for diagnosing and managing biliary complications in posttransplant patients.
Materials and Methods
This retrospective study, reporting 201 MHLA-procedure attempts on 52 patients (including 21 patients from previously published study) [4] from March 2009 to December 2022, was conducted after Institutional Review Board’s approval.
At the authors' institution, MHL patients with suspected biliary pathologies were discussed at monthly, multidisciplinary biliary conferences and referred to IR for diagnostic/therapeutic interventions. The most common indications for MHLA were stricture and biliary leak (Table 1). Supplemental Figure 1 presents the institutional algorithm at the author’s institution for approaching such patients.
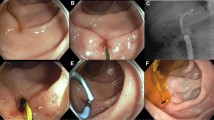
65-year-old man status post deceased donor liver transplant and hepaticojejunostomy with a modified Hutson loop presenting with elevated alkaline phosphatase and bilirubin levels. a Ultrasound demonstrating minimal extrahepatic biliary ductal dilatation (white arrow). b Coronal MRI demonstrating no significant biliary ductal dilatation with debris within the duct. c Image of the bile cast removed from the bile duct. d Cholangiography via the endoscope at the anastomosis (white arrow) demonstrating a patent biliary system (red arrow). The blind-ending limb of the Hutson loop with the wire is seen (black arrow). The blue arrow points to the surgical clips at the modified Hutson loop access site
Technique
The hepatobiliary anatomy was evaluated by reviewing prior imaging and notes as discussed by Riaz et al. [4] Prophylactic antibiotics were administered to all patients, following the Society of Interventional Radiology (SIR) guidelines [15]. Candidacy for the type of anesthesia was determined by Anesthesiology.
Percutaneous Access of the MHL and Catheterizing the Biliary-Enteric Anastomosis
Initial access and catheterization were performed as shown previously [4]. In brief, the MHL was accessed using a 21-gauge needle after identifying under sonographic/fluoroscopic guidance. The intraluminal needle position was confirmed by dilutional contrast fluoroscopy. A 0.018-inch guidewire was introduced into the jejunal lumen and upsized with a transitional dilator to a 0.035-inch system. Afterward, the sheath was oriented in the direction of the liver (retrograde) rather than the jejunojejunostomy (anterograde). In the case of antegrade initial access, a wire was inserted and subsequently, the sheath was retracted over the wire. Subsequently, a catheter positioned side-by-side was employed to propel a wire in a retrograde fashion toward the liver.
Percutaneous Endoscopy in Biliary Interventions via MHLA
In patients where contrast injection via the MHLA could not opacify the biliary system, an endoscope was used to identify the biliary-enteric anastomosis [4]. This endoscope has an outer diameter of 10.5 French, which was introduced through a ≥ 11-French sheath. Various wires, catheters, and devices could be passed through the 3.6 French working channel of the endoscope (SpyGlass Discover, Boston Scientific, Marlborough, MA) (Fig. 1/Video 1) Direct visualization under endoscope aided in identifying the biliary-enteric anastomosis ostium (Video 2). Other cases and techniques (including plastic stent placement and retrieval) have been previously described by Entezari et al. [14].
Biliary Interventions via MHLA
Once the biliary system was cannulated, all patients had a retrograde cholangiogram to evaluate the biliary system. Various therapeutic interventions were performed via the MHLA, including creation of a neo-duct/neo-anastomosis, cholangioplasty, stent/drain placement, and endobiliary radiofrequency ablation (RFA) [4, 16].
A percutaneous transhepatic approach was attempted if the initial MHLA was unsuccessful. This percutaneous transhepatic access could be upsized safely to a 4-French catheter, which can help cross the tight anastomosis and the wire could be snared from the jejunal access to obtain Archimedean (through and through) access (from the skin through the liver, the bile ducts, the hepaticojejunostomy, the jejunum, and out the MHLA). (Supplemental Figure 2).
Removing the Access
Before removing the sheath/catheter from the jejunum, afferent loop obstruction was excluded (by contrast injection into the roux limb to evaluate a) bowel dilatation and b) free flow of contrast towards the jejunojejunostomy) to prevent the formation of an enterocutaneous fistula. Patients were observed for approximately 2 h in the post-anesthesia care unit or outpatient recovery room. A small 6-French drain was placed into the Hutson loop to preserve access for subsequent procedures in 10/52 patients who required PTC access to puncture the Hutson loop (Archimedean access) (Supplemental Figure 3).
Follow-up
Regular follow-ups were scheduled to evaluate the efficacy and safety of the therapeutic intervention. Patients with drains or plastic stents were followed-up every 4–6 weeks for possible removal, assessing the patency, and considering repeat intervention such as stent placement. Total -bilirubin and alkaline phosphatase (ALP) were measured 1–2 months after the last MHL intervention and compared to the pre-intervention value. Adverse events (AEs) were reported per established guidelines [17, 18].
Statistical Analysis
MedCalc® Statistical Software version 20.111 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2022) was utilized to perform all statistical analyses. The significance level was set at 0.05. The numbers are presented as mean, median, and range. The chi-squared test was used to compare the instances of new transhepatic access, and procedural failure rate with and without endoscopy. The Wilcoxon test (paired samples) was used to compare the values of total-bilirubin and ALP pre- and post-interventions.
Result
33 patients (63%) were male. The mean age at the first MHLA attempt was 52 years (20–82 years). The median interval between the biliary-enteric anastomosis creation and the first MHLA attempt was 86 days (1 day–13 years). The underlying diagnoses that resulted in the hepatobiliary surgery with MHL creation are shown in Table 1. Of 52 patients, 43 (83%) had undergone MRCP, and 21 (40%) had failed ERCP before the procedure.
Of 201 procedures, 115 (57%) were performed under general anesthesia, 31 (16%) under monitored anesthesia care, and 55 (27%) under moderate sedation.
Of 201 MHLA attempts in 52 patients, 190 (95%) were technically successful (defined as ability to cannulate bilio-enteric anastomosis and perform diagnostic/therapeutic procedures) in 48 (92%) patients. Figure 2 presents the total number of interventions and procedures. Figure 3 presents the number of procedures/endoscopies per year via the MHL.
The mean number of MHLAs obtained per patient was 3.95 (1–15). Fluoroscopy was used in all procedures. Out of 190 successful MHLAs, 41 (22%; 9 patients) were diagnostic-only, whereas 149 (78%; 29 patients) were therapeutic (e.g., drain insertion, cholangioplasty, stent insertion) (Fig. 2).
There were 11 (5%) unsuccessful MHLAs in eleven patients. In 6/11, the initial percutaneous access was obtained; however, the operator could not visualize/cannulate the bilio-enteric anastomosis. On the other hand, MHLA was not attempted in the remaining 5/11 despite MHL. Therefore, new transhepatic access was obtained in all these cases through which the following interventions were performed: internal–external drain (IE) biliary placement (9/11 [82%]), cholangioplasty (5/11[45%]), neo-anastomosis creation with radiofrequency (RF) wire (1/11 [(9%]), and biliary stent placement (1/11 [(9%]). Compared to unsuccessful MHLAs, new drains were placed only in 18/149 successful therapeutic MHLAs (P < 0.0001). Of 18 drains, 5 were MHL cholangiography/cholangioscopy-assisted IE biliary drains and 13 MHL drains (one retrograde biliary drain inserted percutaneously through MHL into the biliary system, and 12 percutaneous MHL drains to preserve access) (Fig. 2).
Cholangioscopy (endoscopy) was performed in 138/201 (69%) accesses to visualize the biliary-enteric anastomosis and the biliary system. In endoscopy-guided procedures, the technical failure rate was 3/138 (2%), whereas without endoscopy, it was 8/63 (13%) (P = 0.0024). A new percutaneous transhepatic access (in case of MHLA failure, to gain Archimedean access or aid in therapeutic intervention) was obtained in 16/138 (12%) endoscopy-guided procedures compared to 19/63 (30%) procedures without endoscopy (P = 0.0013).
Of the 29 patients with successful therapeutic interventions by IR, twenty-four patients had biliary obstruction, and six had bile leaks (one had an obstruction and a bile leak). On comparison of pre-and post-intervention labs in patients with suspected biliary obstruction undergoing therapeutic interventions via MHL, serum bilirubin decreased by a median of 0.6 mg/dL (pre: 1.55 mg/dL [0.4–24]; post: 0.95 mg/dL [0.4–20], P = 0.3), and serum alkaline phosphatase decreased by a median of 140.5 U/L (pre: 296 U/L [127–805]; post: 155.5 [38–581], P = 0.0047). Four (14%) patients with ischemic cholangiopathy subsequently underwent a repeat liver transplant following repeated MHL interventions for biliary hygiene (clearing of biliary casts and debris) [19]. Among patients (6/29) with a biliary leak, the leak was resolved after undergoing therapeutic interventions (stent placement across the transected duct) via MHL.
Among 19-patients undergoing diagnostic-only procedures, the reasons for not performing any therapeutic intervention were: cholangiography via the MHLA demonstrated no biliary obstruction in 13 patients, no leak in five, and a small leak with no perihepatic fluid collection in one (resolved on the follow-up cholangiogram without any intervention).
In 133/190 (70%) accesses, patients were discharged the same day (outpatient), whereas, in 57/190 (30%), patients were either already admitted (33/57 [58%]) or were admitted after the IR intervention (24/57 [42%]). For patients admitted post-intervention, the mean hospital stay length per patient was 2.5 days (1–10 days).
Of 29 patients with successful therapeutic interventions, a repeat transplant was required only in two (7%) (One had posttransplant rejection with ischemic cholangiopathy, and the other had posttransplant liver failure). The remaining patients (86%) were managed by minimally invasive IR procedures.
Adverse Events
Of 196 MHLAs (excluding five where MHLA was not attempted.), 2 (1%) resulted in major AEs: duodenal/jejunal perforation (SIR class D) requiring surgical correction, and bowel injury (SIR class D) resolving without surgery. In case of duodenal/jejunal perforation, the MHL was accessed 13 days after its creation, and IR inserted a 24 French peel-away sheath to allow interventional GI to visualize the biliary-enteric anastomosis using a 24 French endoscope. In the other patient with bowel injury, the patient was to be discharged the same day, but had to be admitted because of severe abdominal pain postprocedure (Table 2).
There was no procedure-related mortality rate (SIR Class F). Abnormal liver function tests, e.g., deranged bilirubin, ALP, alanine transaminase (ALT), and/or aspartate transaminase (AST) levels were seen in 36/196 (18%) procedures.
Eight AEs resulted in prolonged hospital stay (in patients already admitted for other reasons), of which four resulted in transfer to the intensive care unit (ICU) due to sepsis. Fifteen adverse events resulted in readmission (same day or later), with two resulting in transfer to the ICU (1/2: sepsis; 1/2: contrast allergy-related hypotension). The mean hospital stay duration was 4.7 days (1–18 days) for these readmissions.
Discussion
This study discusses the efficacy and safety of biliary interventions performed via MHLA in 52 consecutive patients. Of 201 procedures, 190 (95%) were technically successful. The technical success rate in this study is similar to previously published studies (88–95%) [4,5,6, 20]. The 95% success rate is also acceptable compared to the success rate thresholds (50–95%) for various procedural outcomes performed through transhepatic access [21]. The use of percutaneous endoscope for directly visualizing the biliary system during the procedure significantly reduced the rate of failure to cannulate the biliary-enteric anastomosis from 13% (8/63) to 2% (3/138). The procedural failure despite using endoscopy was attributed to the inflamed and friable anastomosis (1/3) and high-grade stricture (2/3), preventing cannulation. Therefore, the operator decided to obtain transhepatic access.
Abnormal liver function tests prompt cross-sectional imaging [22]. In hepaticojejunostomies patients, biliary obstruction could occur without significant ductal dilatation. Early intervention can identify an obstruction and prevent secondary biliary cirrhosis [23]. For those with known ischemic cholangiopathy and biliary casts, biliary hygiene (to prevent cholangitis by clearing biliary obstruction) can be achieved via the MHLA until transplant is repeated, eliminating the need for a long-term drains [19]. MHLA provides a minimally invasive way to access the biliary ducts without transversing the transplant liver parenchyma.
At the authors' institution, MHLs are consistently formed in both living and deceased donor liver transplants with biliary-enteric anastomosis and in some other complicated hepatobiliary surgeries. However, they are not routinely created for the pediatric population and the Whipple procedure. Drawing on over 13-year institutional experience, the authors have developed a stepwise algorithm to diagnose and manage biliary complications in patients with MHL (Supplemental Figure 1) [4].
The study’s major AEs rate of 1% and procedure-related mortality rate of 0% align with previously published studies [4,5,6]. In case of duodenal/jejunal perforation, early intervention (within13 days of MHL formation), and use of 24-French endoscope by gastroenterology partially contributed to the perforation. Authors suggest at least one-month interval between MHL creation and MHLA. In another study with a two-decade experience of percutaneous transjejunal biliary access (without MHL), only three jejunal perforation-related AEs occurred with > 4-French tracts, but no such complication occurred with sheath sizes ≤ 12-French [4, 6].
Comparable technical success rates have been reported for liver transplant patients with biliary-enteric anastomosis undergoing biliary interventions via ERCP and PTC. (a) Kohli et al. [29]: Technical success rate of 82.8% (24/29) and 85.7% (6/7) for ERCP and PTC, respectively; (b) Hammad et al. [30]: 76% and 77% for ERCP and PTC, respectively in a 71-patient study (28/71 had liver transplants); (c) Chahal et al. [31]: 71% (22/31) rate in patients undergoing ERCP. A study comparing the safety and efficacy of MHLA, ERCP, and PTC in patients with biliary-enteric anastomoses is needed to consolidate the role of MHLA.
The limitations of this study include a) retrospective nature; b) single-institution study in a center, where modified Hutson loops are routinely formed (as this is not routine in most other centers, this might affect broader applicability); c) no direct comparison between PTC and MHLA; and d) patients with different indications and underlying diagnoses.
In conclusion, percutaneous MHLA can be safely used to perform diagnostic and therapeutic biliary interventions in patients with complex post-surgical biliary anatomy with biliary-enteric anastomoses. Furthermore, percutaneous endoscopy owing to direct visualization of biliary system may improve the success rate of these procedures and decrease the need for new transhepatic access.
Abbreviations
- MHL:
-
Modified Hutson loop
- MHLA:
-
Modified Hutson loop access
- ERCP:
-
Endoscopic retrograde cholangiopancreatography
- PTBD:
-
Percutaneous transhepatic biliary drainage
- PTC:
-
Percutaneous transhepatic cholangiography
- ERC:
-
Retrograde cholangiography
- IR:
-
Interventional radiology
- MRI:
-
Magnetic resonance imaging
- MRCP:
-
Magnetic resonance cholangiopancreatography
- SIR:
-
Society of interventional radiology
- CTCAE:
-
Common terminology criteria for adverse events
- RF:
-
Radiofrequency
- RFA:
-
Radiofrequency ablation
References
Perez-Johnston R, Deipolyi AR, Covey AM. Percutaneous biliary interventions. Gastroenterol Clin North Am. 2018;47(3):621–41. https://doi.org/10.1016/j.gtc.2018.04.008.
Inamdar S, Slattery E, Bhalla R, Sejpal DV, Trindade AJ. Comparison of adverse events for endoscopic vs percutaneous biliary drainage in the treatment of malignant biliary tract obstruction in an inpatient national cohort. JAMA Oncol. 2016;2(1):112–7. https://doi.org/10.1001/jamaoncol.2015.3670.
Al-Kawas F, Aslanian H, Baillie J, et al. Percutaneous transhepatic vs. endoscopic retrograde biliary drainage for suspected malignant hilar obstruction: study protocol for a randomized controlled trial. Trials. 2018;19(1):108. https://doi.org/10.1186/s13063-018-2473-2.
Riaz A, Entezari P, Ganger D, et al. Percutaneous access of the modified Hutson loop for retrograde cholangiography, endoscopy, and biliary interventions. J Vasc Interv Radiol. 2020;31(12):2113-2120.e1. https://doi.org/10.1016/j.jvir.2020.06.011.
Kim D, Bolus C, Iqbal SI, Davison BD, Ahari HK, Flacke S, Molgaard CP. Percutaneous transjejunal biliary access in 60 patients with bilioenteric anastomoses. J Vasc Interv Radiol. 2019;30(1):76-81.e1. https://doi.org/10.1016/j.jvir.2018.06.020.
Fontein DB, Gibson RN, Collier NA, et al. Two decades of percutaneous transjejunal biliary intervention for benign biliary disease: a review of the intervention nature and adverse events. Insights Imaging. 2011;2(5):557–65. https://doi.org/10.1007/s13244-011-0119-y.
Lopera JE, Ramsey GR. Transjejunal biliary interventions: going back to a road less traveled. Acta Radiol. 2014;55(10):1210–8. https://doi.org/10.1177/0284185113515476.
Kuhn JP, Busemann A, Lerch MM, Heidecke CD, Hosten N, Puls R. Percutaneous biliary drainage in patients with nondilated intrahepatic bile ducts compared with patients with dilated intrahepatic bile ducts. AJR Am J Roentgenol. 2010;195(4):851–7. https://doi.org/10.2214/AJR.09.3461.
Morita S, Kitanosono T, Lee D, Syed L, Butani D, Holland G, Waldman D. Comparison of technical success and adverse events of percutaneous transhepatic cholangiography and biliary drainage between patients with and without transplanted liver. AJR Am J Roentgenol. 2012;199(5):1149–52. https://doi.org/10.2214/ajr.11.8281.
Villa NA, Harrison ME. Management of biliary strictures after liver transplantation. Gastroenterol Hepatol (N Y). 2015;11(5):316–28.
Akamatsu N, Sugawara Y, Hashimoto D. Biliary reconstruction, its adverse events and management of biliary adverse events after adult liver transplantation: a systematic review of the incidence, risk factors and outcome. Transpl Int. 2011;24(4):379–92. https://doi.org/10.1111/j.1432-2277.2010.01202.x.
Hutson DG, Russell E, Schiff E, Levi JJ, Jeffers L, Zeppa R. Balloon dilatation of biliary strictures through a choledochojejuno-cutaneous fistula. Ann Surg. 1984;199(6):637–47. https://doi.org/10.1097/00000658-198406000-00002.
Martin EC, Laffey KJ, Bixon R. Percutaneous transjejunal approaches to the biliary system. Radiology. 1989;172(3 Pt 2):1031–4. https://doi.org/10.1148/172.3.1031.
Entezari P, Soliman M, Malik A, et al. How endoscopic guidance augments nonvascular image-guided interventions. Radiographics. 2022;42(6):1845–60. https://doi.org/10.1148/rg.220013.
Chehab MA, Thakor AS, Tulin-Silver S, et al. Adult and pediatric antibiotic prophylaxis during vascular and IR procedures: a society of interventional radiology practice parameter update endorsed by the cardiovascular and interventional radiological society of Europe and the Canadian association for interventional radiology. J Vasc Interv Radiol. 2018;29(11):1483-1501.e2. https://doi.org/10.1016/j.jvir.2018.06.007.
Robins C, Xiao N, Salem R, Malik A, Keswani RN, Riaz A. Percutaneous biliary neo-anastomosis or neo-duct creation using radiofrequency wires. Cardiovasc Intervent Radiol. 2022;45(3):337–43. https://doi.org/10.1007/s00270-022-03059-5.
Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. National institutes of health. Updated 04/19/21. Accessed 8 June 2022. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm
Khalilzadeh O, Baerlocher MO, Shyn PB, et al. Proposal of a new adverse event classification by the society of interventional radiology standards of practice committee. J Vasc Interv Radiol. 2017;28(10):1432-1437.e3. https://doi.org/10.1016/j.jvir.2017.06.019.
Naidu SG, Alzubaidi SJ, Patel IJ, et al. Interventional radiology management of adult liver transplant adverse events. Radiographics. 2022;42(6):1705–23. https://doi.org/10.1148/rg.220011.
McPherson SJ, Gibson RN, Collier NA, Speer TG, Sherson ND. Percutaneous transjejunal biliary intervention: 10-year experience with access via Roux-en-Y loops. Radiology. 1998;206(3):665–72. https://doi.org/10.1148/radiology.206.3.9494484.
Saad WE, Wallace MJ, Wojak JC, Kundu S, Cardella JF. Quality improvement guidelines for percutaneous transhepatic cholangiography, biliary drainage, and percutaneous cholecystostomy. J Vasc Interv Radiol. 2010;21(6):789–95. https://doi.org/10.1016/j.jvir.2010.01.012.
Rodrigues T, Boike JR. Biliary strictures: etiologies and medical management. Semin Intervent Radiol. 2021;38(3):255–62. https://doi.org/10.1055/s-0041-1731086.
Scobie BA, Summerskill WH. Hepatic cirrhosis secondary to obstruction of the biliary system. Am J Dig Dis. 1965;10:135–46. https://doi.org/10.1007/BF02236664.
Perry LJ. Percutaneous biliary drainage in patients with nondilated bile ducts: use of a transjejunal approach. AJR Am J Roentgenol. 2000;175(1):268. https://doi.org/10.2214/ajr.175.1.1750268.
Maroney TP, Ring EJ. Percutaneous transjejunal catheterization of Roux-en-Y biliary-jejunal anastomoses. Radiology. 1987;164(1):151–3. https://doi.org/10.1148/radiology.164.1.3588898.
Sandhu J, Swersky A, Salsamendi J, Abrahams RB, Venkat S, Sleeman D, Mohan P. Utilization of a modified Roux-en-Y anastomosis as an access point for percutaneous transjejunal cholangioplasty of recurrent biliary strictures. Cardiovasc Intervent Radiol. 2019;42(12):1745–50. https://doi.org/10.1007/s00270-019-02335-1.
Committee AT, Enestvedt BK, Kothari S, et al. Devices and techniques for ERCP in the surgically altered GI tract. Gastrointest Endosc. 2016;83(6):1061–75. https://doi.org/10.1016/j.gie.2016.03.018.
Ostroff JW. Management of biliary adverse events in the liver transplant patient. Gastroenterol Hepatol (N Y). 2010;6(4):264–72.
Kohli DR, Aqel BA, Segaran NL, et al. Outcomes of endoscopic retrograde cholangiography and percutaneous transhepatic biliary drainage in liver transplant recipients with a Roux-en-Y biliary-enteric anastomosis. Ann Hepatobiliary Pancreat Surg. 2023;27(1):49–55. https://doi.org/10.14701/ahbps.22-037.
Hammad H, Brauer BC, Smolkin M, Ryu R, Obuch J, Shah RJ. Treating biliary-enteric anastomotic strictures with enteroscopy-ERCP requires fewer procedures than percutaneous transhepatic biliary drains. Digest Dis Sci. 2019;64(9):2638–44. https://doi.org/10.1007/s10620-019-05670-y.
Chahal P, Baron TH, Poterucha JJ, Rosen CB. Endoscopic retrograde cholangiography in post-orthotopic liver transplant population with Roux-en-Y biliary reconstruction. Liver Transpl. 2007;13(8):1168–73. https://doi.org/10.1002/lt.21198.
Funding
The authors received no financial support for preparing this manuscript for publication.
Author information
Authors and Affiliations
Contributions
AM, AH, and AR contributed to the conception and design, data analysis and interpretation, and article drafting. The rest of the authors were involved in critically revising the article for important intellectual content. AR contributed to the final approval of the article.
Corresponding author
Ethics declarations
Conflict of interest
AR and RS are consultants for Boston Scientific. The remaining authors declare that they have no conflict of interest.
Consent for Publication
For this type of study, consent for publication is not required.
Ethical Approval
Formal consent is not required for this type of study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
This study has obtained IRB approval, and the need for informed consent was waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
270_2024_3778_MOESM2_ESM.tif
Fluoroscopic image demonstrating through and through (Archimedean) access. Blue arrow: Modified Hutson loop jejunopexy marked by surgical clips. Black arrow: The through and through wire. White arrow: Endoscope advanced from the percutaneous transhepatic access over the through and through wire to evaluate the biliary-enteric anastomosis that required percutaneous transhepatic access. Red arrow: Percutaneous transhepatic access sheath. Yellow arrows: Two wires placed into additional biliary ducts (separate biliary-enteric anastomosis) from the modified Hutson loop access. Green arrow: Incidental note of coils in the splenic artery from prior splenic artery embolization
270_2024_3778_MOESM3_ESM.tif
Fluoroscopic image demonstrating a 6 French drain (black arrow) in the modified Hutson loop to maintain access for future procedures. The white arrows point towards the plastic stents placed via the modified Hutson loop
Endoscopic video of snaring of the cast from the Hutson loop
Endoscopic video of bile flowing from the patent duct into the bowel
42-year-old woman 1 year status post living donor liver transplant with a modified Hutson loop presenting with elevated alkaline phosphatase. Endoscopic video using a modified Hutson loop access demonstrating the ostium with wire catheterization of the duct
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Husnain, A., Malik, A., Caicedo, J. et al. Percutaneous Biliary Interventions via the Modified Hutson Loop in Patients with Biliary-Enteric Anastomoses: A Retrospective Study. Cardiovasc Intervent Radiol 47, 1083–1092 (2024). https://doi.org/10.1007/s00270-024-03778-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-024-03778-x