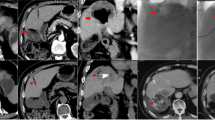
Abstract
Purpose
Approximately, 60–70% of patients with early-stage hepatocellular carcinoma (HCC) globally are ineligible for the recommended first-line procedures. This study aimed to compare conventional transcatheter arterial chemoembolization (cTACE) with a treatment, small drug-eluting bead TACE (DEB-TACE), in patients with stage 0/A HCCs.
Materials and Methods
We retrospectively investigated 76 patients who underwent first-time cTACE (n = 40) or DEB-TACE using 75–150 µm DC Beads® (n = 36) for Barcelona Clinic Liver Cancer (BCLC) stage 0/A HCC < 3 cm at a single tertiary care center between July 2015 and March 2017. Outcome measurements were time to local progression (assessed per modified response evaluation criteria in solid tumors), tumor response at one month and intrahepatic distal recurrence, progression-free survival, overall survival, safety, and toxicity.
Results
The study included 60 (78%) men and 16 (21%) women; participant mean age was 65.8 years. Objective response rates between the cTACE and DEB-TACE groups were similar (p > 0.05). Complete and partial 1-month tumor response rates were 60.0% and 22.5%, respectively, in the cTACE group and 69.4% and 25.0%, respectively, in the DEB-TACE group. The abdominal pain grade was significantly lower with DEB-TACE than with cTACE (p = 0.001). AST and ALT levels after tumor treatment with DEB-TACE were significantly lower than those after treatment with cTACE (p = 0.018 and 0.006). Time to local progression, intrahepatic distal recurrence, progression-free survival, and overall survival were not significantly between the DEB-TACE group and the cTACE group (p > 0.05).
Conclusion
Time to local progression between groups was not significantly different; however, post-embolic syndrome occurred less frequently in the DEB-TACE group. DEB-TACE appears to be a feasible treatment for small HCCs.
Level of Evidence
Level 3.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The first-line recommended treatments for early hepatocellular carcinoma (HCC; Barcelona Clinic Liver Cancer [BCLC] 0/A) is hepatic resection (HR). Second line includes liver transplantation (LT), radiofrequency ablation (RFA), microwave ablation (MWA), transarterial chemoembolization (TACE), transarterial radioembolization (TARE), and radiotherapy [1]. However, approximately 30–40% of patients with early-stage HCC globally are eligible for these procedures [2]. LT is generally limited by the shortage of donor allografts, and HR is widely used as the main choice of treatment for resectable HCC; however, the risk of postoperative hepatic dysfunction often precludes HR [3]. The effectiveness of RFA is equivalent to that of HR for HCCs < 3 cm in size in patients with three or fewer nodules [4]. However, RFA for lesions located close to critical organs, or the liver capsule is often challenging [5, 6]. Consequently, conventional TACE (cTACE) is used frequently for the treatment of early-stage HCC when other curative treatments are not possible.
Although cTACE and TARE is a second-line interventional treatment option widely used nowadays [1], it has not been recommended as a first-line therapy for patients with early-stage HCC, following the results of a single retrospective study [7]. However, other studies have reported good results for cTACE in comparison with other curative therapies for patients with early-stage HCC [8,9,10,11].
TACE with drug-eluting beads (DEB-TACE) is a technique that utilizes microspheres as embolic materials loaded with a chemotherapeutic agent that is gradually released into the target lesion. Previous studies have shown the efficacy and safety of DEB-TACE for the treatment of unresectable HCC [12, 13]. Recently, several international multicenter randomized trials [14,15,16,17,18,19] and retrospective studies [20,21,22,23] compared short-term outcomes of DEB-TACE and cTACE in terms of impact on liver function and radiologic tumor response. Another study [24] reported that the one-month objective response (OR) after DEB-TACE was lower than that after cTACE. However, there is little research comparing cTACE and DEB-TACE in patients with early-stage HCC. Moreover, no study has compared 75–150 µm DEB-TACE with cTACE in such patients.
Thus, the purpose of the study was to compare the time to local progression (TTLP), intrahepatic distal recurrence (IDR), progression-free survival (PFS), overall survival (OS), tumor response, safety, and toxicity of cTACE versus DEB-TACE in patients with BCLC stage 0/A HCC.
Materials and Methods
Patient Population
We retrospectively analyzed the patient database at a single tertiary care center. This retrospective study was approved by the Institutional Review Board at our hospital, which waived the requirement for written informed consent.
The cTACE or DEB-TACE was performed on 178 patients for treatment of BCLC 0/A HCC between July 2015 and March 2017 (Fig. 1). The eligibility criteria of TACE in our institution were (i) HR could not be performed due to risk of postoperative hepatic insufficiency, (ii) tumor could not be visualized on planning US for RFA, (iii) treatment applicability was limited due to risk of thermal injury and heat sink effect, or (iv) patient refused HR or RFA (esp. refusal of invasive treatment or anesthesia or other personal situation).
The diagnosis of HCC was confirmed by using a dynamic CT or/and MR imaging (MRI) based on a typical enhancing pattern [25], in agreement with the consensus of two radiologists. HCCs were histologically confirmed in 12 patients who underwent percutaneous biopsy because of equivocal imaging findings.
The inclusion criteria for TACE were (i) age > 18 years, (ii) bilirubin level < 3 mg/dL, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels < 300 IU/L, (iii) serum creatinine level < 2 mg/dL, (iv) ≤ 3 nodular lesions and lesion sizes ≤ 3 cm, (v) no history of initial treatment for HCC, (vi) Child–Pugh class A/B disease with good performance status (Eastern Cooperative Oncology Group score of 0/1), and (vii) the absence of extrahepatic metastases and/or macrovascular invasion.
Exclusion criteria for TACE were as follows: (i) inadequate target lesion (infiltrative pattern or largest lesion < 1 cm), (ii) previous (within the past 5 years) or concomitant neoplastic disease other than HCC, (iii) contrast medium allergy contraindicating angiography, (iv) history of biliary tract repair or endoscopic biliary treatment, (v) localized or systemic infections, and (vi) pregnancy or breastfeeding.
Finally, 76 patients were included in our study (Fig. 1), including 40 treated with cTACE and 36 treated with DEB-TACE (Table 1). Previous surgery or RFA (n = 72), combined TACE and RFA (n = 14), follow-up loss (n = 11), and others (n = 5) were excluded in our study. The baseline clinical and tumor characteristics in patients of both groups were comparable (Table 1). There were no significant demographic differences between conventional TACE and DEB-TACE, with the exception of AST levels (p = 0.025). The mean tumor size was 1.95 ± 0.54 cm in the cTACE group and 2.06 ± 0.54 cm in the DEB-TACE group.
Chemoembolization Protocols
In the patients evaluated in our study, cTACE was conducted by two interventional radiologists with 11 and 7 years of experience, respectively. After arterial access via the common femoral artery, a 5-Fr catheter (Yashiro; Terumo, Tokyo, Japan) was introduced, and diagnostic angiography of the superior mesenteric artery, celiac axis, and common hepatic artery (including C-arm computed tomography [CT]) was performed to assess arterial anatomy and confirm patency of the portal vein.
Using a generated hepatic vascular map, the artery feeding the tumor was catheterized selectively using a 1.7 or 2.0 Fr microcatheter (Valoute; Asahi Intecc, Aichi, Japan, Progreat; Terumo, Tokyo, Japan) that was coaxially inserted through a 5-Fr catheter. The microcatheter was placed as distally as possible into the vessel supplying the tumor, and the tip of the catheter was advanced into the hepatic artery and feeding branch if the size, location, and blood supply allowed.
Doxorubicin (Adriamycin, Ildong, Seoul, South Korea) dissolved in aqueous nonionic contrast medium was mixed with the iodized oil lipiodol (Guerbet, Roissy, France) in a 1: 4 ratio to form an emulsion using the pumping technique. After appropriate catheter placement, the emulsion (4 mL of lipiodol and 20 mg of doxorubicin) was injected immediately under fluoroscopy. The dose of anticancer agent used for the TACE procedure was determined by the radiologist based on the sizes, numbers, and blood supplies of the target tumors. Chemotherapeutic infusion was discontinued if the antegrade blood flow slowed, and subsequent embolization was performed using 150–300 um calibrated gelatin sponge gel (Cutanplast, Mascia-Brunelli, Spa, Italy). TACE was terminated upon portal vein visualization, complete satiation of the tumor vessels with drug, and the disappearance of the tumor blush on subsequent angiographic imaging.
The DEB-TACE technique was the same as that for conventional TACE. One vial of 75–150 µm DEB agent (DC Bead M1; Biocompatibles UK, Farnham, UK) was loaded with 50 mg of doxorubicin solution, and the preparation was suspended in 30 mL nonionic iodized contrast agent (Xeneticx, Guerbet, Aulnay-sous-Bois, France). All procedures were performed with 75–150 µm DEB particles. The DEB suspension was injected as slowly as possible (the target was > 1 min/mL) to avoid reflux and nontarget embolization; the embolization endpoint was complete stasis.
Follow-up and Evaluation
Contrast-enhanced CT or MRI was performed at 4 ± 1 weeks after TACE and every three months thereafter until tumor recurrence. The tumor response was classified according to the modified response evaluation criteria in solid tumors (mRECIST) [26].
TACE was repeated when radiologic findings were indicative of partial response, stable disease, or progressive disease with residual viable tumor. If no residual tumors were found, TACE was discontinued for a period of time, and follow-up contrast-enhanced CT or MRI was repeated every 3 months ± 2 weeks. The TACE procedure was repeated if tumor recurrences were observed on follow-up CT or MRI. In the second TACE, all patients used the same method as was used in the first TACE; cTACE was used in the third TACE.
All adverse events were graded using the National Cancer Institute Common Terminology Criteria for adverse events (CTCAE), version 4.0 [27]. Toxicity was graded using binary variables (negative: grades 0–1; positive: grades 2–4), taking into account the number of adverse effects and the percentage of patients affected in each TACE group.
For the 1-year follow-up period, all medical events, symptoms, or indicators deemed to be related to the procedure and disease were recorded. The presence of post-embolization syndrome (PES) was assessed during the postprocedural hospital stay [28]. Laboratory findings were recorded for analysis within 2 weeks before the procedure and within three days after; in case of multiple tests, the least favorable value was selected.
For assessment of hepatic complications, clinical medical records and follow-up liver imaging studies were examined for liver abscess, bile duct dilation, biloma, portal vein thrombosis, and liver infarct [29, 30]. Bile duct injury was considered prominent when bile duct dilation was shown in segmental or wider distribution.
The outcome measurement of our study was time to local progression (TTLP), response at one month and intrahepatic distal recurrence (IDR), progression-free survival (PFS), overall survival (OS), safety, and toxicity. Time to local progression (TTLP) was defined as the length of time from the start of treatment for a HCC until the tumor starts to get worse in the same segment of the liver. Progression-free survival (PFS) was defined as the interval between treatment administration and either radiological progression or death; response to treatment was defined based on the mRECIST. Patients who were alive and progression-free at the end of the follow-up period were censored.
Statistical Analysis
Quantitative data were analyzed using descriptive statistics (mean ± standard deviation). The independent t test and Mann–Whitney U test were used to compare continuous variables, and the χ2 or Fisher’s exact tests were used to compare categorical variables between the two groups. Time to local progression (TTLP), intrahepatic distant recurrence (IDR), PFS, and OS rates were determined using the Kaplan–Meier method and life-table analysis, and were compared using the log-rank test. Data were analyzed using statistical software (SPSS, version 22.0, IBM Corp., Armonk, NY, USA). A p value < 0.05 was considered significant for all tests.
Results
The patients’ therapeutic outcomes are summarized in Table 2. cTACE and DEB-TACE were performed 2.00 ± 0.85 and 1.61 ± 0.84 times per patient, respectively (p = 0.142). On the first follow-up after TACE, among the 40 patients receiving cTACE, 24 (60.0%) showed complete response, nine (22.5%) showed partial response, five (12.5%) showed stable disease, and two (5.0%) showed progressive disease. Among the 36 patients who received DEB-TACE, 25 (69.4%) showed complete response, nine (25.0%) showed partial response, and two (5.6%) showed stable disease.
The OR rate after receiving cTACE was 82.5%; although the OR rate with DEB-TACE was higher (94.4%), the difference was not statistically significant (p = 0.376). The 1-year OR rates with cTACE and DEB-TACE were 85% and 88.9%, respectively (p = 0.935); moreover, the cumulative TTLP, IDR, PFS, and OS rates were not significantly different between the two groups (p = 0.290, 0.168, 0.149, and 0.603) (Fig. 2). The most common cause of recurrence after DEB-TACE was local tumor progression (LTP) in six patients (16.7%), followed by LTP and IDR in three (8.3%). The most common cause of recurrence after cTACE was LTP in nine patients (22.5%), followed by IDR in two (5.0%), LTP and IDR in six (15.0%), and LTP, IDR, vascular invasion, and extrahepatic metastasis in one (2.5%). Although the number of patients in the DEB-TACE group was small, progression-free survival rates were not significantly different between the two groups (p = 0.149).
Kaplan–Meier survival curves comparing A overall survival B time to local progression, C intrahepatic distant recurrence, and D progression-free survival rates after first transcatheter therapy between patients who received very small drug-eluting bead (DEB) transcatheter arterial chemoembolization (TACE) (n = 36) versus conventional TACE (cTACE) (n = 40) (p > 0.05)
The incidences of clinically symptomatic adverse events are summarized in Table 3. Clinically symptomatic adverse events occurred in 55.1% of the patients, 60% of the cTACE group, and 52.8% of the DEB-TACE group. The most common adverse event was abdominal pain (n = 42), followed by fever/chills (n = 15) and nausea/vomiting (n = 4) in both groups. All symptoms were mild and classified as grade 1 or 2; no grade 3, 4, or 5 adverse events were recorded. The abdominal pain grade was significantly lower with DEB-TACE than with cTACE (p = 0.001). However, there were no statistically significant differences in terms of fever, nausea, or vomiting (p > 0.05). The mean hospital stay durations after cTACE and DEB-TACE were 3.40 ± 1.59 days and 3.19 ± 0.74 days, respectively. In our institution, patients are usually hospitalized one day before TACE. The procedure is performed the next day. We acquire a blood test 6–12 hours after the procedure and discharge the patients if no complications occurred; while hospital stay was somewhat shorter in the DEB-TACE group, the difference was not statistically significant (p = 0.538).
Based on the CTCAE, we compared the differences between AST, ALT, albumin, total bilirubin, and prothrombin time acquired from blood tests performed before treatment and within 1 week afterward. AST and ALT levels after tumor treatment with DEB-TACE were significantly lower than those after treatment with cTACE (p = 0.018 and 0.006) (Table 4); however, follow-up examination after 1 month showed normalized levels for both enzymes. Only one patient showed deterioration of liver function (3 points on the Child–Pugh score) in the cTACE group; this patient also had progressive disease.
No serious adverse events were reported in the two groups. Focal bile duct dilatation was observed on follow-up imaging in three patients after DEB-TACE, but all were asymptomatic. Systemic adverse events, such as mucositis, alopecia, and bone marrow toxicity, were not observed in either treatment group.
Discussion
Our study found no statistically significant difference in tumor response at 1 month and 12 months between cTACE and DEB-TACE. Meta-analyses by Zou et al. [31] and Huang et al. [32] showed that DEB-TACE produces significantly better tumor responses than cTACE. However, a recent study [24] reported that the 1-month OR after DEB-TACE was lower than that after cTACE. In the latter study, DEB-TACE with 100–300 µm beads was found to not reach the peritumoral portal veins and possibly block the peripheral hepatic arteries without penetrating deeply into the fine tumor vessels in sufficient amounts to induce complete tumor necrosis. However, our study used 75–150 µm beads for treatment of early HCC. Since the diameter of DEB seems to be related to their therapeutic effect, studies on pharmacological kinetics have focused on producing smaller particles that could penetrate deeper into the tumor’s vascular network. The most distal penetration of small DEB reduces the phenomenon of hypoxic-ischemic neoangiogenesis [33]. In our study, opacification of the peritumoral veins was observed in five patients, among whom two showed focal bile duct dilatation. Hence, it is possible to reach the peritumoral portal vein using small beads (75–150 μm), which may explain the favorable OR in small tumors.
In our study, DEB-TACE produced a lower incidence of TTLP and IDR than cTACE (9 vs. 15 and 3 vs. 8, respectively), although the difference was not statistically significant. Nicoliini et al. [20] showed that DEB-TACE was characterized by an intense inflammatory and fibrotic reaction in the area surrounding the tumor tissue; this was not observed in patients receiving cTACE. Additionally, the recurrence rate after liver transplantation (LT) in DEB-TACE was low, and DEB-TACE was identified as an independent predictor of recurrence-free survival on multivariate analysis [20]. The finding that very small beads can pass through the distal artery or peritumoral vein and cause an intense fibrotic reaction may explain the lower recurrence rate of DEB-TACE compared with cTACE.
Little is known about the survival benefit of DEB-TACE compared to cTACE. Burrel et al. [34] reported that the survival expectancy of patients treated with DEB-TACE was higher than previously reported. Sacco et al. [15] and Ferrer et al. [17] found no statistically significant differences between the two groups, although their sample sizes were small. Although the incidence of survival rate among patients in the DEB-TACE group was higher than that in the cTACE group, the PFS rate was not statistically significant in BCLC stage 0/A HCC. Hence, a longer follow-up study is required to more extensively evaluate the survival benefit of DEB-TACE in early HCC.
In some studies, DEB-TACE was associated with fewer common adverse events than cTACE, while serious adverse effects were not significantly different between the two therapeutic groups. PES was the most common complication in both DEB-TACE and conventional TACE patients, although no differences between the groups were observed [15, 17, 21, 22]. Our results also showed that the most common adverse event was PES, with the only significant difference between the groups being the severity of abdominal pain. There were no serious adverse events in both groups, and was comparable to the previously reported incidences of 6–20% [14, 19]. Lammer et al. [14] reported that serious liver toxicity postchemoembolization was also lower in the DC bead group. Postprocedural AST and ALT levels were significantly lower after DEB-TACE than after cTACE. Our study also revealed a significant reduction in serious liver toxicity and side effects caused by doxorubicin with DEB-TACE.
Hepatobiliary injury is a well-known complication after TACE. In recent studies, DEB-TACE was associated with greater hepatobiliary injury than cTACE in patients with intermediate-stage HCC or neuroendocrine tumors [29, 30]. In these studies, hepatobiliary injuries and intrahepatic biloma were more frequently observed after DEB-TACE, suggesting that cTACE might be a more appropriate therapy than DEB-TACE in patients with less advanced cirrhosis. However, the incidences of hepatobiliary injury in BCLC 0/A stage HCC patients who underwent DEB-TACE and cTACE were similar in our study. Only three patients with DEB-TACE had asymptomatic bile duct dilatation and no severe hepatobiliary complications. The reason for the low hepatobiliary complication rate with DEB-TACE in our study was superselective treatment using very small microcatheters (1.7 and 2.0 Fr) for all TACE procedures. Small DEB (75–150 μm) was used in our study to assess the risks of undertreating target lesions and unexpected proximal arterial occlusion, and this technique showed decreased biliary complications.
Varela et al. [13] and Monier et al. [30] reported that high doses of doxorubicin increased the risk of biliary injury. Our study used 20 mg doxorubicin of cTACE and 50 mg doxorubicin of DEB-TACE (as only BCLC 0/A early-stage HCC was treated), and the drug dose was relatively smaller than that in other studies. We speculate that this smaller amount of doxorubicin significantly reduced drug-related adverse effects as well as systemic chemotherapeutic drug concentrations compared to those in other studies, resulting in fewer biliary complications.
The limitations of our study were that the research was based on a retrospective and non-randomized design, and that a small number of subjects were investigated with a relatively short follow-up period at a single tertiary care center. Thus, a randomized clinical trial should be performed on a larger scale to validate our results.
In conclusion, we found no significant difference in OR between patients receiving cTACE and those undergoing DEB-TACE. There was less abdominal pain and a lower impact on AST and ALT levels with DEB-TACE. Therefore, DEB-TACE can be used as a feasible and promising approach for the curative treatment of patients with early-stage (lesions < 3 cm) HCC.
References
Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, staging, and management of hepatocellular carcinoma: 2019 practice guidance by American association for the study of liver diseases. Hepatology. 2018;66(2):732–50. https://doi.org/10.1002/hep.29913.
Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, Kudo M, Johnson P, Wagner S, Orsini LS, Sherman M. Global pattern of hepatocellular carcinoma management from diagnosis to death: the BRIDGE study. Liver Int. 2015;35:2155–66. https://doi.org/10.1111/liv.12818.
Bruix J, Castells A, Bosch J, Feu FA, Fuster JO, Garcia-Pagan JC, Visa JO, Bru CO, Rodes JO. Surgical resection of hepatocellular carcinoma in cirrhotic patients: Prognostic value of preoperative portal pressure. Gastroenterology. 1996;111:1018–22. https://doi.org/10.1016/S0016-5085(96)70070-7.
Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–8. https://doi.org/10.1097/01.sla.0000201480.65519.b8.
Cho YK, Kim JK. Sustained complete response and low complication rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis. Hepatology. 2008;47:1791. https://doi.org/10.1002/hep.22245.
Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: Complications encountered in a multicenter study. Radiology. 2003;226:441–51. https://doi.org/10.1148/radiol.2262012198.
Arii S, Yamaoka Y, Futagawa S, Inoue K, Kobayashi K, Kojiro M, Makuuchi M, Nakamura Y, Okita K, Yamada R. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology. 2000;32:1224–9. https://doi.org/10.1053/jhep.2000.20456.
Hsu KF, Chu CH, Chan DC, Yu JC, Shih ML, Hsieh HF, Hsieh TY, Yu CY, Hsieh CB. Superselective transarterial chemoembolization vs hepatic resection for resectable early-stage hepatocellular carcinoma in patients with Child-Pugh class a liver function. Eur J Radiol. 2012;81:466–71. https://doi.org/10.1016/j.ejrad.2010.12.058.
Bargellini I, Sacco R, Bozzi E, Bertini M, Ginanni B, Romano A, Cicorelli A, Tumino E, Federici G, Cioni R, Metrangolo S. Transarterial chemoembolization in very early and early-stage hepatocellular carcinoma patients excluded from curative treatment: a prospective cohort study. Eur J Radiol. 2012;81:1173–8. https://doi.org/10.1016/j.ejrad.2011.03.046.
Yang H-J, Lee J-H, Lee DH, Yu SJ, Kim YJ, Yoon JH, Kim HC, Lee JM, Chung JW, Yi NJ, Lee KW. Small single-nodule hepatocellular carcinoma: Comparison of transarterial chemoembolization, radiofrequency ablation, and hepatic resection by using inverse probability weighting. Radiology. 2014;271:909–18. https://doi.org/10.1148/radiol.13131760.
Kim HC, Suk KT, Kim DJ, Yoon JH, Kim YS, Baik GH, Kim JB, Kim CH, Sung H, Choi JY, Han KH. Transarterial chemoembolization in Barcelona Clinic Liver Cancer Stage 0/A hepatocellular carcinoma. World J Gastroenterol. 2014;20:745–54. https://doi.org/10.3748/wjg.v20.i3.745.
Malagari K, Chatzimichael K, Alexopoulou E, Kelekis A, Hall B, Dourakis S, Delis S, Gouliamos A, Kelekis D. Transarterial chemoembolization of unresectable hepatocellular carcinoma with drug eluting beads: Results of an open-label study of 62 patients. Cardiovasc Intervent Radiol. 2008;31:269–80. https://doi.org/10.1007/s00270-007-9226-z.
Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, Ayuso C, Castells L, Montañá X, Llovet JM, Bruix J. Chemoembolization of hepatocellular carcinoma with drug eluting beads: Efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474–81. https://doi.org/10.1016/j.jhep.2006.10.020.
Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S, Benhamou Y. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: Results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41–52. https://doi.org/10.1007/s00270-009-9711-7.
Sacco R, Bargellini I, Bertini M, Bozzi E, Romano A, Petruzzi P, Tumino E, Ginanni B, Federici G, Cioni R, Metrangolo S. Conventional versus doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2011;22:1545–52. https://doi.org/10.1016/j.jvir.2011.07.002.
Vogl TJ, Lammer J, Lencioni R, Malagari K, Watkinson A, Pilleul F, Denys A, Lee C. Liver, gastrointestinal, and cardiac toxicity in intermediate hepatocellular carcinoma treated with PRECISION TACE with drug-eluting beads: Results from the PRECISION V randomized trial. Am J Roentgenol. 2011;197:W562–70. https://doi.org/10.2214/AJR.10.4379.
Ferrer Puchol MD, la Parra C, Esteban E, Vaño M, Forment M, Vera A, Cosin O. Comparison of doxorubicin-eluting bead transarterial chemoembolization (DEB-TACE) with conventional transarterial chemoembolization (TACE) for the treatment of hepatocellular carcinoma. Radiologia. 2011;53:246–53. https://doi.org/10.1016/j.rx.2010.07.010.
Recchia F, Passalacqua G, Filauri P, Doddi M, Boscarato P, Candeloro G, Necozione S, Desideri G, Rea S. Chemoembolization of unresectable hepatocellular carcinoma: Decreased toxicity with slow-release doxorubicin-eluting beads compared with lipiodol. Oncol Rep. 2012;27:1377–83. https://doi.org/10.3892/or.2012.1651.
Golfieri R, Giampalma E, Renzulli M, Cioni R, Bargellini I, Bartolozzi C, Breatta AD, Gandini G, Nani R, Gasparini D, Cucchetti A. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014;111:255–64. https://doi.org/10.1038/bjc.2014.199.
Nicolini D, Svegliati-Baroni G, Candelari R, Mincarelli C, Mandolesi A, Bearzi I, Mocchegiani F, Vecchi A, Montalti R, Benedetti A, Risaliti A. Doxorubicin-eluting bead vs conventional transcatheter arterial chemoembolization for hepatocellular carcinoma before liver transplantation. World J Gastroenterol. 2013;19:5622–32. https://doi.org/10.3748/wjg.v19.i34.5622.
Wiggermann P, Sieron D, Brosche C, Brauer T, Scheer F, Platzek I, Wawrzynek W, Stroszczynski C. Transarterial Chemoembolization of Child-A hepatocellular carcinoma: drug-eluting bead TACE (DEB TACE) vs. TACE with cisplatin/lipiodol (cTACE). Med Sci Monit 2011;17:CR189-95. https://doi.org/10.12659/MSM.881714
Song MJ, Chun HJ, Song DS, Yoo SH, Park CH, Bae SH, Choi JY, Im Chang U, Yang JM, Lee HG, Yoon SK. Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol. 2012;57:1244–50. https://doi.org/10.1016/j.jhep.2012.07.017.
Dhanasekaran R, Kooby DA, Staley CA, Kauh JS, Khanna V, Kim HS. Comparison of conventional transarterial chemoembolization (TACE) and chemoembolization with doxorubicin drug eluting beads (DEB) for unresectable hepatocelluar carcinoma (HCC). J Surg Oncol. 2010;101:476–80. https://doi.org/10.1002/jso.21522.
Lee M, Chung JW, Lee KH, Won JY, Chun HJ, Lee HC, Kim JH, Lee IJ, Hur S, Kim HC, Kim YJ. Korean multicenter registry of transcatheter arterial chemoembolization with drug-eluting embolic agents for nodular hepatocellular carcinomas: six-month outcome analysis. J Vasc Interv Radiol. 2017;28:502–12. https://doi.org/10.1016/j.jvir.2016.08.017.
Bruix J, Sherman M. American association for the study of liver disease. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–2. https://doi.org/10.1002/hep.24199.
Lencioni R, Llovet JM. Modified recist (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. https://doi.org/10.1055/s-0030-1247132.
National Institute of Cancer. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed June 10 2018.
Leung DA, Goin JE, Sickles C, Raskay BJ, Soulen MC. Determinants of postembolization syndrome after hepatic chemoembolization. J Vasc Interv Radiol. 2001;12:321–6. https://doi.org/10.1016/S1051-0443(07)61911-3.
Guiu B, Deschamps F, Aho S, Munck F, Dromain C, Boige V, Malka D, Leboulleux S, Ducreux M, Schlumberger M, Baudin E. Liver/biliary injuries following chemoembolisation of endocrine tumours and hepatocellular carcinoma: Lipiodol vs. drug-eluting beads. J Hepatol. 2012;56:609–17. https://doi.org/10.1016/j.jhep.2011.09.012.
Monier A, Guiu B, Duran R, Aho S, Bize P, Deltenre P, Dunet V, Denys A. Liver and biliary damages following transarterial chemoembolization of hepatocellular carcinoma: comparison between drug-eluting beads and lipiodol emulsion. Eur Radiol. 2017;27:1431–9. https://doi.org/10.1007/s00330-016-4488-y.
Zou JH, Zhang L, Ren ZG, Ye SL. Efficacy and safety of cTACE versus DEB-TACE in patients with hepatocellular carcinoma: a meta-analysis. J Dig Dis. 2016;17:510–7. https://doi.org/10.1111/1751-2980.12380.
Huang K, Zhou Q, Wang R, Cheng D, Ma Y. Doxorubicin-eluting beads versus conventional transarterial chemoembolization for the treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29:920–5. https://doi.org/10.1111/jgh.12439.
Greco G, Cascella T, Facciorusso A, Nani R, Lanocita R, Morosi C, Vaiani M, Calareso G, Greco FG, Ragnanese A, Bongini MA, Marchianò AV, Mazzaferro V, Spreafico C. Transarterial chemoembolization using 40 μm drug eluting beads for hepatocellular carcinoma. World J Radiol. 2017;9(5):245–52.
Burrel M, Reig M, Forner A, Barrufet M, de Lope CR, Tremosini S, Ayuso C, Llovet JM, Real MI, Bruix J. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design. J Hepatol. 2012;56:1330–5. https://doi.org/10.1016/j.jhep.2012.01.008.
Funding
This study was not supported by any funding.
Author information
Authors and Affiliations
Contributions
YYJ and YJK conceived and conducted the study, and BCL and HOK performed the analyses, the interpretation of results, and the drafting of the manuscript. YJK and SBC collected the data and conducted the study and assisted with interpretation of results and drafting of the manuscript. YYJ, NYI, and JKK assisted with the analyses, the interpretation of results, and drafting of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and Animal Rights
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Ethical Approval
For this type of study, formal consent is not required. The study was reviewed and approved by the Chonnam National University Hwasun Hospital Institutional Review Board.
Informed Consent
For this type of study, informed consent is not required.
Consent for Publication
For this type of study, consent for publication is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kang, Y.J., Lee, B.C., Kim, J.K. et al. Conventional Versus Small Doxorubicin-eluting Bead Transcatheter Arterial Chemoembolization for Treating Barcelona Clinic Liver Cancer Stage 0/A Hepatocellular Carcinoma. Cardiovasc Intervent Radiol 43, 55–64 (2020). https://doi.org/10.1007/s00270-019-02349-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-019-02349-9