Abstract
Introduction
To evaluate feasibility, safety and efficacy of fusion imaging in order to guide endovascular revascularization of iliac steno-occlusive disease.
Materials and Methods
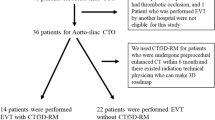
Retrospectively, we identified twenty-six patients (20 male, mean age 63 ± 8y; Rutherford II-V) who underwent revascularization of a chronic total occlusion (n = 6; 23%) or severe stenosis (n = 20; 77%) of the common and/or external iliac artery. Median lesion length was 33 mm (IQR 20–60). In one group of patients (NEW; n = 11), fusion imaging with 2-D/3-D registration was used to guide revascularization. No baseline digital subtraction angiography (DSA) had been acquired in these patients. In another group of patients (OLD; n = 15), no fusion imaging had been utilized and at least one DSA run had been performed to guide the procedure. In both groups, final DSA of the treated lesions was performed. Number of DSA runs, radiation and contrast medium exposure, technical success (residual stenosis < 30%) and complications were analyzed.
Results
Median DSA runs needed in OLD for guidance were n = 2 (IQR 2–3) and in NEW n = 0 (IQR 0–0; p = 0.001). Compared to OLD, median dose area product (DAP) was reduced by 17,118 mGy*cm2 (IQR 10,407–23,614; p = 0.016) if fusion imaging guidance had been used (NEW). Based on the median DAP of the final angiogram in NEW, median DAP reduction was 6007 mGy*cm2 (IQR 5012–16,105; p = 0.1). Median total contrast medium volume injected in NEW was 45 ml (IQR 30–90) and in OLD 120 ml (IQR 100–140; p = 0.001). Technical success was 100% for both groups. In 1/27 patients (3.7%) a minor complication (embolism) occurred.
Conclusion
Fusion imaging proved to be feasible as well as safe and significantly reduces radiation and contrast medium exposure during endovascular revascularization of iliac steno-occlusive disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, computed tomography angiography (CTA)- or magnetic resonance angiography (MRA)-based fusion imaging (FI) has been developed basically to guide (complex) endovascular aortic repair (EVAR) procedures. For those, FI technique has shown significant reduction in the cumulative radiation dose and contrast medium (CM) exposure [1,2,3,4], which may decrease the risk for especially long-term side effects of radiation, and has the potential to decrease the burden on renal function as well as may decrease the risk for acute kidney injury [5,6,7,8,9].
2D-3D and 3D-3D FI are established methods to guide interventions such as EVAR or chemoembolization. In the case of 2D-3D FI, pre-interventional CTA, MRA or cone beam CT (CBCT) (on table) images are co-registered with two fluoroscopic orthogonal images. Thereby, the CTA or MRA images are superimposed on the live fluoroscopic images. On the other side, 3D-3D FI is performed by fusing intra-operative CBCT with preoperative MRA/CTA images to guide the intervention [10, 11].
Till today, three studies with a limited number of patients and without a control group have focused on FI to guide peripheral artery interventions in peripheral artery disease (PAD) patients, using 3D-3D registration [12,13,14].
The present study was designed to evaluate feasibility, safety and efficacy of fusion imaging with 2D-3D registration to guide endovascular revascularization of iliac steno-occlusive disease.
Materials and Methods
Patient Selection and Study Design
Retrospectively, we identified 26 consecutive PAD patients (20 male, mean age 63 ± 8y) with iliac stenosis (n = 20, 77%) or occlusion (n = 6, 23%). Of those, 11 patients underwent endovascular revascularization of the iliac artery after FI had become available in the interventional radiology (IR) department in 10/2017. This collective represents the FI study group (NEW group). During the time span of 6 months prior to the establishment of FI, we identified another group of 15 consecutive patients who underwent endovascular revascularization for iliac artery steno-occlusive disease. This group represents the control group (OLD group). Patient characteristics as well as characteristics of lesions treated and devices used are summarized in Tables 1 and 2.
In the OLD group (control group, n = 15), iliac target lesions had traditionally been visualized by a baseline DSA prior to treatment. In the NEW group (n = 11) instead of performing this baseline DSA, FI was used during crossing and (pre-) dilatation of the target lesion as well as to guide stent implantation.
We evaluated differences in dose area products (DAP, mGy*cm2) and volumes of contrast media (volume (ml)) injected as well as in the number of digital subtraction angiography runs (n) between both groups. Furthermore, we compared the following variables between the two groups: technical success, which was defined as successful intra- or sub-intimal revascularization with residual stenosis < 30% at completion angiography; clinical success, which was defined as an increase in Rutherford category by at least one stage; hemodynamic success, which was defined as ABI improvement (> 0.2) as compared to the baseline value. Furthermore, major complications and adverse events within 24 h from the intervention were evaluated.
Preoperative Imaging
In the NEW group, all patients had a routinely acquired preoperative CTA. The CTA had a scan range extending from the costo-diaphragmatic recess to the forefoot and was conducted on a 128-slice CT scanner (Somatom Definition®, Siemens Healthcare, Erlangen, Germany). The CT was acquired using the following parameters: tube voltage 120 kV, reference tube current–time product 200 mAs with tube current modulation (CareDose®, Siemens Healthcare, Erlangen, Germany), rotation time 0.3 s and collimation 0.6 mm. CTA was performed with the patient in supine position. A dose of 100 ml of Iomeprol 300 mg I/ml (Imeron 300®, Bracco, Milano, Italy), followed by a 30 ml saline flush, both at a flow rate of 4.0 ml/s, was power injected (Ct motion®, Ulrich medical, Ulm, Germany) via a 20 G or larger intravenous cannula placed preferably in an antecubital vein. Arterial phase images were obtained 15 s after bolus detection in the suprarenal aorta (threshold 150 HU, CareDose®). For vascular assessment, images were first reconstructed using a medium soft kernel (B30f) with a field of view of 100/500 mm and an effective slice thickness of 1.0 mm. Second, images were reconstructed with the same field of view and a slice thickness of 5.0 mm. In addition, in every patient sagittal and coronal reformations were done.
Endovascular Procedure Using Fusion Imaging (NEW Group)
CTA images were routinely acquired less than 3 months before the intervention. These preoperative images (source images with a slice thickness of 1 mm) were imported to a stand-alone workstation for FI in the angiography suite (Allura Xper® FD20/15, software version 3.4, Philips Healthcare, Hamburg, Germany). The software for FI was the VesselNavigator® (Philips Healthcare, Hamburg, Germany). Prior to revascularization, three steps were performed to prepare utilization of FI.
-
1.
Planning
Using the stand-alone workstation, vessel segmentation from the pre-interventional images was performed semiautomatically. Optimal c-arm angulations were simulated manually (e.g., for optimal visualization of the iliac or femoral bifurcation) and preset. This step takes about 5 min.
-
2.
Registration
In the angio-suite, two fluoroscopic images were acquired in 45° LAO and RAO projections (2D registration) and automatically co-registered with the preoperative CTA scan. This step takes approximately 1 min).
-
3.
Manual correction
Manual correction of the fusion images derived from the pre-interventional images and fluoroscopic images was performed. This step of manual correction took approximately 1 min. Primarily, bone borders of the pelvis and lumbar spine were used as landmarks.
After that, a live 3D vascular model was shown on an additional screen next to the conventional live fluoroscopy monitor and the preparation was finalized.
All procedures were carried out under local anesthesia. Lesion crossing and (pre-) dilatation as well as final stent implantation was performed by FI guidance only. The vessel reference diameter was determined pre-interventionally using 3D post-processing software in order to select the appropriate size of balloons and stents. At the completion of the procedure, a digital subtraction angiography was performed (injector parameters: 10 ml/s, 20 ml volume). The contrast consisted of Iomeprol (Imeron® 300; Bracco Imaging, Konstanz, Germany). The femoro-popliteal and below-the-knee runoff was examined by DSA using manual contrast injection via the sheath. Figures 1A–C and 2A–C illustrate two examples of FI-guided procedures.
A CTA shows a short calcified stenosis of the proximal left common iliac artery (red arrow). B In this case, the wire straightened the vessel, which made it more difficult to optimally fuse the CTA with the fluoroscopy images. After conventional PTA, a self-expandable stent was implanted and post-dilated. C The final angiogram shows no residual stenosis
Endovascular Procedure Without Fusion Imaging (OLD Group)
Percutaneous access was achieved after local anesthesia. After wire passage of the lesion using fluoroscopy guidance, a pigtail catheter was placed in the infra-renal aorta above the bifurcation and a DSA was performed (injection parameters see above). Another DSA (injection parameters see above) in an oblique projection with magnification of the region with the target lesion was acquired. More than two initial angiograms were only performed to optimally visualize the internal iliac artery in case of lesions reaching the iliac bifurcation or cross-angled and tortuous lesions. Using an overlay image (SmartMask®, Philips Healthcare, Hamburg, Germany) derived from the baseline DSA, PTA followed by stent implantation was performed. After that, a final DSA (injection parameters see above) was acquired to confirm technical success and to rule out complications. The femoro-popliteal and below-the-knee runoff was examined by DSA using manual contrast injection via the sheath.
Data Collection and Statistical Analysis
The total DAP (mGy*cm2), the cumulative DAP for DSA (mGy*cm2), cumulative DAP for fluoroscopy (mGy*cm2) as well as cumulative fluoroscopy time (min) and the DAP for each DSA run (mGy*cm2) were obtained from the automatically produced dose protocol of the angiography unit. In both groups, the acquisition parameters for fluoroscopy and for DSA runs, e.g., pulse rate for fluoroscopy and frame rate for DSA acquisition, were identical. The total CM volume and the CM volume per DSA run injected during the procedures were counted for each group. Data were compared between the two groups. The median DAP of the baseline DSA runs of the OLD group was calculated and compared to the median baseline DAP of the NEW group (“inter-group” comparison). Furthermore, the median DAP of the final angiogram of the NEW group was calculated. To achieve a second statistical value, illustrating the potential DAP reduction by the use of FI and to calculate the median DAP reduction in the NEW group itself, the DAP of the final angiogram was set as the DAP of a potential baseline DSA run, which had not been performed owing to the use of FI (“intra-group” comparison). Median CM volume in both groups was compared. After testing for normal distribution, the differences between the OLD and the NEW group were tested by using the nonparametric Mann–Whitney U test. Statistical significance was set at p < 0.05. Statistical evaluation of the data was performed by dedicated statistical software (SPSS 25 for Windows; IBM, Armonk, NY, USA).
Local ethic review committee approval was granted (18-216A).
Results
Total median DAP in the NEW group was 28,742 mGy*cm2 (IQR 19,668–42,172) and in the OLD group 43,791 mGy*cm2 (IQR 27,966–84,633) (p = 0.048). DAP from fluoroscopy in the NEW group was 82,146 mGy*cm2 (IQR 3703–14,393) and in the OLD group 8763 mGy*cm2 (IQR 2327–15,798) (p = 0.82). Data of DAPs and CM for both groups are shown in Table 3. Median baseline DSA runs performed in the OLD group were n = 2 (IQR 2–3) and in the NEW group 0 (0–0) (p = 0.001). Inter-group median DAP reduction based on saved baseline DSA runs was 17,118 mGy*cm2 (IQR 10,407–23,614) (p = 0.016). In the NEW group intra-group median DAP reduction was (6007 mGy*cm2; IQR 5012–16,105) (p = 0.1) (Fig. 3). Median cumulative contrast medium volume injected in the NEW group was 45 ml (IQR 30–90) and in the OLD group 120 ml (IQR 100–140) (p = 0.001). Median cumulative CM volume for baseline and final DSA runs was 20 ml (IQR 20–20) in the NEW group and 60 (IQR 40–80) in the OLD group (p = 0.001).
Technical success was 100%. Rutherford category post-interventionally increased by at least one category in all patients (clinical success 100%). ABI post-interventionally increased in the NEW group by 0.25 (p = 0.01) and in the OLD group by 0.3 (p = 0.001) (hemodynamic success: 100%). One procedure-associated complication was reported in the NEW group (peripheral thromboembolism). In this case, the patient was a Rutherford category III patient with a 70-mm-long occluded lesion of the left external iliac artery, which showed no calcified plaques. The CTA was performed 6 weeks prior to the lesion. This was successfully handled during a secondary procedure via an antegrade access using manual aspiration thrombectomy. Table 4 summarizes the results.
Discussion
Fusion imaging for endovascular guidance has proven to be valuable in complex EVAR procedures and to guide selection of the target vessel during embolization procedures of the liver [2, 3, 15, 16]. These studies showed that FI derived from CTA images significantly reduces CM volume and radiation exposure as well as procedure time. Reduction in radiation exposure during (complex) endovascular interventions is important to patients and staff not only because of short-term deterministic but mostly because of long-term stochastic effects, which might induce cancer years or decades from the intervention [5, 6, 17,18,19]. By FI, DSA runs can be limited to a minimum, resulting not only in reduction in radiation exposure but also in a significant reduction in CM. It is known that CM exposure can lead to decreased renal function and is associated with acute kidney injury [7,8,9, 20].
In theory, FI should be helpful during endovascular revascularization of peripheral arteries as well. However, the literature regarding this topic is limited to three studies to the best of our knowledge. Those that have been published have evaluated image fusion with 3D-3D registration in patients with iliac or femoro-popliteal steno-occlusive disease.
Ierardi et al. evaluated preoperative CTA and MRA with fluoroscopy for road mapping in five patients with aorto-iliac steno-occlusive disease [13]. All cases were technically successful, and a significant reduction in CM was achieved. Furthermore, it was stated that fusion imaging has a potential of radiation reduction in iliac procedures. Sailer and colleagues evaluated fusion guidance in 17 patients with PAD [12]. In 12 patients, an iliac stenosis or occlusion was found. In 3 patients out of 17, movement of the patient hindered the use of the fusion road mapping. Furthermore, PTA and stenting only based on fusion guidance were successfully performed in the half of patients (8 out of 17). Accurate stent placement and the iliac runoff were examined by conventional DSA at the end of the intervention. Sailer et al. also concludes that fusion imaging can reduce contrast medium volume. Goudeketting et al. validated the reliability of 3D-3D FI for revascularization of iliac artery obstructions and in particular, the accuracy of FI in catheterized and non-catheterized iliac vessels [14].
Our study is the only one published yet in which 2D-3D FI had been used for registration prior to FI guidance of iliac artery procedures in PAD patients. We compared a NEW group, in which iliac stenosis or occlusion was revascularized by fusion guidance to an OLD group, in which at least one initial angiogram for guidance purposes had been performed. All procedures in both groups were technically and hemodynamically successful. In the NEW group, median DAP reduction of 17,118 mGy*cm2 (IQR 10,407–23,614) (p = 0.016) was achieved by saving at least one DSA run compared to the OLD group. Furthermore, to illustrate the potential to reduce radiation by using FI, the DAP of the final angiogram in the NEW group was set to equal the DAP of a potential baseline angiogram in the same group, which had not been performed owing to the use of FI. Therefore, a potential DAP reduction of 6007 mGy*cm2 (IQR 5012–16,105) (p = 0.1) was attained in the NEW group. Furthermore, by reducing DSA runs, the amount of CM was equally reduced. Although patients were not sedated, compared to the report by Sailers et al. [12], no relevant body movement during the intervention was detected neither did physiological breathing of the patients relevantly disturb fusion accuracy. This is probably explained by the retroperitoneal pelvic anatomy of the iliac vessels, which are less vulnerable to abdominal movement compared to the abdominal aorta or the visceral arteries. Nonetheless, anxious patients could be sedated in order to avoid unnecessary and procedure-delaying movement.
An issue we faced in one patient (Fig. 2A–C) was straightening of the target vessel even by a soft guidewire, which interfered with fusing the CTA with fluoroscopy images. In this case, technical success was nevertheless achieved, since the short lesion was located in the middle segment of the common iliac artery with sufficient distal and proximal landing zones. Moreover, there was no case in which re-adjustment of the fused images had to be done. Goudeketting et al. validated the accuracy of FI in catheterized and non-catheterized iliac vessels and found no significant difference in both groups [14]. As in our study, they only used soft guidewires and catheters to probe the vessels, which apparently in most cases will not change the configuration of the vessel anatomy to an extent that would compromise the match. We believe that a slight inaccuracy of FI is only relevant in parts of the vessel where absolute optimal landing of a stent is necessary, e.g., at the aortic/iliac bifurcation or at the distal part of the external iliac artery. In order not to risk technical success in those cases, a conventional approach should be considered.
An alternative approach to 2D-3D registration used in this study is 3D-3D registration, in which pre-procedural contrast-enhanced MRA or CTA is segmented and semiautomatically registered to a cone beam computed tomography image acquired at the beginning of the procedure (“on table”). In the case of EVAR, it has been shown that this method is more accurate than 2D-3D registration [21]. Nevertheless, at least for the patient acquisition of a cone beam CT scan contributes to a higher cumulative DAP and consequently to a greater risk for stochastic or deterministic effects of radiation.
Regarding radiation exposure during iliac artery revascularization procedures, the optimal combination might be represented by combining 2D-3D registration based on an MRA. However, owing to logistics in our clinic in most patients, a CTA is available as pre-procedural imaging.
Lalys et al. recently published a study in which the accuracy of a new image fusion system with 2D-2D registration was compared to 2D-3D registration during revascularization of femoro-popliteal arteries of PAD patients [22]. Both methods were equally accurate. Therefore, 2D-3D image fusion may not only be a supportive tool during iliac procedures but also during femoro-popliteal interventions.
As in our study, Sailer and Ierardi performed a final conventional angiogram to confirm correct stent position and to rule out potential complications [12, 13]. In future cases, Ierardi suggested replacing the final DSA run by measuring aortic and iliac pressure distal and proximal from the treated lesion [13]. Hereby, pressure differences < 10 mmHg could indicate a hemodynamically successful procedure. In our opinion, this technique might verify an improved flow within the treated vessel segment. However, it cannot confirm correct deployment of a stent or rule out complications (e.g., embolism). Another radiation-sparing alternative to DSA would be IVUS, which can detect incorrect stent deployment or residual stenosis.
Nevertheless, even though a final angiogram might be needed in most cases to rule out complications and document technical success, a baseline angiogram, which is still common practice during almost every intervention in most departments, can easily be substituted by the use of FI in the future.
The limitations of this study are the retrospective and non-randomized study design as well as the small study population. Moreover, a general awareness for radiation and contrast medium exposure has been reported to influence radiation and contrast dose, which might have influenced the results [23].
Conclusion
In conclusion, CTA-based fusion imaging using 2D-3D registration is feasible and safe for guidance of endovascular treatment of iliac steno-occlusive disease. Compared to the traditional procedure, it significantly reduces the radiation dose and contributes to minimizing operators’ and patients’ risk for potential short- and long-term effects of radiation exposure. Moreover, it significantly reduces contrast medium volume and could thereby limit the patients’ risk of renal dysfunction.
References
Maurel B, Hertault A, Sobocinski J, et al. Techniques to reduce radiation and contrast volume during EVAR. J Cardiovasc Surg (Torino). 2014;55:123–31.
Hertault A, Maurel B, Sobocinski J, et al. Impact of hybrid rooms with image fusion on radiation exposure during endovascular aortic repair. Eur J Vasc Endovasc Surg. 2014;48:382–90.
Hertault A, Rhee R, Antoniou GA, et al. Radiation dose reduction during EVAR: results from a prospective multicentre study (The REVAR Study). Eur J Vasc Endovasc Surg. 2018;56:426–33.
Kobeiter H, Nahum J, Becquemin JP. Zero-contrast thoracic endovascular aortic repair using image fusion. Circulation. 2011;124:e280–2.
Kuhelj D, Zdesar U, Jevtic V, et al. Risk of deterministic effects during endovascular aortic stent graft implantation. Br J Radiol. 2010;83:958–63.
Weerakkody RA, Walsh SR, Cousins C, Goldstone KE, Tang TY, Gaunt ME. Radiation exposure during endovascular aneurysm repair. Br J Surg. 2008;95:699–702.
Schwab SJ, Hlatky MA, Pieper KS, et al. Contrast nephrotoxicity: a randomized controlled trial of a nonionic and an ionic radiographic contrast agent. N Engl J Med. 1989;320:149–53.
Rudnick MR, Goldfarb S, Wexler L, et al. Nephrotoxicity of ionic and nonionic contrast media in 1196 patients: a randomized trial. The Iohexol Cooperative Study. Kidney Int. 1995;47:254–61.
Andreucci M, Solomon R, Tasanarong A. Side effects of radiographic contrast media: pathogenesis, risk factors, and prevention. Biomed Res Int. 2014. https://doi.org/10.1155/2014/741018.
Abi-Jaoudeh N, Kobeiter H, Xu S, Wood BJ. Image fusion during vascular and nonvascular image-guided procedures. Tech Vasc Interv Radiol. 2013;16:168–76.
Dijkstra ML, Eagleton MJ, Greenberg RK, Mastracci T, Hernandez A. Intraoperative C-arm cone-beam computed tomography in fenestrated/branched aortic endografting. J Vasc Surg. 2011;53:583–90.
Sailer AM, de Haan MW, de Graaf R, et al. Fusion guidance in endovascular peripheral artery interventions: a feasibility study. Cardiovasc Intervent Radiol. 2015;38:314–21.
Ierardi AM, Duka E, Radaelli A, Rivolta N, Piffaretti G, Carrafiello G. Fusion of CT angiography or MR angiography with unenhanced CBCT and fluoroscopy guidance in endovascular treatments of aorto-iliac steno-occlusion: technical note on a preliminary experience. Cardiovasc Intervent Radiol. 2016;39:111–6.
Goudeketting SR, Heinen SG, van den Heuvel DA, et al. The use of 3D image fusion for percutaneous transluminal angioplasty and stenting of iliac artery obstructions: validation of the technique and systematic review of literature. J Cardiovasc Surg (Torino). 2018;59:26–36.
Bargellini I, Turini F, Bozzi E, et al. Image fusion of preprocedural CTA with real-time fluoroscopy to guide proper hepatic artery catheterization during transarterial chemoembolization of hepatocellular carcinoma: a feasibility study. Cardiovasc Intervent Radiol. 2013;36:526–30.
Sailer AM, de Haan MW, de Graaf R, et al. Fusion guidance in endovascular peripheral artery interventions: a feasibility study. Cardiovasc Intervent Radiol. 2015;38:314–21.
Marx MV. The radiation dose in interventional radiology study: knowledge brings responsibility. J Vasc Interv Radiol. 2003;14:947–51.
Kocinaj D, Cioppa A, Ambrosini G, et al. Radiation dose exposure during cardiac and peripheral arteries catheterisation. Int J Cardiol. 2006;113:283–4.
Rahimi SA, Coyle BW, Vogel TR, Haser PB, Graham AM. Acute radiation syndrome after endovascular AAA repair. Vasc Endovascular Surg. 2011;45:178–80.
Parfrey PS, Griffiths SM, Barrett BJ, et al. Contrast material-induced renal failure in patients with diabetes mellitus, renal insufficiency, or both. A prospective controlled study. N Engl J Med. 1989;320:143–9.
Stangenberg L, Shuja F, Carelsen B, Elenbaas T, Wyers MC, Schermerhorn ML. A novel tool for three-dimensional roadmapping reduces radiation exposure and contrast agent dose in complex endovascular interventions. J Vasc Surg. 2015;62:448–55.
Lalys F, Favre K, Villena A, Durrmann V, Colleaux M, Lucas A, et al. A hybrid disease. Int J Comput Assist Radiol Surg. 2018;13:997–1007.
Stansfield T, Parker R, Masson N, Lewis D. The endovascular preprocedural run through and brief: a simple intervention to reduce radiation dose and contrast load in endovascular aneurysm repair. Vasc Endovascular Surg. 2016;50:241–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and Animal Rights
This article does not contain any studies with animals performed by any of the authors.
Informed Consent
Written, informed consent was not applicable, as this was a retrospective study involving no human subjects. Local ethic review committee approval was granted (18-216A).
Consent for Publication
For this type of study, consent for publication is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Stahlberg, E., Sieren, M., Anton, S. et al. Fusion Imaging Reduces Radiation and Contrast Medium Exposure During Endovascular Revascularization of Iliac Steno-Occlusive Disease. Cardiovasc Intervent Radiol 42, 1635–1643 (2019). https://doi.org/10.1007/s00270-019-02250-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-019-02250-5