Abstract
Surgical curettage is currently the standard of care for the chondroblastoma, but in peri-articular tumors it is limited by its morbidity. In this preliminary report, we evaluate the feasibility of percutaneous cryotherapy as an alternative ablative treatment for chondroblastoma. Three patients with a chondroblastoma treated by CT scan-guided cryotherapy are presented in this article. Pain permanently disappeared 2 days after the procedure. No local tumor recurrence or cartilage damage was observed by MRI performed 1 year after the intervention. This preliminary case report suggests that percutaneous cryotherapy may be a possible alternative to the current standard of care in chondroblastoma. Further studies are needed to evaluate if this technique offers similar anti-tumoral efficacy while providing better pain relief and less morbidity than curettage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chondroblastoma (CB) is a rare benign bone tumor that accounts for 1–2% of all primary bone tumors and 9% of all benign bone tumors. It usually develops in young patients aged between 5 and 25. Malignant transformation has rarely been described. 50–75% of CB are located on long bones, and it mainly affects the proximal tibia or femur, distal femur, and proximal humerus. The major symptoms of CB are chronic pain, local swelling, and decreased joint mobility [1].
The typical X-ray presentation of CB is an epiphyseal, well-demarcated, eccentric, and osteolytic lesion with a thin peripheral rim of sclerotic bone (Lodwick classification 1A–1C).
CT scan imaging shows a peripheral rim of sclerotic bone, a lacelike matrix mineralization with scalloped borders, and a periosteal reaction.
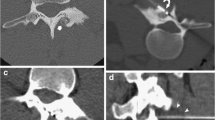
MRI imaging shows an important bone inflammation with a high T2 signal marrow edema that surrounds a low to intermediate T2 signal of the tumor. T1 signal is low in the tumor [2] (Fig. 1).
Patient 2 initial CT scan and MRI: A Knee CT scan before the procedure shows an osteolytic lesion of the distal femur in the intercondylar notch, surrounded by a thin rim of sclerosis (white arrow). B MRI before the procedure (T2-weighted through the knee in three planes) shows a bone edema in the distal femur. C T1-weighted (through the knee in coronal and sagittal planes) shows the contact of the tumor with the cartilage (white arrow)
Currently, complete surgical bone curettage is the standard of care. Surgical bone curettage offers a high cure rate, but it is limited by its morbidity and local recurrences (local recurrence rates reported vary from 10 to 35%) [1].
A previous study has suggested that combining curettage with adjuvant cryotherapy might decrease local recurrence rates [3]. In this article, we evaluate the feasibility of using percutaneous cryotherapy alone as an alternative ablative treatment in patients with CB.
Case Report
Three patients (two males and one female) were addressed to the Leon BERARD Hospital, Lyon, France, for a strong suspicion of CB (young age, inflammatory pain, no biological inflammatory syndrome, epiphyseal bone tumor, cartilaginous matrix, and bone edema).
All lesions were in touch with the cartilage, but epiphysis was closed for all patients.
Two patients were 17 years old and one was 16 years old.
All patients had a consultation with an orthopedic surgeon or an interventional radiologist with a CT scan and an MRI imaging to characterize the bone tumor. All patients were discussed during a multidisciplinary treatment conference. Percutaneous cryotherapy was proposed to patients if an interventional radiologist considered it technically feasible and if a surgeon considered surgery to be possibly too morbid to the joint (Table 1).
Bone biopsy and cryotherapy were performed during the same procedure, under general anesthesia with CT scan guidance.
A bone access was first performed using a motorized bone biopsy system (Arrow® OnControl®) with a bone access needle in 11G. Next, a bone biopsy was made using a bone needle in 12G and completed with a Quick-Core® biopsy in 14G. The CB was histologically proven for all patients.
Cryotherapy (GALIL Medical®) was performed after by using needles and generator’s power that were chosen according to the size and the localization of the chondroblastoma.
Each treatment was composed of two cycles and each cycle lasted 10 min (Fig. 2).
Patient 2 interventional CT scan and MRI after treatment: Transverse, coronal MIP, and sagittal control CT scan after placement of three cryotherapy needles (three Ice Sphere needles) (patient 2). Sagittal MRI (T2-weighted) after 3 months shows a peripheral halo in low signal (white arrows) which delimits the congelation zone
Three and 12 months after, patients were evaluated radiologically by MRI.
Two patients (patient 2 and 3) underwent one treatment, whereas the third patient (patient 1) underwent two procedures because of a local recurrence.
Each hospitalization lasted 3 days. All patients returned to their daily activities without any disability 2 weeks after the procedure.
All patients reported a permanent disappearance of the pain (VAS = 0) 2 days after the procedure.
After 3 months, MRI showed no residual tumor for patient 2 and 3.
After 6 months, MRI showed a progression of tumor volume and edema, in favor of local recurrence for patient 1. This patient was retreated by the second round of cryotherapy and total ablation of the tumor was observed.
All three MRIs performed 1 year after the procedure showed no cartilage damage and no local recurrence.
Discussion
The traditional treatment for CB is surgical curettage. While highly effective, this technique is limited by its various complications (tumor relapses, fractures, long hospital stays, and functional recoveries) [4].
Radiofrequency is an alternative treatment modality that has been used in patients with CB, but unfortunately it can cause delayed complications, like subchondral bone plate infraction or osteonecrosis/chondrolysis that usually occur 6 months after treatment [5].
Compared to radiofrequency, cryotherapy is a more reproducible and conformational technique and the simultaneous use of several needles allows the treatment of larger lesions (> 2.5 cm) [6].
A recent study has suggested that if the addition of cryotherapy to a surgical curettage might decrease local recurrence rates, it does not decrease its morbidity [3].
Compared to surgical curettage, cryotherapy has the advantage of avoiding the presence of a scar, and it might be associated with decreased morbidity, faster rehabilitation, and fewer functional impairments. In our case report, percutaneous cryotherapy was used both on the lesion and on the surrounding bone tissue to insure an adequate destruction of the tumor. No complications were observed 1 year after the procedure, but a longer follow-up is necessary to assess the possibility of delayed complications (like subchondral bone plate infraction) and to evaluate post-treatment complete ossification.
In our case reports, one patient had to be treated twice by percutaneous cryotherapy. It was the first patient to be treated in our hospital and he was probably initially treated sub-optimally by fear of potential cartilage damage. One month after treatment, an MRI showed that the percutaneous cryotherapy had not covered the whole tumor volume. The second treatment permitted a satisfactory ablation, and no recurrence was observed after a 1-year follow-up.
Cryotherapy ice balls are barely visible by CT scan in bone tissues. MRI guidance is already used for percutaneous cryotherapy in soft tissues (breast, lung, or prostate). It may be more accurate than a CT scan to evaluate the size of the ice ball and the ablation volume during the procedure, but it would require special MRI compatible equipment [7, 8].
In conclusion, after a 1-year follow-up, percutaneous ablation of CB using cryotherapy seems to be a feasible, effective, and minimally invasive procedure that needs to be further evaluated in more patients and directly compared to surgical curettage.
Long-term recurrence rates and the medico-economical burden of this procedure also need to be assessed in comparison with surgical curettage.
References
Xu H, Nugent D, Monforte HL, Binitie OT, Ding Y, Letson GD, et al. Chondroblastoma of bone in the extremities: a multicenter retrospective study. J Bone Joint Surg Am. 2015;97(11):925–31.
Steffner R. Benign bone tumors. Cancer Treat Res. 2014;162:31–63.
Mashhour MA, Abdel Rahman M. Lower recurrence rate in chondroblastoma using extended curettage and cryosurgery. Int Orthop. 2014;38(5):1019–24.
Tiefenboeck TM, Stockhammer V, Panotopoulos J, Lang S, Sulzbacher I, Windhager R, et al. Complete local tumor control after curettage of chondroblastoma—a retrospective analysis. Orthop Traumatol Surg Res. 2016;102(4):473–8.
Lalam RK, Cribb GL, Tins BJ, Cool WP, Singh J, Tyrrell PNM, et al. Image guided radiofrequency thermo-ablation therapy of chondroblastomas: should it replace surgery? Skeletal Radiol. 2014;43(4):513–22.
Rybak LD, Rosenthal DI, Wittig JC. Chondroblastoma: radiofrequency ablation—alternative to surgical resection in selected cases. Radiology. 2009;251(2):599–604.
Kaiser WA, Pfleiderer SOR, Baltzer PAT. MRI-guided interventions of the breast. J Magn Reson Imaging. 2008;27(2):347–55.
Liu S, Ren R, Liu M, Lv Y, Li B, Li C. MR imaging-guided percutaneous cryotherapy for lung tumors: initial experience. J Vasc Interv Radiol. 2014;25(9):1456–62.
Author information
Authors and Affiliations
Contributions
All of the authors substantively contributed to this work and agree with its content. A. Bouhamama, F. Pilleul, A. Thibaut have designed the study. A. Bouhamama, A. Ricoeur, A. Thibaut have gathered the data. A. Thibaut and A. Bouhamama have analyzed the data. A. Bouhamama vouches for the data and the analysis. A. Thibaut, A. Boespflug, A. Bouhamama, C. Mastier wrote the paper. All authors have decided to publish the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Thibaut, A., Bouhamama, A., Boespflug, A. et al. Percutaneous Cryotherapy for Treatment of Chondroblastoma: Early Experience. Cardiovasc Intervent Radiol 42, 304–307 (2019). https://doi.org/10.1007/s00270-018-2085-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-018-2085-y