Abstract
Endovascular aneurysm repair (EVAR) is first-choice treatment for many patients with abdominal aortic aneurysms. Complications unique to endovascular treatment include endoleak and endotension, which can eventually lead to rupture. We present two cases of late aortic rupture after EVAR, where both patients had recent preceding catheter-directed thrombolysis with urokinase for acute limb ischemia. These cases suggest a relation between thrombolytic therapy and aortic rupture after EVAR, and we should therefore be aware of this possible complication.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endovascular aneurysm repair (EVAR) plays a key role in the treatment of abdominal aortic aneurysms (AAA). Since its introduction by Parodi et al. [1] in 1991 EVAR has been extensively studied and compared to open surgical repair [2]. These studies conclude that short-term mortality favors EVAR; however, re-intervention rates are believed to be higher in the EVAR group. A reason for re-intervention could be any type of endoleak or in rarer cases endotension. Distinction of these types of complications is often hard, especially between type 3, 4 and endotension. White et al. [3] defined endotension as persistent or recurrent pressurization of an aneurysm sac after EVAR, without evidence of endoleak. Consequently, there is, usually slow, continued growth of the aneurysm sac [4]. Despite sometimes being classified as type V endoleak, this term should be avoided as it is in fact not an endoleak.
One recently published case described a patient developing acute endotension after repeated treatment with catheter-directed thrombolytic therapy because of an occluded iliac graft limb [5]. Rupture did not occur and was therefore treated conservatively. To our knowledge rupture of the aneurysm sac after catheter-directed thrombolytic therapy in patients after EVAR has not been described before, which is why we present two cases: First case is a type 3 endoleak, while the second case involves the phenomenon endotension.
Case Presentation
Case 1
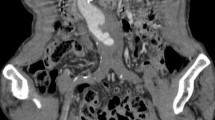
In January 2012, a 65-year-old male presented at the emergency department with a cold and painful left lower leg. Two years prior, an EVAR (Zenith low profile, Cook Medical inc, Bloomington, USA) had been performed for an infrarenal AAA. Further medical history was unremarkable. Contrast-enhanced computed tomography (CECT) revealed occlusion of the left iliac leg graft, with an otherwise normal aspect of the stent graft and aneurysm sac. Consequently, he was set up for catheter-directed urokinase treatment (Cragg-McNamara, Covidien, Mansfield, Massachusetts) with a bolus of 250.000 IE urokinase and continuous infusion at 50.000 IE/h for 24 h. Repeating angiography the next day showed a patent left iliac leg graft; however, endoleak was suspected, presumably a type 3 considering the contrast extravasation at the level of the flow divider (Fig. 1A). This was supported by CECT. There was no relevant growth of the aneurysm sac and no sign of rupture. Close follow-up was proposed: Four days later, laboratory results showed marked decrease of Hb from 6.0 mmol/l to 4.5 mmol/l. CECT was repeated, which showed no more sign of endoleak, but instead revealed a contained rupture (Fig. 1B). Treatment was necessary and resulted in open surgical repair involving removal of the aortic stent graft.
Case 1; A angiography after urokinase treatment for acute limb ischemia shows a patent graft. Notice the endoleak on the left side close to the flow divider (black arrow). B CECT in arterial phase 4 days after angiography shows no sign of endoleak. However, there is a contained rupture. Notice the disruption of the intimal calcification of the left dorsal aspect of the aneurysm sac with adjacent retroperitoneal stranding (white arrow)
Case 2
Since 2002, a 76-year-old female with a history of hypertension and peripheral artery disease was followed up for an AAA and bilateral popliteal aneurysms. The right popliteal aneurysm was thrombosed and stable; however, the left popliteal aneurysm required surgical repair with a venous interponate in 2004. In 2007, the AAA surpassed the 5-cm threshold and underwent elective EVAR using an Excluder stent graft (Excluder, W.L. Gore & Associates inc., Flagstaff, USA). The procedure was uncomplicated, and shrinkage of the aneurysm sac was obtained during routine follow-up in the years thereafter until no measurable sac remained.
In July 2015, she presented at the emergency department with a cold and painful left foot, increasing over the past 2 weeks. CECT and subsequent angiogram confirmed an occluded anterior tibial artery (ATA); hence, catheter-directed thrombolytic therapy was initiated conform protocol similar to case 1. Repeat angiogram the next day showed revascularization of the ATA. Symptoms reduced accordingly, and recovery was uneventful. Therefore, she was discharged on the fourth day after admission.
However, the next day she returned to the emergency department with increasing abdominal discomfort in the left lower quadrant. She was hemodynamically stable. Blood pressure was 185/92 mmHg with a pulse of 89 bpm. On palpation there was abdominal tenderness. The left groin area and left foot were unremarkable, both with strong pulsations. Laboratory results showed a hemoglobin (Hb) level of 5.7 mmol/l, hematocrit (Ht) of 0.27 and mean cell volume (MCV) of 89.
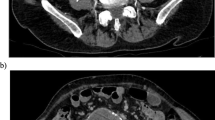
CECT in arterial and venous phase demonstrated a patent aortic stent graft, but with an aneurysm sac of 65 mm in diameter (Fig. 2A), whereas the CT 5 days prior showed no measurable aortic sac (Fig. 2B). Furthermore, retroperitoneal fat stranding and hematoma signaled rupture (Fig. 2A). There was no endoleak on CT, nor did the stent graft change in (mechanical) configuration. Based on clinical and radiologic findings, endotension with contained aortic aneurysm rupture was suggested.
Case 2; A CECT displays an enlarged aneurysm sac around the stent graft measuring 65 mm in AP diameter. Notice the retroperitoneal stranding on the left side indicating rupture of the aneurysm. B Five days before rupture, CECT remarkably shows no measurable aneurysm sac around the stent graft, despite being at comparable anatomical height
Endovascular graft relining, an accepted treatment for endotension [6], was proposed. After gaining access through both common femoral arteries, an angiogram was performed, which confirmed the absence of any clear endoleak. A 23 mm × 3.3 cm Excluder aortic extender (Excluder, W.L. Gore & Associates inc., Flagstaff, USA) was placed directly below the lowest renal artery. Next, both iliac limb Excluder stent grafts, a 20 mm × 13.5 cm and 18 mm × 13.5 cm, were placed in the cuff and deployed, thereby completely covering the old stent graft. Completion angiogram after post-dilatation with a noncompliant balloon showed no endoleak or occlusion. Both access points were closed with closure devices (Prostar XL, Abott, Illinois, USA). A few days after, she was discharged in good health. Follow-up CT after 3 weeks showed marked decrease in size of the aneurysm sac, measuring 57 mm in diameter. Seven months after treatment, CT showed no measurable aneurysm sac.
Discussion
EVAR plays a major role in the treatment of AAA, but also introduces complications unique to EVAR compared to open surgical alternatives. These complications include endoleak and endotension [2, 3]. The pathology of endoleaks is quite well described, and improvement in the new age endovascular aortic stent grafts results in a lower incidence of the complication. Endotension is a rare and late complication of EVAR, defined as increased intrasac pressure without evidence of endoleak [3]. Though not diagnostic, on CECT this translates into growth of the aneurysm sac without signs of endoleak. In the literature endoleak has been described masquerading as endotension, where no contrast leakage is seen on CT, however, present at surgery [7].
The exact etiology of endotension remains unknown. Numerous causes of increased intrasac pressure have been hypothesized [4]. Pressure is assumed to develop within the sac, e.g., in infection or hygroma, or is transmitted to the aneurysm sac either through thrombus around the ends of the graft, through the graft wall or from branch vessels. In particular grafts with increased permeability, for instance, the original permeable Gore Excluder devices are prone to develop endotension [8]. It is believed that this high permeability leads to microleaks, transudation or exudation and eventually to aortic sac expansion and rupture.
One previous case report described a possible relation between catheter-directed thrombolysis and endotension. A Cook Zenith Flex was used as stent graft and recombinant tissue plasminogen activating factor (r-tPA) as thrombolytic agent to treat an occluded iliac limb [5]. The aneurysm sac grew from 54 to 66 mm without signs of rupture and thus was successfully treated conservatively. The authors proposed that thrombolytic agents contribute in an increased graft porosity.
In our second case a late low-permeability Excluder device was used for EVAR in 2007. This type device was manufactured and proven successful to prevent microleakage leading to endotension [9]. Moreover, thrombolysis was directed at the ATA. Nonetheless our patient developed endotension with extremely rapid sac enlargement, growing from no measurable sac to 65 mm in 5 days, which is unusually rapid for endotension.
This second case supports the presumed relation between thrombolysis and endotension, while our first case shows that also endoleak could result from thrombolytic treatment. However, this case is less understood with the theory of increased graft porosity, since instead of endotension there was a rather large endoleak. Nevertheless, in both cases thrombolytic therapy led to rupture, which in this context has not been described before.
Treatment was necessary in both cases. Open surgery and endovascular graft relining are the most common options [6, 10]. In uncomplicated endotension direct sac aspiration may well be a feasible option [10]. Relining has successfully been used to treat endoleak and endotension and even graft tears [6]. However, no comparative study has been performed between open surgery and relining; hence, the treatment of choice remains debatable.
This report does not imply that thrombolytic agents should be avoided when an endovascular aortic stent graft is present; however, surgeons and interventional radiologists should be aware of this rare complication and possibilities in treatment. When a patient develops abdominal pain after thrombolytic therapy, further assessment with CT is advised. In case of an aortic aneurysm rupture following endoleak or endotension, relining is among other treatment options. However, comparative studies between open surgery and endovascular treatment are necessary to determine mainstay treatment.
References
Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. 1991;5(6):491–9.
Paravastu SC, Jayarajasingam R, Cottam R, Palfreyman SJ, Michaels JA, Thomas SM. Endovascular repair of abdominal aortic aneurysm. Cochrane Database Syst Rev. 2014;1:CD004178.
White GH, May J. How should endotension be defined? History of a concept and evolution of a new term. J Endovasc Ther. 2000;7(6):435–8.
Toya N, Fujita T, Kanaoka Y, Ohki T. Endotension following endovascular aneurysm repair. Vasc. Med. 2008;13(4):305–11.
Belchos J, Wheatcroft M, Prabhudesai V, Moloney T. Development of endotension after multiple rounds of thrombolysis after endovascular aneurysm repair. J. Vasc. Surg. Cases. 2015;1(1):24–7.
Filippi F, Tirotti C, Stella N, Rizzo L, Taurino M. Endotension-related aortic sac rupture treated by endograft relining. Vascular. 2013;21(2):113–5.
Yoshitake A, Hachiya T, Itoh T, Kitahara H, Kasai M, Kawaguchi S, Shimizu H. Nonvisualized type III endoleak masquerading as endotension: a case report. Ann Vasc Surg. 2015;29(3):595–7.
Fillinger M. Three-dimensional analysis of enlarging aneurysms after endovascular abdominal aortic aneurysm repair in the gore excluder pivotal clinical trial. J Vasc Surg. 2006;43(5):888–95.
Haider SE, Najjar SF, Cho JS, Rhee RY, Eskandari MK, Matsumura JS, Makaroun MS, Morasch MD. Sac behavior after aneurysm treatment with the Gore Excluder low-permeability aortic endoprosthesis: 12-month comparison to the original excluder device. J Vasc Surg. 2006;44(4):694–700.
Uthoff H, Katzen BT, Gandhi R, Pena CS, Benenati JF, Geisbusch P. Direct percutaneous sac injection for postoperative endoleak treatment after endovascular aortic aneurysm repair. J Vasc Surg. 2012;56(4):965–72.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical Approval
For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Sudiono, D.R., Hoencamp, R., Ottevanger, J. et al. Aortic Aneurysm Rupture After Urokinase Treatment for Acute Limb Ischemia in Two Patients After EVAR. Cardiovasc Intervent Radiol 40, 1641–1644 (2017). https://doi.org/10.1007/s00270-017-1671-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-017-1671-8