Abstract
K-Ca double carbonates recently identified in inclusions in diamonds, as well as associated alkali-carbonate melts can play an important role in the deep carbon cycle. We studied pressure-induced changes in the crystal structure of high-pressure α-K2Ca3(CO3)4 phase up to 20 GPa using synchrotron single-crystal x-ray diffraction in diamond anvil cell. At ~ 7 GPa at room temperature the orthorhombic P212121 phase of α-K2Ca3(CO3)4 undergoes displacive phase transition into monoclinic P1121 phase. Despite the phase transition, PV-curve does not demonstrate any irregularities so that both phases can be described by the same 4th order Birch-Murnaghan equation of state with V0 = 1072.5(3) Å3, K0 = 51.1(8) GPa, K’0=3.7(3), K’’0=0.12(6).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The findings of Na-Ca and K-Ca double carbonates as inclusions in superdeep (Kaminsky et al. 2009) and lithospheric (Golovin et al. 2016; Logvinova et al. 2019) diamonds together with the experimental observations of these phases in carbonated eclogites (Thomson et al. 2016) and pelites (Grassi and Schmidt 2011) subjected to the PT-conditions of mantle transition zone demonstrate the geological relevance of Na2CO3-K2CO3-CaCO3 system at conditions of the Earth’s mantle. Although the systematic studies of this system still do not exceed pressures of 6 GPa (Shatskiy et al. 2015a, b; Podborodnikov et al. 2018; Arefiev et al. 2019), they allowed to describe a surprisingly large number of new crystal structures of double alkali-calcium carbonates (Gavryushkin et al. 2014; Rashchenko et al. 2017, 2018, 2021) which can potentially play a role in the global carbon cycle. In addition, recently alkali – alkali earth double carbonates were shown to be promising materials for nonlinear optical (NLO) and ultraviolet (UV) applications (Luo et al. 2016; Song et al. 2017).
Among the new high-pressure phases discovered in the K2CO3-CaCO3 system, two polymorphic modifications of K2Ca3(CO3)4 are of particular crystallographic interest. The α-K2Ca3(CO3)4, synthesized at pressure of 3 GPa, is characterized by an orthorhombic unit cell (space group P212121) and ordered K-Ca cation distribution among crystallographic sites (Rashchenko et al. 2021). However, at pressure of 6 GPa, the same stoichiometry crystallizes as a different polymorphic modification, β-K2Ca3(CO3)4 (space group Pnma), characterized by the same topology, (nearly) the same parameters of orthorhombic unit cell, but K and Ca cations disordered in mixed crystallographic sites (Rashchenko et al. 2024). Both structures are homeotypic to REE orthoborate type compounds, promising as solid-state laser materials and phosphors (Ma et al. 2005; Si and Cai 2013).
In order to reveal the crystal chemical mechanisms underlying this example of both unusual K-Ca isomorphism and unusual response of crystal structure to high pressure, we performed a structural study of ordered α-K2Ca3(CO3)4 modification upon metastable compression at room temperature up to 20 GPa.
Experimental
The α-K2Ca3(CO3)4 sample was synthesized by a solid-state reaction from a stoichiometric mixture of pure K2CO3 and CaCO3 (Wako) using DIA-type 1500-ton press ‘Discoverer’ at IGM SB RAS (Novosibirsk, Russia). During the synthesis, the sample was compressed to a pressure of 3 GPa at room temperature, heated to 975 °C at a rate of 100 °C/min, annealed at constant pressure and temperature for 4 h, quenched with a rate of 300–400 °C/s, followed by 4-hour decompression. The stoichiometry of the recovered sample was confirmed by an energy-dispersive Xray microanalysis (EDS): 25.40(17) mol% K2CO3 and 74.60(17) mol% CaCO3 (for more details on the sample synthesis and EDS, see (Arefiev et al. 2019).
The in situ high-pressure single-crystal diffraction experiments were performed at the station ID015b at the European Synchrotron Radiation Facility. Membrane-driven LeToullec type diamond anvil cell (DAC) was used, equipped with Boehler-Almax anvils with an opening half-angle of 32o. The culet diameter was 600 μm. Stainless steel was used as the gasket material, indented to about 80 μm and drilled to obtain a sample chamber with diameter of 300 μm. A small chip of the above mentioned single-crystal fragment was placed inside the sample chamber along with ruby sphere. Hydrostatic conditions were provided by the DAC loading with a helium pressure-transmitting medium. Pressures were determined using ruby fluorescence scale (Shen et al. 2020). A measurement at ambient pressure was made first, and then the DAC was loaded with He and sequentially pressurized from 0.2 to 20.4 GPa.
Monochromatic X-ray diffraction measurements were performed at a wavelength of 0.41505 Å with a beam size of 10 × 10 μm (Merlini and Hanfland 2013). Diffraction patterns were collected using a EIGER2 XE CdTe 9 M hybrid pixel detector during ± 32° rotation of the DAC in 0.5° steps. The patterns were then transferred into CrysAlisPro software using ESPERANTO protocol (Rothkirch et al. 2013) for indexing and integration. The structure solution and refinement was performed using SUPERFLIP and JANA2020 software (Palatinus and Chapuis 2007; Petříček et al. 2023). The evolution of unit cell parameters with pressure are given in Table 1; more data is available in the supplementary CIF files. Parameters of pressure-volume equations of state were obtained by nonlinear least squares fitting using EoSFit7 software (Angel et al. 2014; Gonzalez-Platas et al. 2016).
Results and discussion
Orthorhombic to monoclinic phase transition
The most evident effect of applied high pressure on the crystal structure of α-K2Ca3(CO3)4 is indeed a second-order phase transition from orthorhombic (P212121) to monoclinic (P1121) phase at pressure around 7 GPa. The phase transition is manifested in clear splitting of reflections in reciprocal space due to non-merohedral twinning (Fig. 1).
The observed phase transition may indicate either existence of stability field of monoclinic P21 phase at pressures around 7 GPa, or metastable nature of this phase in the stability field of β-K2Ca3(CO3)4. To establish the exact phase relations, additional high-pressure and high-temperature experiments are required.
A remarkable feature of the P212121 modification is its anisotropic compression with nearly incompressible b axis, which shrinks less than by 0.5% in the pressure range up to 7 GPa compared with ~ 4% and ~ 6% for c and a axes, respectively (Fig. 2).
Equations of state
Although there is a clear change in the anisotropy of compression of the structure at the phase transition (Fig. 2a) there are no detectable anomalies in the volume variation. Therefore the pressure-volume curves for α-K2Ca3(CO3)4 were fitted with one equation of state to both phases simultaneously. We tested Birch-Murnaghan equations of state of 3rd and 4th order for fitting PV-curves (Fig. 3, Table 2) and found that K’’ value of 4th order equation of state differs by more than 3σ from implied K’’ value of 3rd order equation. Together with lower w-χ2 it indicates that 4th order is statistically more reliable (Fig. 3.).
Compressibility of cation polyhedra
Refinement of crystal structure showed that the phase transition from orthorhombic to monoclinic is displacive and preserves the overall topology of the crystal structure including K and Ca cation ordering (Fig. 4). The latter is evident from the preservation of the average distance in cation polyhedra in both phases (Table 3).
The response of different cation polyhedra in the crystal structure of α-K2Ca3(CO3)4 to compression is visualized in Fig. 5a. Although each initial cation site splits after the P212121 → P1121 transition into two independent ones, the compression of corresponding polyhedra follows the same trend.
According to Hazen and Finger (Hazen and Finger 1979), who reviewed 103 compounds belonging to 19 structural type, polyhedron compressibility (1/KP) linearly correlates with its volume according to the following equation: KP·d3/s2·za·zc = 750 ± 20 GPa·Å3, where d is mean bond length in a given polyhedron, s2 – «ionicity» of the bond, za and zc – formal charge of anion and cation. The only exception from the trend mentioned by Hazen and Finger is CsCl- type compounds with large cations.
In order to compare compressibility of cation polyhedra in α-K2Ca3(CO3)4 with the above trend, we evaluated bulk moduli of individual cation polyhedra in P212121 and P1121 phases using 2rd order Birch-Murnaghan equations of state (Table 4) and plotted them in the 1/KP vs. d3/zc plot.
In the P212121 structure at ambient pressure K polyhedra clearly deviates to the bottom side (Fig. 5b), while Ca – to the top side of the line (Fig. 5c). When pressure increases to 6.25 GPa Ca polyhedra approach to the line while K polyhedra move even further from the line. Phase transition does not significantly changes properties of polyhedra.
(a) Compressibility of K and Ca polyhedra in the α-K2Ca3(CO3)4 crystal structure (the corresponding 2nd order Birch-Murnaghan equations of state for P212121 and P1121 modifications are also shown). (b) Bulk moduli of Ca polyhedra compared with KP·d3/zc = 750 GPa·Å3 line. (c) Bulk moduli of K polyhedra compared with KP ·d3/zc = 750 GPa·Å3 line. For P212121 phase changes in cation polyhedra upon pressure increase from 1 atm to 6.25 GPa are shown with arrows. Arg – aragonite, butsch – butschliite
To compare cation polyhedra compressibility in similar compounds we also plot data for CaO9 polyhedron in aragonite (Palaich et al. 2016) and CaO6 and KO9 polyhedra in butschliite (Zeff et al. 2024). It is clear that CaO9 polyhedron in aragonite lies close to the KP ·d3/zc = 750 GPa·Å3 line (Fig. 5b), however, CaO6 and KO9 polyhedra in butschliite also deviates from the line (Fig. 5b, c).
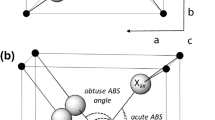
The observed deviation of compressibility vs. volume of large K polyhedra from KP ·d3/zc = 750 GPa·Å3 trend resembles that of CsCl-type halides, reported by Hazen and Finger (1979) (Fig. 6a). The authors explained this deviation by non-negligible interaction of cations with their second coordination sphere which is also typical for large K polyhedra in the structure of α-K2Ca3(CO3)4. For example, in the case of K3 coordination (Fig. 6b) the gap between first and second coordination spheres (3.6–3.9 Å) is smaller than width of the first coordination sphere (2.6–3.6 Å).
(a) Compressibility of K and Ca polyhedra in α-K2Ca3(CO3)4 (legend same as in Fig. 5) in comparison with KP ·d3/zc = 750 GPa·Å3 line and trend for anomalous polyhedra in CsCl-type halides – grey dashed line according to Hazen and Finger (1979). (b) Variation of bond lengths of 16 nearest oxygen atoms to K3 with pressure for P212121 phase
Conclusions
The performed high-pressure – room temperature study of α-K2Ca3(CO3)4 revealed mechanical instability of ordered orthorhombic P212121 phase which undergoes displacive phase transition into monoclinic P1121 phase at ~ 7 GPa at room temperature. Compressibilities of K polyhedra in the α-K2Ca3(CO3)4 structure suggest the presence of a systematic deviation from the known trend KP·d3/zc = 750 GPa·Å3 for polyhedra with large (≥ 8) coordination number.
Data availability
No datasets were generated or analysed during the current study.
References
Angel RJ, Alvaro M, Gonzalez-Platas J (2014) EosFit7c and a Fortran module (library) for equation of state calculations. Z Für Krist - Cryst Mater 229:405–419. https://doi.org/10.1515/zkri-2013-1711
Arefiev AV, Shatskiy A, Podborodnikov IV et al (2019) The system K2CO3–CaCO3 at 3 GPa: link between phase relations and variety of K–Ca double carbonates at ≤ 0.1 and 6 GPa. Phys Chem Min 46:229–244. https://doi.org/10.1007/s00269-018-1000-z
Gavryushkin PN, Bakakin VV, Bolotina NB et al (2014) Synthesis and Crystal Structure of New Carbonate Ca 3 na 2 (CO 3) 4 Homeotypic with orthoborates M 3 ln 2 (BO 3) 4 (M = ca, Sr, and ba). Cryst Growth Des 14:4610–4616. https://doi.org/10.1021/cg500718y
Golovin AV, Sharygin IS, Korsakov AV (2016) Origin of alkaline carbonates in kimberlites of the siberian craton: evidence from melt inclusions in mantle olivine of the Udachnaya-East pipe. Chem Geol. https://doi.org/10.1016/j.chemgeo.2016.10.036
Gonzalez-Platas J, Alvaro M, Nestola F, Angel R (2016) EosFit7-GUI: a new graphical user interface for equation of state calculations, analyses and teaching. J Appl Crystallogr 49:1377–1382. https://doi.org/10.1107/S1600576716008050
Grassi D, Schmidt MW (2011) The melting of Carbonated pelites from 70 to 700 km depth. J Petrol 52:765–789. https://doi.org/10.1093/petrology/egr002
Hazen RM, Finger LW (1979) Bulk modulus—volume relationship for cation‐anion polyhedra. J Geophys Res: Solid Earth 84(B12):6723–6728. https://doi.org/10.1029/JB084iB12p06723
Kaminsky F, Wirth R, Matsyuk S et al (2009) Nyerereite and nahcolite inclusions in diamond: evidence for lower-mantle carbonatitic magmas. Mineral Mag 73:797–816. https://doi.org/10.1180/minmag.2009.073.5.797
Logvinova AM, Shatskiy A, Wirth R et al (2019) Carbonatite melt in type Ia gem diamond. Lithos 342–343:463–467. https://doi.org/10.1016/j.lithos.2019.06.010
Luo M, Song Y, Lin C et al (2016) Molecular Engineering as an Approach To Design a New Beryllium-Free Fluoride Carbonate as a deep-Ultraviolet Nonlinear Optical Material. Chem Mater 28:2301–2307. https://doi.org/10.1021/acs.chemmater.6b00360
Ma P, Chen J, Hu Z et al (2005) Structure of Ba3Y2(BO3)4 crystal. Mater Res Innovations 9:63–64. https://doi.org/10.1080/14328917.2005.11784895
Merlini M, Hanfland M (2013) Single-crystal diffraction at megabar conditions by synchrotron radiation. High Press Res 33:511–522. https://doi.org/10.1080/08957959.2013.831088
Palaich SEM, Heffern RA, Hanfland M, Lausi A, Kavner A, Manning CE, Merlini M (2016) High-pressure compressibility and thermal expansion of aragonite. Am Mineral 101(7):1651–1658. https://doi.org/10.2138/am-2016-5528
Palatinus L, Chapuis G (2007) SUPERFLIP–a computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J Appl Crystallogr 40:786–790
Petříček V, Palatinus L, Plášil J, Dušek M (2023) Jana2020 – a new version of the crystallographic computing system Jana. Z Für Krist - Cryst Mater 238:271–282. https://doi.org/10.1515/zkri-2023-0005
Podborodnikov IV, Shatskiy A, Arefiev AV et al (2018) The system Na2CO3–CaCO3 at 3 GPa. Phys Chem Min 45:773–787. https://doi.org/10.1007/s00269-018-0961-2
Rashchenko SV, Bakakin VV, Shatskiy AF et al (2017) Noncentrosymmetric Na2Ca4(CO3)5 carbonate of m13M23XY3Z Structural Type and Affinity between Borate and Carbonate structures for Design of New Optical materials. Cryst Growth Des 17:6079–6084. https://doi.org/10.1021/acs.cgd.7b01161
Rashchenko SV, Ignatov MA, Shatskiy AF, Arefiev AV, Litasov KD (2024) Coupling between cation and anion disorder in β-K2Ca3(CO3)4. J Appl Crystallogr 57(3):665–669. https://doi.org/10.1107/S1600576724002292
Rashchenko SV, Shatskiy AF, Arefiev AV et al (2018) Na 4 ca(CO 3) 3: a novel carbonate analog of borate optical materials. CrystEngComm 20:5228–5232. https://doi.org/10.1039/C8CE00745D
Rashchenko SV, Shatskiy AF, Ignatov MA et al (2021) High-pressure synthesis and crystal structure of non-centrosymmetric K2Ca3(CO3)4. CrystEngComm 23:6675–6681. https://doi.org/10.1039/D1CE00882J
Rothkirch A, Gatta GD, Meyer M et al (2013) Single-crystal diffraction at the Extreme conditions beamline P02.2: procedure for collecting and analyzing high-pressure single-crystal data. J Synchrotron Radiat 20:711–720. https://doi.org/10.1107/S0909049513018621
Shatskiy A, Borzdov YM, Litasov KD et al (2015a) Phase relationships in the system K2CO3-CaCO3 at 6 GPa and 900–1450 C. Am Mineral 100:223–232. https://doi.org/10.2138/am-2015-5001
Shatskiy A, Gavryushkin PN, Litasov KD et al (2015b) Na-Ca carbonates synthesized under upper-mantle conditions: Raman spectroscopic and X-ray diffraction studies. Eur J Mineral 27:175–184. https://doi.org/10.1127/ejm/2015/0027-2426
Shen G, Wang Y, Dewaele A et al (2020) Toward an international practical pressure scale: a proposal for an IPPS ruby gauge (IPPS-Ruby2020). High Press Res 1–16. https://doi.org/10.1080/08957959.2020.1791107
Si J-Y, Cai G-M (2013) Synthesis and characterization of powder four borate Sr3Sm2(BO3)4. Powder Diffr 28:262–268. https://doi.org/10.1017/S0885715613000420
Song Y, Luo M, Zhao D et al (2017) Explorations of new UV nonlinear optical materials in the Na2CO3–CaCO3 system. J Mater Chem C 5:8758–8764. https://doi.org/10.1039/C7TC02789C
Thomson AR, Walter MJ, Kohn SC, Brooker RA (2016) Slab melting as a barrier to deep carbon subduction. Nature 529:76–79. https://doi.org/10.1038/nature16174
Zeff G, Kalkan B, Armstrong K, Kunz M, Williams Q (2024) High pressure raman spectroscopy and X-ray diffraction of K2Ca(CO3)2 bütschliite: multiple pressure-induced phase transitions in a double carbonate. Phys Chem Minerals 51(1). https://doi.org/10.1007/s00269-023-01262-5
Acknowledgements
This work is financially supported by the joint RFBR-FWF grant project (#21-55-14001 and I 5046-N) and performed on state assignment of IGM SB RAS (No. 122041400176-0) and GEOKHI RAS. MAI acknowledge support by the strategic academic leadership program “Priority 2030” at the Novosibirsk State University. We acknowledge the European Synchrotron Radiation Facility (ESRF) for provision of synchrotron radiation facilities (proposal ES-1061) and we would like to thank Prof. Michael Hanfland and Davide Comboni for assistance and support in using beamline ID015b.
Author information
Authors and Affiliations
Contributions
A.S., A.A. and K.L. synthesized the studied sample, S.R., A.L. and A.R. performed high-pressure x-ray diffraction experiment, M.I. processed the obtained x-ray diffraction data and S.R. wrote the main manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ignatov, M.A., Rashchenko, S.V., Likhacheva, A.Y. et al. High-pressure structural behavior of α-K2Ca3(CO3)4 up to 20 GPa. Phys Chem Minerals 51, 30 (2024). https://doi.org/10.1007/s00269-024-01292-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00269-024-01292-7