Abstract
Aim
To compare the efficacy and safety of synthetic and biological meshes in ventral hernia repair (VHR) and abdominal wall reconstruction (AWR).
Methods
We screened all clinical trials that reported the application of synthetic and biological meshes in VHR and AWR using Medline, Web of Science, and Embase (Ovid). Only comparative studies with similar baselines such as age, sex, body mass index, degree of wound contamination, and hernia defects between the intervention and control groups were included. Effect sizes with 95% confidence were pooled using a random- or fixed-effects model based on the size of heterogeneity. A sensitivity analysis was performed to test the stability of the results.
Results
Ten studies with 1305 participants were included. Biological meshes were associated with significantly higher recurrence rate (OR, 2.09; 95% CI 1.42–3.08; I2 = 50%), surgical site infection (OR, 1.47; 95% CI 1.10–1.97; I2 = 30%), higher re-admission rate (OR, 1.51; 95% CI 1.05–2.17; I2 = 50%), and longer length of hospital stay (SMD, 0.37; 95% CI 0.10–0.65; I2 = 72%). Similar surgical site occurrence, re-operation rate, and mesh explantation rate were observed among biological and synthetic meshes. Biological meshes have no difference in recurrence rate as compared to synthetic meshes, between the clean-contaminated, and contamination-infected fields (OR, 1.41; 95% CI 0.41–4.87 vs 3.00; 95% CI 1.07–8.46; P = 0.36).
Conclusion
Synthetic meshes are a safe alternative to biological meshes for VHR and AWR. Considering the high cost of biological meshes, synthetic meshes are more appropriate for the VHR and AWR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ventral hernia repair (VHR) is a commonly performed surgical procedure in clinical practice. In the United States, it is estimated that on average, more than 600, 000 VHR procedures are performed annually [1]. Compared to suture repairs, the use of a mesh can significantly reduce the recurrence of ventral hernia from 8.2 to 2.7% [2].
Several types of prosthetic meshes have been used for VHR and abdominal wall reconstruction (AWR), including biological and synthetic meshes. Synthetic mesh is most commonly used for hernia repair, because of its low cost and high strength. However, multiple studies have argued that using synthetic materials in VHR and AWR results in a high rate of wound complications, including wound infection and surgical removal of previously planted mesh [3,4,5]. Consequently, prior studies have suggested using biological mesh as an alternative to synthetic mesh in contaminated incisions, because biological mesh seems to show advantages in anti-infection and biocompatibility [6, 7].
There is no consensus regarding the materials to be used in VHR and AWR. Several randomized controlled trials (RCT) published recently have shown that the use of synthetic mesh in abdominal hernia repair has similar or lower recurrence and complication rates than biological mesh [8,9,10].
Several meta-analyses have compared the efficacy and safety of synthetic and biological meshes in VHR [11, 12]. The evidence from these meta-analyses is weak, mainly because most of the included clinical trials had non-comparative designs. In addition, the baseline of the included studies was significantly different. The degree of wound contamination and the mean size of the defect area were significantly different between the biological mesh group and the synthetic mesh group in the same study. Therefore, the pooled results of studies with significantly different baseline values may have been misleading. Recently, several high-quality randomized controlled trials have been published [8,9,10], and it seems prudent to conduct an updated rigorous meta-analysis comparing biological and synthetic meshes for VHR and AWR.
Methods
This meta-analysis and systematic review were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. After a preliminary literature search, this project was registered on the PROSPERO website (ID: CRD42022380412), and all subsequent analyses were performed using the registered protocol.
Search strategy and selection criteria
We systematically searched MEDLINE, Web of Science, and Embase (Ovid) to identify relevant studies published before November 17, 2022. Only studies written in English were included in this meta-analysis. We also carefully read the reference lists of the included studies and reviews to avoid missing any potential studies. The main search terms and their combinations were ventral hernia, abdominal wall reconstruction, synthetic mesh, and biological meshes. The detailed literature retrieval syntax strategy is presented in Supplementary Table 1.
Study selection
Two researchers (Dongchao Yang and Wenpei Dong) filtered the relevant studies by screening titles and abstracts from the results of a systematic search. Disputes were resolved through discussion with a third reviewer (Hekai Shi). The inclusion criteria were as follows: (1) head-to-head clinical trials; (2) patients from biological and synthetic groups had similar baselines, such as age, BMI (body mass index), degree of wound contamination distribution, and hernia defect size (3) patients received nonabsorbable synthetic mesh or absorbable biological mesh in VHR or abdominal wall reconstruction (4) reported at least one of following outcomes: surgical site infection (SSI), surgical site occurrence (SSO), hernia recurrence (HR), length of hospital stay, re-admission, re-operation, and mesh explantation. Studies that failed to meet these criteria were excluded.
Data extraction and risk of bias assessment
Two investigators (Wenpei Dong and Dongchao Yang) extracted the following information from the included studies: name of the first author, publication year, sample size, basic demographic data of the patients, degree of wound contamination, and outcomes of interest mentioned previously. Centers for Disease Control and Prevention (CDC) wound classification was used to describe wound contamination. CDC 1–4 were corresponding to clean, clean-contaminated, contaminated, and dirty, respectively. Any conflicts between the two investigators were resolved through discussion with a third reviewer (Hekai Shi).
We defined SSO as events occurring at the surgical site, including seroma, hematoma, and cellulitis. If a study reported multiple SSI and SSO at different time points, we only included the data reported at or closest to 1 month after the operation. The long-term outcome was HR. Short-term outcomes included SSI, SSO, length of hospital stay, re-admission, re-operation, and mesh explantation.
Two reviewers independently evaluated the quality of included studies. We used the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) to assess the quality of the non-randomized trials. The Risk of Bias 2 (ROB2) tool was used for randomized controlled clinical trials. If graded as low or moderate risk in ROBINS-I or ROB2, we considered this study to be of relatively high quality.
Statistical analysis
We conducted this meta-analysis using R software (GitHub, San Francisco, US; version 4.1.2) with the “meta” package (version 5.2–0). We used I2 statistics to evaluate the size of heterogeneity, and I2 > 50% and P < 0.05 was considered significant. If there was significant heterogeneity, we used a random-effects model. Otherwise, a fixed effects model was used. All effect sizes with 95% CI were calculated using a random-effects or fixed-effects model based on the size of heterogeneity. A sensitivity analysis was performed to determine whether any study included in this meta-analysis had a significant impact on the overall findings. We conducted a subgroup analysis to identify HR with synthetic and biological meshes in clean-contaminated and contamination-infected fields. P < 0.05 (two-tailed) was considered statistically significant in all analyses.
Results
Study characteristics
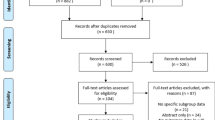
After a systematic search, 3564 relevant studies were preliminarily included. Finally, we identified 10 studies with 1305 patients based on the previously established inclusion and exclusion criteria (Fig. 1) [8,9,10, 13,14,15,16,17,18,19]. The one of the most common reason for exclusion was that the studies had an apparent difference at baseline between the biological and synthetic mesh groups. Supplementary Table 2 lists these studies in reference format.
Table 1 presents the characteristics of the included studies. The mean patient age ranged from 51 to 62.3 years old. The mean BMI of the included patients was relatively high, from 26.9 (standard deviation, SD:5.7) to 38.6 (SD:9.6). Four studies were randomized controlled trials [8,9,10, 18] while the remaining six were retrospective [13,14,15,16,17, 19]. Nine studies reported the wound class in detail, and 30.1% of the patients had clean wounds (n = 346). Seven studies examined polypropylene-based meshes [8,9,10, 13, 15, 18, 19], two examined polyester/polypropylene/polytetrafluoroethylene meshes [14, 17], and one study did not report the specific material of the synthetic meshes [16]. For the biological mesh, six studies used a porcine dermis-derived matrix [8, 9, 16,17,18,19], two studies examined porcine small intestinal submucosa-derived material [10, 15], and two studies partly used human dermis/bovine pericardium mesh [13, 14]. The mean hernia size varied from 52.55 (SD:56.53) to 356 (SD:251) mm2.
Operative techniques
Five studies reported the location of the mesh [13, 14, 17,18,19], and the majority of the mesh position were retro muscular (n = 357, 70.6%), followed by preperitoneal (n = 106, 20.9%). The surgical approach was open in six studies [8, 9, 16,17,18,19], one study used both open and laparoscopic surgical techniques [10], and three studies did not report this. Overall, there was considerable variation in fascial closure rates across studies, as well as in component separation rates. Five studies reported fascial closure in 89.3% (n = 626) of the patients. The fascial closure rate ranged from 40 to 100% in the included studies [8, 9, 16, 18, 19]. Five studies reported on component separation, with a component separation technique performed in 52.2% of patients (n = 283) [8, 15, 17,18,19]. The component separation rates ranged from 16.7 to 89.5%. Eight studies reported a mean operative time of 217 min.
Risk of bias assessment
The results of the ROBINS-I and ROB2 showed that most of the included studies were of moderate quality [9, 13,14,15,16,17,18,19]. Common sources of bias for non-RCTs included the selection of participants and missing data. For the RCTs, the common sources of bias were the randomization process and missing outcome data. Supplemental Table 3 presents the results of the quality assessment.
Hernia recurrence
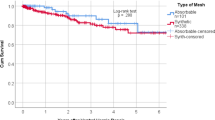
Seven studies with 875 patients compared the incidence of HR for synthetic and biological meshes in head-to-head clinical trials (Fig. 2). Our pooled results showed that biological meshes had significantly higher HR (odds ratio, OR, 2.09; 95% CI 1.42–3.08) than synthetic meshes. There was moderate heterogeneity, with an I2 value of 50% (P = 0.06). The HR rate for biological mesh was 16.7% (95% CI 9.1–28.5%) and that for synthetic mesh was 10.4% (95% CI 6.2–17.0%). Subgroup analyses were performed based on the degree of wound contamination (Supplementary Fig. 1). Biological meshes do not show advantages in decreasing recurrence rates compared to synthetic meshes in CDC 1–2 and CDC 3–4 groups (OR, 1.41; 95% CI 0.41–4.87 vs 3.00; 95% CI 1.07–8.46; P = 0.36).
SSI and SSO
Ten studies with 1356 patients revealed that biological meshes have a significantly higher SSI than synthetic mesh in ventral hernia repair (OR, 1.47; 95% CI 1.10–1.97; Fig. 3a) with low heterogeneity (I2 = 30%, P = 0.17). The pooled SSI rates for biological and synthetic meshes were 21.5% (95% CI 14.76–30.2%) and 14.5% (95% CI 9.1–22.5%), respectively.
Six studies involving 842 patients reported the occurrence of SSO during ventral hernia repair. The SSO of the biological mesh was similar to the synthetic mesh (OR, 1.46; 95% CI:0.8–2.67; Fig. 3b). Moderate heterogeneity was observed (I2 = 64%, P = 0.02). SSO rate for biological mesh was 33.5% (95% CI 15.2–58.6%), and 24.3% (95% CI 15.8–35.6%) for synthetic mesh.
Mesh explantation and length of hospital stay
Six studies with 842 participants reported 23 mesh explantations (Fig. 4a). Compared to synthetic mesh, the mesh explantation of biological mesh did not reach a significant level (OR, 0.52; 95% CI 0.22–1.2) with low heterogeneity (I2 = 0%, P = 0.47). Mesh explantation for biological mesh was 33.5% (95% CI 15.2–58.6%), and 24.3% (95% CI 15.8–35.6%) for synthetic mesh.
Pooled results from ten studies with 1356 patients suggested that compared to synthetic meshed, biologic meshes have a significantly longer length of hospital stay (standardized mean difference, SMD: 0.37; 95% CI 0.10–0.65; Fig. 4b) with high heterogeneity (I2 = 72%, P < 0.01). LOS for biological mesh was 10.04 (95% CI 6.96–13.13) days and 7.27 (95% CI 5.86–8.69) days for synthetic mesh.
Re-admission and re-operation
Seven studies (751 patients mentioned a total of 171 cases of re-admission. Pooled results indicated that biological meshes significantly increase the incidence of re-admission (OR, 1.51; 95% CI 1.05–2.17; Fig. 5a) with acceptable heterogeneity (I2 = 50%, P = 0.06). The pooled incidence of re-admission for biological mesh was 26.4% (95% CI 19.0–35.5%), and 15.6% (95% CI 6.9–31.9%) for synthetic mesh.
Five studies, involving 597 patients, reported 92 unplanned surgeries. Biological meshes tend to increase the chances of re-operation but did not reach the significant level (OR 1.51, 95% CI 0.94–2.41; Fig. 5b) with negligible heterogeneity (I2 = 11%, P = 0.34). The incidence of re-operation for biological mesh was 17.9% (95% CI 11.3–27.4%), and 13.1% (95% CI 5.7–27.5%) for synthetic mesh.
Sensitivity analysis
The results of the sensitivity analysis showed that excluding one study and analyzing the remaining studies, the results of recurrence rate, mesh implantation, re-operation, and length of stay showed good stability. However, the pooled effect sizes of SSI, SSO, and re-admission results were unstable and favorable to synthetic meshes in some conditions (Supplementary Fig. 2).
Discussion
This meta-analysis included ten studies (four RCTs) with 1356 patients, suggesting that biological and synthetic meshes have similar SSO, and mesh explantation. However, biological mesh was associated with significantly higher recurrence rate, SSI, re-admission, and length of hospital stay. In addition, biological meshes have trend to increase the re-operation rate. Data from our subgroup analysis indicated no difference in recurrence rates between synthetic and biological meshes in CDC 1–2 and CDC 3–4 wounds. Additionally, the cost of biological meshes is significantly higher than synthetic meshes [2]. Therefore, a synthetic mesh is more appropriate for abdominal wall hernia repair.
Regardless of the type of prosthesis used, wound complications can occur after hernia repair, particularly in contaminated fields. Bacterial load and colonization can inhibit mesh integration, eventually resulting in unplanned mesh explantation. The morphological characteristics of meshes can affect bacterial adhesion. Sanders performed an in vitro experimental study and found that a polytetrafluoroethylene polymer, multifilament meshes, increased mesh weight, and smaller mean pore size were associated with higher bacterial adherence [20]. Therefore, lightweight meshes with large pores are preferred. Pérez-Köhler et al. reported that polymers loaded with chlorhexidine reduced the risk of infection [21]. Blatnik et al. further showed that monofilament polypropylene and polyester meshes could clear a large percentage of methicillin-resistant S. aureus (MRSA) contaminants [22]. In our analysis, most of the included studies used synthetic meshes with macroporosity and light to medium weight, which may have contributed to the lower SSI.
Previous studies have suggested that using a biological mesh can improve bacterial clearance [23, 24]. In addition, Baumann et al. suggested that biological meshes in the host remodel tissue through neocellularization, neovascularization, and collagen deposition, instead of scar tissue and encapsulation [24]. However, our results showed that the recurrence and SSI rate of biological meshes was significantly higher than that of synthetic meshes. Silva analyzed 14 porcine mesh explants and detected no evidence of xenograft remodeling [25]. After MRSA or Escherichia coli colonization, the biomechanical properties of the biological meshes decreased significantly [26, 27]. Majumder et al. assumed that synthetic meshes serve as a scaffold to provide internal reinforcement rather than as a large continuous laminar flow barrier such as a biological prosthesis [17]. These structural differences may lead to slower integration and greater foreign body reactions in the bioprosthesis [17].
Our study builds on several previously published meta-analyses [11, 12, 28]. Morales-Conde et al. conducted a single-arm meta-analysis of articles reporting the outcomes of biological and synthetic meshes [11]. This study had several limitations. Most of the included studies were not randomized controlled trials, and the pooled results were the percentages of complications derived from different meshes. The quality of the included studies was low and only two were randomized controlled trials. Another meta-analysis conducted by Morris et al. included only six head-to-head clinical trials comparing the efficacy and safety of synthetic and biological meshes, and these included studies had statistically different baselines, such as hernia size, and wound contamination, which may cause significant confounding biases in the pooled results [12].
The advantage of this analysis was that a large number of head-to-head studies were included. Confounding bias was avoided in the included studies. The experimental and control groups in the same study had similar ages, BMI, sex percentages, defect areas, and wound contamination. Our results are more robust than those of previous meta-analyses. Homogeneous pooled data suggest that biological meshes lead to higher SSI.
This study has some limitations. First, only a few types of mesh materials were included. We did not include absorbable synthetic meshes (e.g., meshes made of polyglycolic acid, trimethylene carbonate materials, poly-4-hydroxybutyrate, and so on) and biosynthetic meshes. Most of the biological meshes used in the included studies were derived from porcine dermis, and we did not compare the outcomes of different types of biological meshes. Second, heterogeneity was high for some pooled outcomes such as SSO (I2 = 64), and LOS (I2 = 72%). Third, the included studies used different wound contamination grading systems, and the effectiveness of these systems may differ. We divided studies into “CDC 1–2” and “CDC 3–4” groups based on the degree of contamination of the majority of included patients, which may cause potential bias. Lastly, because the number of included studies for most outcomes was less than 10, we did not calculate the publication bias.
Data availability
Not applicable. No datasets were generated during the production of this work.
References
Schlosser KA, Renshaw SM, Tamer RM, Strassels SA, Poulose BK (2023) Ventral hernia repair: an increasing burden affecting abdominal core health. Hernia 27(2):415–421. https://doi.org/10.1007/s10029-022-02707-6
Nguyen MT, Berger RL, Hicks SC, Davila JA, Li LT, Kao LS, Liang MK (2014) Comparison of outcomes of synthetic mesh vs suture repair of elective primary ventral herniorrhaphy: a systematic review and meta-analysis. JAMA Surg 149(5):415–421
Leber GE, Garb JL, Alexander AI, Reed WP (1998) Long-term complications associated with prosthetic repair of incisional hernias. Arch Surg (Chicago, Ill. : 1960) 133(4):378–382.
Halm JA, de Wall LL, Steyerberg EW, Jeekel J, Lange JF (2007) Intraperitoneal polypropylene mesh hernia repair complicates subsequent abdominal surgery. World J Surg 31(2).
Rosen MJ, Krpata DM, Ermlich B, Blatnik JA (2013) A 5-year clinical experience with single-staged repairs of infected and contaminated abdominal wall defects utilizing biologic mesh. Ann Surg 257(6):991–996
Slater NJ, van der Kolk M, Hendriks T, van Goor H, Bleichrodt RP (2013) Biologic grafts for ventral hernia repair: a systematic review. Am J Surg 205(2):220–230
Hiles M, Record Ritchie RD, Altizer AM (2009) Are biologic grafts effective for hernia repair?: a systematic review of the literature. Surg Innovat 16(1):26–37.
Harris HW, Primus F, Young C, Carter JT, Lin M, Mukhtar RA, Yeh B, Allen IE, Freise C, Kim E, Sbitany H, Young DM, Hansen S (2021) Preventing recurrence in clean and contaminated hernias using biologic versus synthetic mesh in ventral hernia repair: the PRICE randomized clinical trial. Ann Surg 273(4):648–655
Rosen MJ, Krpata DM, Petro CC, Carbonell A, Warren J, Poulose BK, Costanzo A, Tu C, Blatnik J, Prabhu AS (2022) Biologic vs synthetic mesh for single-stage repair of contaminated ventral hernias: a randomized clinical trial. JAMA Surg 157(4):293–301
Miserez M, Lefering R, Famiglietti F, Mathes T, Seidel D, Sauerland S, Korolija D, Heiss M, Weber G, Agresta F, Steup W-H, Śmietański M, Ribeiro R, Cuccurullo D, Catena F, Rudroff C, Rosanelli G, Schön F, Smet B, Wenger F, Saad S, Naver L, Neugebauer E (2021) Synthetic versus biological mesh in laparoscopic and open Ventral Hernia Repair (LAPSIS): results of a multinational, randomized, controlled, and double-blind trial. Ann Surg 273(1):57–65
Morales-Conde S, Hernández-Granados P, Tallón-Aguilar L, Verdaguer-Tremolosa M, López-Cano M (2022) Ventral hernia repair in high-risk patients and contaminated fields using a single mesh: proportional meta-analysis. Hernia J Hernias Abdominal Wall Surg 26(6):1459–1471
Morris MP, Mellia JA, Christopher AN, Basta MN, Patel V, Qiu K, Broach RB, Fischer JP (2021) Ventral hernia repair with synthetic mesh in a contaminated field: a systematic review and meta-analysis. Hernia J Hernias Abdominal Wall Surg 25(4):1035–1050.
Chamieh J, Tan WH, Ramirez R, Nohra E, Apakama C, Symons W (2017) Synthetic versus biologic mesh in single-stage repair of complex abdominal wall defects in a contaminated field. Surg Infect 18(2):112–118
Herrero A, Gonot Gaschard M, Bouyabrine H, Perrey J, Picot MC, Guillon F, Fabre JM, Souche R, Navarro F (2022) Comparative study of biological versus synthetic prostheses in the treatment of ventral hernias classified as grade II/III by the Ventral Hernia Working Group. J Visceral Surg 159(2):98–107.
Koscielny A, Widenmayer S, May T, Kalff J, Lingohr P (2018) Comparison of biological and alloplastic meshes in ventral incisional hernia repair. Langenbecks Arch Surg 403(2):255–263
Liang MK, Berger RL, Nguyen MT, Hicks SC, Li LT, Leong M (2014) Outcomes with porcine acellular dermal matrix versus synthetic mesh and suture in complicated open ventral hernia repair. Surg Infect 15(5):506–512
Majumder A, Winder JS, Wen Y, Pauli EM, Belyansky I, Novitsky YW (2016) Comparative analysis of biologic versus synthetic mesh outcomes in contaminated hernia repairs. Surgery 160(4):828–838
Olavarria OA, Bernardi K, Dhanani NH, Lyons NB, Harvin JA, Millas SG, Ko TC, Kao LS, Liang MK (2021) Synthetic versus biologic mesh for complex open ventral hernia repair: a pilot randomized controlled trial. Surg Infect 22(5):496–503
Shao JM, Ayuso SA, Deerenberg EB, Elhage SA, Prasad T, Colavita PD, Augenstein VA, Heniford BT (2022) Biologic mesh is non-inferior to synthetic mesh in CDC class 1 & 2 open abdominal wall reconstruction. Am J Surg 223(2):375–379
Sanders D, Lambie J, Bond P, Moate R, Steer JA (2013) An in vitro study assessing the effect of mesh morphology and suture fixation on bacterial adherence. Hernia 17(6):779–789. https://doi.org/10.1007/s10029-013-1124-5
Pérez-Köhler B, Fernández-Gutiérrez M, Pascual G, García-Moreno F, San Román J, Bellón JM (2016) In vitro assessment of an antibacterial quaternary ammonium-based polymer loaded with chlorhexidine for the coating of polypropylene prosthetic meshes. Hernia J Hernias Abdominal Wall Surg 20(6):869–878.
Blatnik JA, Krpata DM, Jacobs MR, Gao Y, Novitsky YW, Rosen MJ (2012) In vivo analysis of the morphologic characteristics of synthetic mesh to resist MRSA adherence. J Gastrointest Surg Offic J Soc Surg Aliment Tract 16(11):2139–2144
Katzen M, Ayuso SA, Sacco J, Ku D, Scarola GT, Kercher KW, Colavita PD, Augenstein VA, Heniford BT (2023) Outcomes of biologic versus synthetic mesh in CDC class 3 and 4 open abdominal wall reconstruction. Surg Endosc 37(4):3073–3083
Baumann DP, Butler CE (2012) Bioprosthetic mesh in abdominal wall reconstruction. Semin Plast Surg 26(1):18–24
De Silva GS, Krpata DM, Gao Y, Criss CN, Anderson JM, Soltanian HT, Rosen MJ, Novitsky YW (2014) Lack of identifiable biologic behavior in a series of porcine mesh explants. Surgery 156(1):183–189
Bellows CF, Wheatley BM, Moroz K, Rosales SC, Morici LA (2011) The effect of bacterial infection on the biomechanical properties of biological mesh in a rat model. PLoS ONE 6(6):e21228
Cole WC, Balent EM, Masella PC, Kajiura LN, Matsumoto KW, Pierce LM (2015) An experimental comparison of the effects of bacterial colonization on biologic and synthetic meshes. Hernia J Hernias Abdominal Wall Surg 19(2):197–205.
Atema JJ, de Vries FEEE, Boermeester MA (2016) Systematic review and meta-analysis of the repair of potentially contaminated and contaminated abdominal wall defects. Am J Surg 212(5).
Acknowledgements
There are no acknowledgments.
Funding
This work is supported by the National Natural Science Foundation of China (Number: 81970455, 82170526).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, H., Wang, R., Dong, W. et al. Synthetic Versus Biological Mesh in Ventral Hernia Repair and Abdominal Wall Reconstruction: A Systematic Review and Recommendations from Evidence-Based Medicine. World J Surg 47, 2416–2424 (2023). https://doi.org/10.1007/s00268-023-07067-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-023-07067-5