Abstract
Background
Clinically relevant postoperative pancreatic fistula (POPF) occurs in 15–20% of patients after pancreaticoduodenectomy (PD) and reintervention in the setting of Grade C POPF remains associated with a mortality rate of up to 25%. In patients at high risk of POPF, PD with external wirsungostomy (EW) could be a safe alternative that avoids pancreatico-enteric anastomosis while preserving the remnant pancreas.
Methods
Of the 155 consecutive patients who underwent PD from November 2015 to December 2020, 10 patients were managed using an EW, all with a fistula risk score (FRS) ≥ 7 and BMI ≥30 kg/m2, and/or major associated abdominal surgery. The pancreatic duct was cannulated with a polyethylene tube to allow good external drainage of the pancreatic fluid. We retrospectively analyzed postoperative complications and endocrine and exocrine insufficiencies.
Results
The median alternative FRS was 36.9% [22.1–45.2]. There was no postoperative death. The 90-day overall severe complication (grade ≥3) rate was 30% (n = 3 patients), no patient required reoperation, and 2 hospital readmissions occurred. 3 patients experienced Grade B POPF (30%), managed using image-guided drainage for 2 patients. The external pancreatic drain was removed after a median drainage time of 75 days [63–80]. Two patients presented with late symptoms (> 6 months) warranting interventional management (pancreaticojejunostomy and transgastric drainage). Six patients experienced significant weight loss (> 2 kg) 3 months after surgery. One year after surgery, 4 patients still complained of diarrhea and were treated with transit-delaying drugs. One patient presented new-onset diabetes one year after surgery, and 1 of the 4 patients with preexisting diabetes experienced worsening disease.
Conclusion
EW after PD might be a solution to reduce post-operative mortality following PD in high-risk patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Even though the safety of pancreaticoduodenectomy (PD) has greatly improved over the years, the postoperative mortality rate still ranges from 0 to 5% in high-volume centers [1,2,3,4] and was 6.9% in a French nationwide analysis of 12,333 patients [5].
Among the complications that can lead to death, clinically relevant postoperative pancreatic fistula (CR-POPF) remains the most challenging [6]. In a large series, CR-POPF occurred in around 15–20%, including 5% of Grade C POPF after PD [7, 8]. Although the relaparotomy rate is decreasing with time and increasing access to minimally invasive drainage, the mortality rate remains high, at around 15% after catheter drainage [9] and between 23 and 36% after relaparotomy [7, 9, 10]. In addition, obesity has traditionally been considered a cause of increased surgical complexity and poor outcomes following abdominal surgery and especially PD, with a twofold increase in risk of major complications and POPF [11,12,13,14].
Several approaches have been developed to reduce the incidence and severity of POPF after PD and especially avoidance of performing pancreatico-enteric anastomosis (occlusion of the main pancreatic duct with glue [15], a two-stage procedure [16, 17], total pancreatectomy [18] or external wirsungostomy (EW) [19]).
Funovics et al. [20] first described a technique of pancreatocutaneous drainage after PD in 1987. Two-stage PD was then proposed in 1994 by Miyagawa et al. [21], and the procedure included PD with tube wirsungostomy for complete external drainage of pancreatic juice, followed by late-stage reconstruction. EW alone was then described as a pancreas-preserving salvage procedure for POPF after PD by Denost et al. [19]. Therefore, in patients with a high-risk for POPF, PD with EW could be a safe alternative surgical technique by avoiding pancreatico-enteric anastomosis and externally draining pancreatic juice, while preserving the remnant pancreas.
The objective of this study was to report our experience of PD with external wirsungostomy in patients at high risk of POPF (fistula risk score (FRS) ≥ 7 and BMI ≥ 30 kg/m2, and/or major associated abdominal surgery) and to evaluate the short-term results of this procedure.
Methods
Patient selection
The first EW was performed in 2016 in our department, in a so-called salvage situation during a heavy surgical procedure for a retroperitoneal sarcoma associating PD to, right colectomy, right nephrectomy, right surrenalectomy, resection of the vena cava with prosthetic reconstruction and prosthetic reimplantation of the right renal vein. According to Denost et al. [19], no re-establishment of pancreatic continuity was proposed in first intent, considering that peripancreatic adhesions limit the risk of pancreatic leak. The external drain was removed at postoperative day (POD) 67. The patient presented an isolated uncomplicated acute pancreatitis at POD 86, without any other late complication (pseudocyst, recurrence of acute pancreatitis). Following this experience, this approach was proposed as an option for patients at high risk of POPF, and especially obese.
From November 2015 to December 2020, 155 consecutive patients underwent PD in our department, including 10 with EW.
The study complied with French National Health guidelines on research involving human subjects. The current study was performed with the approval of the institutional ethics committee review board (Study number E2021-48). Informed consent was obtained from all individual participants included in this study.
External-wirsungostomy indication
The fistula risk score (FRS)[6] and the alternative fistula risk score (a-FRS)[22] were calculated online (https://www.pancreasclub.com/calculators/fistula-risk-score-calculator/). The two scores were validated to accurately predict the risk of developing CR-POPF. Patients were considered as candidates for EW in the setting of a FRS ≥ 7 for patients with BMI ≥ 30 kg/m2 and/or associated high risk surgery. We believed that in such an extended procedure (cytoreductive surgery with HIPEC in particular) the occurrence of a POPF would have been catastrophic.
Surgical procedure
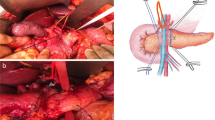
The proximal third of the remaining pancreatic duct was cannulated with a polyethylene tube (Escat drain®, Coloplast-Holtedam 1-3-DK-3050 Humlebaek-Denmark), without passing the tube through the jejunal loop (the cut plane of the pancreas was fixed to the jejunal) [17]. The tube was pushed delicately to a depth of 5–6 cm. The size of the catheter was chosen according to the diameter of the main pancreatic duct. The catheter was then sewn to the pancreas with a PDS®4/0 suture with two interrupted U-shaped sutures, and the free end was passed through the abdominal wall and sewn to the skin. Two silicone drains were placed. An omental flap was fashioned and interposed between the pancreatic stump and the celiac and mesenteric vessels that had been dissected during PD. The pancreatic drain was cut after a course of 10 cm from the skin and left open in a draining pouch until its removal on weeks 8–10.
All patients received single-shot intravenous antibiotic prophylaxis upon induction of anesthesia. Continuous injection of somatostatin Eumedica® 6 mg/24 h (Chemin de la Nauwelette 1, 7170 Manage, Belgium) was initiated during the operation once resection had been decided and was continued for 7 days and then relayed by an intramuscular injection of somatostatin LAR® 30 mg (NovartisPharmaSAS).
Follow up
Follow-up was performed every 3 months according to the French guidelines. The presence of endocrine insufficiency was evaluated by measuring venous glucose, with postprandial glycemic sticks and by glycated hemoglobin levels. De novo diabetes was defined as diabetes after surgery (with the need for insulin therapy) in previous nondiabetic patients. Worsened diabetes was defined as a patient with overt diabetes at the time of surgery who then needed increased treatment after surgery. Exocrine pancreatic insufficiency was not monitored because it was assumed that it would be present in all patients. All patients received daily pancreatic enzyme therapy. However, the frequency of daily stool was monitored, and diarrhea was defined by having loose stools three or more times a day.
Results
The characteristics of the 10 patients are summarized in Table 1. The median age was 65 years (39–76), and 1 patient had overt diabetes at the time of surgery. Median operative time was 360 min (240–600). All the patients presented an FRS over 7 and the median a-FRS was 36.9 (22.1–45.2). Our first patient (patient 1) underwent right compartment surgery for retroperitoneal sarcoma with right colectomy, right nephrectomy, pancreaticoduodenectomy, and prosthetic replacement of the inferior vena cava. Four other patients had associated visceral and/or vascular resection (Table 1).
There were no postoperative deaths, but all patients experienced at least one complication (Table 2). The 90-day overall severe complication (grade ≥ 3) rate was 30% (n = 3 patients), with 3 percutaneous imaging guided drainages (PIGDs) for intraabdominal collection (patient 1 and 7) or chemical peritonitis after accidental displacement of the pancreatic drain (patient 4). 1 of these 3 patients was admitted to the ICU for septic shock secondary to central line infection (patient 7). No patient required reoperation or completion of a pancreatectomy. Ninety-day hospital readmission occurred for 2 patients (patient 1 and 10, respectively, for acute pancreatitis and retrogastric collection).
Regarding specific complications, 3 patients (30%) experienced clinically relevant POPF (Grade B). Abdominal peripancreatic drain might have been left in place for extended periods of 23, 25 and 32 days. Two patients presented (at POD 93 and 102, respectively), a peripancreatic collection following pancreatic drain removal, managed using image-guided drainage (patient 7 and 10). 4 patients suffered from delayed gastric emptying, including 2 Grade B patients. One patient had recurring late acute pancreatitis on the pancreatic stump.
The pancreatic drain was removed after a median drainage time of 75 days (63–80).
Two patients suffered from self-limited acute abdominal pain immediately after drain removal.
One patient required a second-stage reoperation with the construction of a pancreaticojejunostomy one year after PD to manage recurrent episodes of Balthazar A pancreatitis (patient 3). Another patient developed tenderness of the upper part of the abdomen due to a pseudocyst from the pancreas section managed by EUS-guided transgastric drainage (patient 7), 6 months after PD.
Long-term results are summarized in Table 2. Six patients had significant weight loss with a median weight loss of 4 kg (2–12) but with normal albumin levels at 6 months. Four of them regained their preoperative weight within 14 months. All patients received daily pancreatic enzyme therapy. One year after surgery, four of them still complained of diarrhea and were treated with transit-delaying drugs (loperamide).
Concerning endocrine insufficiency, one patient presented with new onset diabetes mellitus one year after surgery, and 1 of the 4 patients with preexisting diabetes at the time of surgery experienced worsening of their diabetes.
Discussion
The choice to perform an EW may have been an effective strategy, in patients at high risk of POPF with an FRS > 7, presenting morbid obesity. The predicted clinically relevant fistula rate expected with the a-FRS calculator in the current cohort was 36.9% (22.1–45.2), while we observed no Grade C fistula neither post-operative mortality.
Pancreatic anastomosis is the Achilles heel of PD, and the management of the pancreatic stump is still the primary issue due to the frequency of its related complications. The major concern is that POPF following PD is not a pancreatic fistula, such as those that can be seen in complicated chronic pancreatitis with ascites and pleural effusions or after left-sided pancreatectomies. POPF following PD corresponds to a pancreatico-enteric fistula, and this point clearly makes the difference. Pancreatic juice contains the proenzymes trypsinogen, chymotrypsinogen, procarboxypeptidases, and proelastase, all of which are activated by trypsin in the intestinal lumen when the pancreatic juice enters the digestive fluid or bile [23,24,25,26]. The pancreaticoenteric anastomosis is not watertight in many patients, and a leak of activated pancreatic enzymes through this anastomosis may increase leakage with possible autodigestion of the anastomosis itself and also of the peripancreatic tissue, celiac, and mesenteric vessels that have been dissected during PD and the stump of gastroduodenal artery that have been ligated.
The purpose of EW was to prevent activation of the pancreatic juice by avoiding contact with the enteric contents. This is possible by external drainage of the main pancreatic duct and by practices aimed at reducing the amount of pancreatic fluid production with the use of somatostatin therapy. A key point is also to separate the pancreatic stump from the other anastomoses and the tissues at risk (arteries and veins) using an omental flap. Indeed, a concomitant biliary fistula could activate the pancreatic juice and cause harmful autodigestion.
The use of a EW has been described in 2-stage PD in patients at high risk of fistula and as a salvage procedure after PD. One of the limitations in the setting of 2-stage PD was the performance of a complex re-laparotomy and removal of adhesions. Indeed, firm tissue adhesions are expected in the setting of fistulization around drainage tubes in abdominal surgery, and the adhesions are typically severe and lead to anatomic disorientation during the reoperations. Following salvage EW, Denost et al. [19] demonstrated the feasibility of removing the pancreatic drain without the need for subsequent re-establishment of pancreatic continuity, nor percutaneous/endoscopic drainage. Only one patient was reoperated for pancreaticojejunal anastomosis in our cohort (patient 3). In case of persistent output of the EW after 3 months, different proposals could be made, to avoid recourse to a re-intervention (2 patients in the current cohort), such as “wait and see” and no removal of the EW, gluing of the drain path, or possibly radiotherapy [27, 28].
Several surgical techniques have been compared and evaluated to prevent POPF, but in the setting of high risk of POPF, the two accepted approach are total pancreatectomy and PD with externalized stent.
Total pancreatectomy (TP) could be performed either as a treatment for a surgical complication or as a prevention of anastomotic leakage. Capretti et al. [29] reported a comparative study in 62 patients with a fistula risk score above or equal to 7; 35 patients were managed with PD and PJ, and 27 were managed with TP. The overall complication rate was significantly higher in the PD group, with a nonsignificantly higher rate of major complications; in the PD group, 49% of the patients developed clinically relevant POPF. TP could be an option to prevent POPF in well-selected patients, as confirmed recently by Marchegiani et al. [30] However, major concerns after TP remain the management of the apancreatic state with its attendant total endocrine and exocrine insufficiency, pending clinical trials evaluating islet autotransplantation for malignant disease (PAN-IT trial NCT01346098; TPIAT1 trial NCT05116072). Currently, islet autotransplantation should be combined with TP only for benign disease to prevent severe diabetes [18, 31]. In Verona’s experience, although general QoL was comparable following TP and PD in high-risk patients, TP patients had worse diabetes-specific QoL [30]. Improvements in postoperative management including advances in insulin formulations, and the use of glucagon rescue therapy, allow much tighter control of blood glucose than previously possible; this markedly lessens the risk of life-threatening hypoglycemia and decreases the risk of long-term complications.
The placement of a stent through the pancreatic anastomosis is also an attractive strategy to reduce the POPF rate by diverting the pancreatic enzymes from the pancreatic anastomotic area. Following others [32], Pessaux et al. [33] reported that external stenting decreased the POPF rate from 42 to 20% in high risk situations. Guo et al. [34] confirmed in a meta-analysis of randomized controlled trials that placing an external stent across pancreaticojejunal anastomosis could significantly reduce the incidence of POPF (RR = 0.61, 95% CI 0.43–0.86, p = 0.005). Ecker et al. [35] regarding optimal management of these very high-risk cases, indicate that an approach of pancreatojejunostomy with externalized stent placement and without octreotide can reduce high-risk cases from 30 to 40% CR-POPF rates down to the more acceptable 13–15% range. Then, according to recent literature, in the setting of high-risk situation for POPF, pancreaticojejunal anastomosis with externalized stent seems to be the most consensual approach [35, 36]. In obese patients, even in high volume centers, mortality rate following PD was significantly high, as reported by Di Gioia et al. (6% vs. 3.1% in non-obese patients, p < 0.05). In the subgroup of patients with nonmalignant tumors, obesity was described as the only independent predictor of failure to rescue, highlighting the particularity of post-operative management of obese patients and finding alternative options such as EW or perhaps in the near future preventive pancreatic irradiation to decrease pancreatic secretion (NCT01656486).
Total pancreatectomy and external drainage reduce the rate of severe complications and POPF, and EW possibly combines the advantages of these two techniques by avoiding the creation of a pancreatico-enteric anastomosis and by draining the pancreatic fluid away from the operative site. Although this is the first cohort reported on the subject, to our knowledge, several limitations must be declared. One of the main limitations of the study is its small size, which reduces the reliability of the results. Regarding the low number of included patients, a comparative study could not be done during the same period of inclusion. The comparison to a historical cohort did not seem appropriate to us because of the important changes in practice since 2016 with the implementation of the ERAS recommendations in our center and the systematic realization of a standardized physical, respiratory, and nutritional preparation.
Conclusions
External wirsungostomy might be a option following PD to limit POPF and associated mortality, especially in obese patient; however, evaluation must be continued before it can be considered an alternative in patients at high risk for POPF, especially to reduce post-operative mortality.
References
Birkmeyer JD, Sun Y, Wong SL, Stukel TA (2007) Hospital volume and late survival after cancer surgery. Ann Surg 245:777–783. https://doi.org/10.1097/01.sla.0000252402.33814.dd
Winter J, Cameron J, Campbell K et al (2006) 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience☆. J Gastrointest Surg 10:1199–1211. https://doi.org/10.1016/j.gassur.2006.08.018
Smits FJ, Henry AC, Besselink MG et al (2022) Algorithm-based care versus usual care for the early recognition and management of complications after pancreatic resection in the Netherlands: an open-label, nationwide, stepped-wedge cluster-randomised trial. Lancet 399:1867–1875. https://doi.org/10.1016/S0140-6736(22)00182-9
Kulshrestha S, Sweigert PJ, Tonelli C et al (2022) Textbook oncologic outcome in pancreaticoduodenectomy: do regionalization efforts make sense? J Surg Oncol 125:414–424. https://doi.org/10.1002/jso.26712
El Amrani M, Clement G, Lenne X et al (2018) Failure-to-rescue in patients undergoing pancreatectomy: is hospital volume a standard for quality improvement programs? Nationwide analysis of 12,333 patients. Ann Surg 268:799–807. https://doi.org/10.1097/SLA.0000000000002945
Bassi C, Marchegiani G, Dervenis C et al (2017) The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery 161:584–591. https://doi.org/10.1016/j.surg.2016.11.014
Stoop TF, Fröberg K, Sparrelid E et al (2022) Surgical management of severe pancreatic fistula after pancreatoduodenectomy: a comparison of early versus late rescue pancreatectomy. Langenbecks Arch Surg 407:3467–3478. https://doi.org/10.1007/s00423-022-02708-0
Groen JV, Smits FJ, Koole D et al (2021) Completion pancreatectomy or a pancreas-preserving procedure during relaparotomy for pancreatic fistula after pancreatoduodenectomy: a multicentre cohort study and meta-analysis. Br J Surg 108:1371–1379. https://doi.org/10.1093/bjs/znab273
Smits FJ, van Santvoort HC, Besselink MG et al (2017) Management of severe pancreatic fistula after pancreatoduodenectomy. JAMA Surg 152:540. https://doi.org/10.1001/jamasurg.2016.5708
Ma T, Bai X, Chen W et al (2019) Surgical management and outcome of grade-C pancreatic fistulas after pancreaticoduodenectomy: a retrospective multicenter cohort study. Int J Surg 68:27–34. https://doi.org/10.1016/j.ijsu.2019.05.019
Di Gioia A, Giuliani T, Marchegiani G et al (2022) Pancreatoduodenectomy in obese patients: surgery for nonmalignant tumors might be deferred. HPB (Oxford) 24:885–892. https://doi.org/10.1016/j.hpb.2021.10.018
Yamane H, Abe T, Amano H et al (2018) Visceral adipose tissue and skeletal muscle index distribution predicts severe pancreatic fistula development after pancreaticoduodenectomy. Anticancer Res 38:1061–1066. https://doi.org/10.21873/anticanres.12323
Shamali A, Shelat V, Jaber B et al (2017) Impact of obesity on short and long term results following a pancreatico-duodenectomy. Int J Surg 42:191–196. https://doi.org/10.1016/j.ijsu.2017.04.058
Tang T, Tan Y, Xiao B et al (2021) Influence of body mass index on perioperative outcomes following pancreaticoduodenectomy. J Laparoendosc Adv Surg Tech A 31:999–1005. https://doi.org/10.1089/lap.2020.0703
Gong J, He S, Cheng Y et al (2018) Fibrin sealants for the prevention of postoperative pancreatic fistula following pancreatic surgery. Cochrane Database Syst Rev 6:009621. https://doi.org/10.1002/14651858.CD009621.pub3
Tuech JJ, Pessaux P, Regenet N et al (2001) Emergency pancreaticoduodenectomy with delayed reconstruction for bleeding: a life saving procedure. Int J Pancreatol 29:59–62. https://doi.org/10.1385/IJGC:29:1:59
Hasegawa K, Kokudo N, Sano K et al (2008) Two-stage pancreatojejunostomy in pancreaticoduodenectomy: a retrospective analysis of short-term results. Am J Surg 196:3–10. https://doi.org/10.1016/j.amjsurg.2007.05.050
Shahbazov R, Naziruddin B, Salam O et al (2020) The impact of surgical complications on the outcome of total pancreatectomy with islet autotransplantation. Am J Surg 219:99–105. https://doi.org/10.1016/j.amjsurg.2019.04.007
Denost Q, Pontallier A, Rault A et al (2012) Wirsungostomy as a salvage procedure after pancreaticoduodenectomy. HPB (Oxford) 14:82–86. https://doi.org/10.1111/j.1477-2574.2011.00406.x
Funovics JM, Zöch G, Wenzl E, Schulz F (1987) Progress in reconstruction after resection of the head of the pancreas. Surg Gynecol Obstet 164:545–548
Miyagawa S, Makuuchi M, Kawasaki S, Ogiwara M (1994) Second-stage pancreatojejunostomy following pancreatoduodenectomy in high-risk patients. Am J Surg 168:66–68. https://doi.org/10.1016/s0002-9610(05)80073-x
Mungroop TH, van Rijssen LB, van Klaveren D et al (2019) Alternative fistula risk score for pancreatoduodenectomy (a-FRS): design and international external validation. Ann Surg 269:937–943. https://doi.org/10.1097/SLA.0000000000002620
Wüster C, Shi H, Kühlbrey CM et al (2020) Pancreatic inflammation and proenzyme activation are associated with clinically relevant postoperative pancreatic fistulas after pancreas resection. Ann Surg 272:863–870. https://doi.org/10.1097/SLA.0000000000004257
Green GM, Nasset ES (1980) Importance of bile in regulation of intraluminal proteolytic enzyme activities in the rat. Gastroenterology 79:695–702
Hadorn B, Hess J, Troesch V et al (1974) Role of bile acids in the activation of trypsinogen by enterokinase: disturbance of trypsinogen activation in patients with intrahepatic biliary atresia. Gastroenterology 66:548–555. https://doi.org/10.1016/S0016-5085(74)80043-0
Seth TN (1924) The activation of pancreatic juice by enterokinase. Biochem J 18:1401–1416. https://doi.org/10.1042/bj0181401
Pieroni PL, Rudick J, Adler M et al (1976) Effect of irradiation on the canine exocrine pancreas. Ann Surg 184:610–614. https://doi.org/10.1097/00000658-197611000-00013
Gemici C, Yaprak G, Ozdemir S et al (2018) Volumetric decrease of pancreas after abdominal irradiation, it is time to consider pancreas as an organ at risk for radiotherapy planning. Radiat Oncol 13:238. https://doi.org/10.1186/s13014-018-1189-5
Capretti G, Donisi G, Gavazzi F et al (2021) Total pancreatectomy as alternative to pancreatico-jejunal anastomosis in patients with high fistula risk score: the choice of the fearful or of the wise? Langenbecks Arch Surg 406:713–719. https://doi.org/10.1007/s00423-021-02157-1
Marchegiani G, Perri G, Burelli A et al (2021) High-risk pancreatic anastomosis vs. total pancreatectomy after pancreatoduodenectomy: postoperative outcomes and quality of life analysis. Ann Surg. https://doi.org/10.1097/SLA.0000000000004840
Balzano G, Maffi P, Nano R et al (2016) Autologous islet transplantation in patients requiring pancreatectomy: a broader spectrum of indications beyond chronic pancreatitis. Am J Transplant 16:1812–1826. https://doi.org/10.1111/ajt.13656
Poon RTP, Fan ST, Lo CM et al (2007) External drainage of pancreatic duct with a stent to reduce leakage rate of pancreaticojejunostomy after pancreaticoduodenectomy: a prospective randomized trial. Ann Surg 246:425–433. https://doi.org/10.1097/SLA.0b013e3181492c28. (Discussion 433–435)
Pessaux P, Sauvanet A, Mariette C et al (2011) External pancreatic duct stent decreases pancreatic fistula rate after pancreaticoduodenectomy: prospective multicenter randomized trial. Ann Surg 253:879–885. https://doi.org/10.1097/SLA.0b013e31821219af
Guo C, Xie B, Guo D (2022) Does pancreatic duct stent placement lead to decreased postoperative pancreatic fistula rates after pancreaticoduodenectomy? A meta-analysis. Int J Surg 103:106707. https://doi.org/10.1016/j.ijsu.2022.106707
Ecker BL, McMillan MT, Asbun HJ et al (2018) Characterization and optimal management of high-risk pancreatic anastomoses during pancreatoduodenectomy. Ann Surg 267:608–616. https://doi.org/10.1097/SLA.0000000000002327
Andrianello S, Marchegiani G, Malleo G et al (2020) Pancreaticojejunostomy With externalized stent vs pancreaticogastrostomy with externalized stent for patients with high-risk pancreatic anastomosis: a single-center, phase 3, randomized clinical trial. JAMA Surg 155:313–321. https://doi.org/10.1001/jamasurg.2019.6035
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Codjia, T., Roussel, E., Papet, E. et al. Can the Realization of an External Wirsungostomy be an Option for High-Risk Pancreatic Anastomosis After Pancreaticoduodenectomy?. World J Surg 47, 1533–1539 (2023). https://doi.org/10.1007/s00268-023-06927-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-023-06927-4