Abstract
Background
Acute acalculous cholecystitis (AAC) is often diagnosed in critically ill patients. Percutaneous cholecystostomy tube (PCT) placement facilitates less invasive gallbladder decompression in patients who are poor surgical candidates. Specific guidelines for optimal management of AAC patients following PCT placement remain to be defined. We hypothesize that AAC patients are at lower risk of recurrent cholecystitis than acute calculous cholecystitis (ACC) patients and do not require cholecystectomy after PCT placement.
Methods
A retrospective review of patients who underwent PCT placement for AAC or ACC between 6/1/2007 and 5/31/2019 was performed. Primary outcome was recurrent cholecystitis and interval cholecystectomy for patients surviving 30 days after PCT placement. Secondary outcome was 30 day mortality. A cox regression model calculated the adjusted hazard ratio (AHR) for the outcomes.
Results
Eighty-four AAC and 85 ACC patients underwent PCT placement. Compared to ACC patients, more AAC patients were male (72.6 vs. 48.2%; p < 0.01), younger (median age 62 vs. 73 years; p < 0.01), and required intensive care (69.0 vs. 52.9%; p = 0.04), with lower median Charlson Comorbidity Index (4.0 vs. 6.0; p < 0.01). 30 day mortality was higher among AAC patients than ACC patients (45.2 vs. 21.2%; p < 0.01). 2/24 (8.3%) AAC patients and 5/31 (16.1%) ACC patients developed recurrent cholecystitis at a median 208.0 days (IQR:64.0–417.0) after PCT placement and 115.0 days (IQR:7.0–403.0) following PCT removal. Cox regression analysis demonstrated that AAC patients had lower likelihood of interval cholecystectomy compared to ACC patients (AHR 2.35; 95% CI:1.11,4.96).
Conclusion
Recurrent cholecystitis is rare in patients surviving 30 days following PCT placement. When compared with ACC patients, fewer AAC patients require cholecystectomy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute acalculous cholecystitis (AAC) is an inflammatory disease of the gallbladder in the absence of cholelithiasis, with a complex pathogenesis involving gallbladder hypoperfusion leading to ischemia and subsequent reperfusion injury [1,2,3,4]. Accounting for 10% of all cases of cholecystitis, AAC is associated with high morbidity and mortality rates up to 30% [3, 5]. Percutaneous cholecystostomy tube (PCT) placement is a less invasive method of gallbladder decompression in critically ill patients who are poor candidates for cholecystectomy due to concurrent medical conditions and clinical status [6]. PCT placement as a definitive treatment of AAC versus a temporizing measure until an interval cholecystectomy is performed remains a topic of debate [7]. Often, findings and experience with management of ACC are extrapolated to AAC and vice versa. However, how these two entities differ in regards to certain outcomes, including an interval cholecystectomy, is poorly studied.
The purpose of this study was to evaluate the clinical characteristics of patients with AAC requiring PCT placement and compare their outcomes to patients undergoing PCT placement for ACC. We hypothesized that patients with AAC undergoing PCT placement are at lower risk of recurrent cholecystitis than ACC patients and that routine interval cholecystectomy is not necessary after PCT placement.
Materials and methods
Patients admitted to a single, quaternary care, medical center from 6/1/2007 to 5/31/2019 who required PCT placement for AAC or ACC were identified. Patients with repeat PCTs, gallbladder perforation, prior biliary stents, or PCT placement for underlying malignancy or masses were excluded. Due to the low sensitivity of gallstone detection with computed tomography (CT) imaging, patients with CT as the sole diagnostic imaging were also excluded [8, 9]. This study was approved by the Institutional Review Board; the need for consent was waived given its retrospective nature and the minimal risk its conduction posed to participants. Data were analyzed using the IBM SPSS statistics for windows, version 25.0 (IBM Corp., Amonk, NY).
Baseline demographics and clinical characteristics included age, sex, location [ward vs. intensive care unit (ICU)] and laboratory values prior to PCT placement [white blood cell count (WBC), liver function tests, and lactic acid]. Results of relevant imaging studies [ultrasound (US), magnetic resonance cholangiopancreatography (MRCP) and hepatobiliary iminodiacetic acid (HIDA) scan] prior to PCT placement were recorded. The Charlson Comorbidity Index (CCI) was calculated for each patient [10], as was the risk for mortality and major complications based on the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) surgical risk calculator for mortality and complications (defined as cardiac arrest, myocardial infarction, pneumonia, progressive renal insufficiency, acute renal failure, pulmonary embolism, deep venous thrombosis, return to the operating room, deep incisional surgical site infection, organ space surgical site infection, systemic sepsis, unplanned intubation, urinary tract infection, or wound disruption) for a laparoscopic cholecystectomy at the time of PCT placement [11].
PCT placements were performed by board-certified interventional radiologists under US or CT guidance, as previously described [12]. Timing of PCT insertion and removal was documented, as was the timing of interval cholecystectomy, if it occurred. Patients were followed until death, interval cholecystectomy, or last documented follow-up in the electronic medical record.
Patients without gallstones seen on US or MRCP prior to PCT placement were considered to have AAC, while those with gallstones were considered to have ACC. AAC patients were compared to their ACC counterparts. The primary outcomes were interval cholecystectomy and recurrent cholecystitis in patients who survived beyond 30 days following PCT placement. Secondary outcome was 30 day mortality. To minimize survival bias, the secondary outcome was analyzed after excluding patients who died within 30 days from PCT placement.
Student’s t test or Mann–Whitney U test was utilized to compare means between patients with acalculous and calculous cholecystitis. Fisher’s exact test and chi-squared test were used to compare proportions. A Kaplan–Meier curve was created to depict the primary and secondary outcomes in relation to time, and a log rank test was calculated. A cox regression model incorporating variables that were statistically different between the two cohorts at a p < 0.20 level was utilized to calculate the adjusted hazard ratio (AHR) and 95% confidence interval (CI) for 30 day mortality, interval cholecystectomy and recurrent cholecystitis. All reported p values were two-sided with p < 0.05 considered statistically significant.
Results
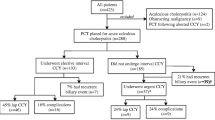
We identified 223 patients admitted over a 13 year period who underwent PCT placement. Of these, 54 patients were excluded due to PCTs placed for underlying mass or malignancy, presence of prior biliary stents, or diagnosis of cholecystitis based solely on CT (Fig. 1). Of the 169 patients included in our analysis, 102 (60.4%) patients were male with a median age of 66.0 years (Table 1). The median CCI score was 5.0 and the median NSQIP risk of serious complications, any complications, or mortality were predicted to be 8.0, 9.9 and 3.5%, respectively. At the time of PCT placement, 103/169 (60.9%) patients were in the ICU. A total of 56/169 (33.1%) patients required vasopressor support and 60/169 (35.5%) patients were on mechanical ventilation.
The cohort was almost equally divided into those with AAC (49.7%, n = 84/169) and those with ACC (50.3%, n = 85/169) (Fig. 1). AAC patients were significantly more likely to be male (72.6 vs. 48.2%, p < 0.01) and younger than their ACC counterparts (median age 62.0 vs. 73.0 years, p < 0.01). Although AAC patients had lower median CCI scores (4.0 vs. 6.0, p < 0.01), the NSQIP-predicted risks of complications and mortality were similar between both groups (Table 1). Compared to ACC patients, AAC patients at the time of PCT placement more likely to be located in the ICU (69.0 vs. 52.9%, p = 0.04) and require mechanical ventilation (44.0 vs. 27.1%, p = 0.03) but had no significant difference in vasopressor requirement (38.1 vs. 28.2%, p = 0.19), or liver function tests (Table 1). The median duration of indwelling PCT was 54.5 days and similar for the compared cohorts (median 62.5 days for AAC and 52.0 days for ACC, p = 0.20) (Table 2).
The majority of gallbladder fluid cultures sampled at the time of PCT placement had no growth (58.4%, n = 94). The remainder cultures were mostly polymicrobial (16.8%, n = 27), while the most common isolates were Enterococcus (12.4%, n = 20), Escherichia coli (12.4%, n = 20), and Klebsiella (11.8%, n = 19) (Table 3). There were no differences in microbiology between AAC and ACC patients. The most common complication associated with PCT was tube dislodgment (10.7%, n = 18/169), followed by tube clotting (4.7%, n = 8/169), and leakage around the insertion site (2.4%, n = 4/169).
The overall 30 day mortality was 33.1% (n = 56) and significantly higher for AAC patients (45.2 vs. 21.2%, p < 0.01) (Table 2). Figure 2 depicts the Kaplan–Meier curve for 30 day mortality for the two cohorts, indicating significantly higher mortality for AAC patients (log rank p < 0.01.) A cox regression model, however, adjusting for sex, age, location at the time of PCT placement (ICU vs. ward), CCI, RUQ pain, vasopressor status, and intubation status indicated that although ACC patients tended to have a lower AHR for 30 day mortality, this difference did not reach statistical significance (AHR: 0.60; 95% CI: 0.32, 1.13; adjusted p = 0.12). The area under the curve (AUC) for the model was 0.775.
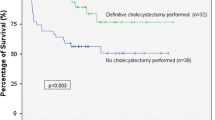
Excluding patients who died within 30 days after PCT placement (n = 56), we identified 113 patients for additional analysis: 46 AAC (40.7%) and 67 ACC (59.3%) patients. Of the patients who survived 30 days beyond PCT placement, AAC patients compared to ACC patients had similar ICU status (60.9 vs. 47.8%, p = 0.19), vasopressor requirement (26.1 vs. 20.9%, p = 0.65), and need for mechanical ventilation (37.0 vs. 19.4%, p = 0.05) (Table 4). The median duration of PCT was similar between AAC and ACC patients (63.0 vs. 52.0 days, p = 0.18) (Table 5). Cholangiogram was performed prior to PCT removal in 46.1% (n = 41) patients overall (AAC 45.7%, ACC 46.3%). Rate of interval cholecystectomy was also similar (AAC: 30.4% vs. ACC: 46.3%, p = 0.12). Overall median follow-up was 57.0 days (IQR: 31.5, 108.0) (Table 5). Figure 3 depicts the Kaplan–Meier curve for interval cholecystectomy for AAC and ACC patients (log rank p = 0.05). The AHR for interval cholecystectomy was significantly higher for ACC patients based on a cox regression model adjusting for sex, age, CCI, location (ICU vs. ward), RUQ pain, and intubation status (AHR: 2.35; 95% CI: 1.11, 4.96; adjusted p = 0.03). The AUC for the model was 0.676. Additionally, the proportion of patients who underwent interval cholecystectomy was similar between PCT patients from the ward (55.5%) and PCT patients from the ICU (44.7%) (p = 0.352).
Recurrent cholecystitis occurred in five (16.1%) ACC and two (8.3%) AAC patients who survived beyond 30 days after PCT placement and had PCT removed without concurrent cholecystectomy (Fig. 4) at a median follow-up of 208.0 days (IQR 64.0, 417.0 days) after PCT placement and a median of 115.0 days (IQR 7.0, 403.0 days) after PCT removal. Of the ACC patients with recurrent cholecystitis, three (60.0%) underwent interval cholecystectomy (1 open, 2 laparoscopic). No ACC patients underwent conversion from laparoscopic to open cholecystectomy. Of the AAC patients with recurrent cholecystitis, both patients underwent cholecystectomy (1 laparoscopic, 1 laparoscopic converted to open).
Discussion
In this large case series from a quaternary referral center, we found that approximately half of patients requiring PCT placement for AAC died within 30 days. The majority of microbiological cultures obtained had no growth. The duration of PCT approximated 2 months. The majority of these patients did not undergo an interval cholecystectomy after approximately 2 months and of those who survived beyond 30 days after PCT placement, only a small fraction experienced recurrent cholecystitis during the follow-up period. These findings may be utilized to question the utility of interval cholecystectomy following PCT placement for AAC patients, especially in the early period of recovery from their disease, given their very high mortality risk.
PCTs are often utilized in patients who are at high risk of morbidity or mortality from cholecystectomy, such as patients with AAC who are often located in the ICU requiring vasopressors and mechanical ventilation and have concurrent systemic diseases [13]. Our study cohort had a high rate of mortality overall and at 30 days following PCT placement, reflecting a more critically ill study population. One of the reasons for this may be based on institutional practice. For example, more aggressive practice in performing cholecystectomy and reserving PCTs for only those who are critically ill or at an exceptionally high risk of surgery may account for the high mortality rate in our patient cohort. Furthermore, this could support and explain the fact that we do not routinely perform interval cholecystectomies on patients who receive PCTs.
Despite the common use of PCTs for AAC, associated outcomes remain to be defined [7]. Anderson et al. observed greater mortality risk in AAC patients undergoing PCT placement compared to emergent cholecystectomy [14, 15], while other groups such as Simorov et al. demonstrated no difference in mortality between AAC and ACC patients with PCTs, although placement for AAC was associated with lower morbidity overall [16]. Similarly, Kirkegard et al. suggested PCT insertion as a definitive therapy for AAC as it was found to be associated with low mortality risk and low interval cholecystectomy rate [17]. Furthermore, in a large single-center study of > 400 patients by Boules et al. most patients treated with PCT placement and interval cholecystectomy were younger and had lower comorbidity indices, leading to the conclusion that older, high-risk patients should be considered for PCT placement as definitive management of cholecystitis [18].
Although AAC patients had lower median CCI scores and similar predicted surgical risks, they were more likely than ACC patients to be located in the ICU on mechanical ventilation, with significantly greater odds of 30 day mortality. After regression analysis, we no longer observed a statistically significant difference in 30 day mortality between AAC and ACC patients, similar to studies such as by Bhatt et al. [19]. The discordance between mortality rate and low median CCI scores in our study’s AAC patients may be related to the fact that patients with malignancy were excluded, while malignancy is a variable that contributes to a higher CCI [10]. Additionally, several studies have suggested that CCI is a poor predictor of mortality and may not accurately represent patient risk, as well as other risk-adjustment scores such as NSQIP [20, 21].
In further investigating patients who survived beyond 30 days, we found that less AAC patients underwent interval cholecystectomy than ACC patients. The rate of recurrent cholecystitis after PCT removal was low in both AAC and ACC patients, consistent with existing literature [2, 19, 22]. Cholangiogram to assess duct patency prior to PCT removal is a practice that varies between providers and institutions, and current studies suggest that routine cholangiography may not be necessary [23, 24]. Loftus et al. in a retrospective cohort study found that although routine surveillance cholangiography identified cystic duct filling defects in more patients than those who underwent cholangiography only if symptomatic, there was no significant difference in clinical outcomes including rates of recurrent cholecystitis or cholangitis [23]. Similarly, Hung et al. did not identify significant differences in clinical outcomes for patients with PCTs who underwent cholangiogram prior to PCT removal compared to patients who underwent PCT clamp trials, suggesting that routine cholangiography does not provide clinically significant benefits [24]. We did not investigate results of cholangiograms prior to PCT removal and thus cannot comment on duct patency and its potential effects on the rate of recurrent cholecystitis in our patients.
Our observation that AAC patients have lower likelihood of interval cholecystectomy after PCT placement are consistent with existing literature such as from Colonna et al. [25] and Chung et al. [2], suggesting PCT as a feasible definitive AAC treatment. Similarly, a retrospective analysis by Byrne et al. suggested that PCT could be a safe alternative to surgery for high-risk patients with either ACC or AAC [26]. The findings of our study suggest that recommending an interval cholecystectomy routinely to these patients may not be justified. Watchful waiting may in fact be a safer alternative, given the very small risk for recurrent symptoms.
The limitations of our study include its retrospective nature, small sample size of patients with recurrent cholecystitis, and lack of long-term follow up. In addition, it was impossible to determine whether the patients’ condition improved due to the initial PCT placement, and therefore whether it was a procedure that was truly indicated or done in the process of ruling out a septic source. Nevertheless, our study provides insight into both the characteristics and outcomes of patients with AAC who undergo PCT placement. It also gives rise to interesting points of future studies such as long-term morbidity and mortality in AAC patients undergoing PCT placement. Future research directions should focus on further identifying the characteristics of patients who may benefit the most from PCT placement, the ideal duration, and its management.
In conclusion, PCT placement at our institution was associated with a significantly high mortality, likely indicating the restricted use of this procedure to the sickest of patients with the highest surgical risk. The risk for recurrent cholecystitis in AAC patients is extremely low, therefore, a routine interval cholecystectomy may not be required. Further research is required to validate whether the findings are similar at lower volume institutions or institutions with different practices in selecting patients for PCT. Furthermore, given the variability in practice, guidelines for selection of patients for PCT, their post-placement management, and the indication for an interval cholecystectomy are required.
Change history
24 June 2022
A Correction to this paper has been published: https://doi.org/10.1007/s00268-022-06632-8
References
Barie PS, Eachempati SR (2003) Acute acalculous cholecystitis. Curr Gastroenterol Rep 5:302–309. https://doi.org/10.1007/s11894-003-0067-x
Chung YH, Choi ER, Kim KM, Kim MJ, Lee JK, Lee KT et al (2012) Can percutaneous cholecystostomy be a definitive management for acute acalculous cholecystitis? J Clin Gastroenterol 46:216–219. https://doi.org/10.1097/MCG.0b013e3182274375
Kim SB, Gu MG, Kim KH, Kim TN (2020) Long-term outcomes of acute acalculous cholecystitis treated by non-surgical management. Med (United States) 99:1–4. https://doi.org/10.1097/MD.0000000000019057
Huffman JL, Schenker S (2010) Acute acalculous cholecystitis: a review. Clin Gastroenterol Hepatol 8:15–22. https://doi.org/10.1016/j.cgh.2009.08.034
Barie PS, Eachempati SR (2010) Acute acalculous cholecystitis. Gastroenterol Clin North Am 39:343–357. https://doi.org/10.1016/j.gtc.2010.02.012
Treinen C, Lomelin D, Krause C, Goede M, Oleynikov D (2015) Acute acalculous cholecystitis in the critically ill: risk factors and surgical strategies. Langenbeck’s Arch Surg 400:421–427. https://doi.org/10.1007/s00423-014-1267-6
Elsharif M, Forouzanfar A, Oaikhinan K, Khetan N (2018) Percutaneous cholecystostomy why, when, what next? A systematic review of past decade. Ann R Coll Surg Engl 100:618–31. https://doi.org/10.1308/rcsann.2018.0150
Ratanaprasatporn L, Uyeda JW, Wortman JR, Richardson I, Sodickson AD (2018) Multimodality imaging, including dual-energy CT, in the evaluation of gallbladder disease. Radiographics 38:75–89. https://doi.org/10.1148/rg.2018170076
Bortoff GA, Chen MYM, Ott DJ, Wolfman NT, Routh WD (2000) Gallbladder stones: imaging and intervention. Radiographics 20:751–766. https://doi.org/10.1148/radiographics.20.3.g00ma16751
Laor A, Tal S, Guller V, Zbar AP, Mavor E (2016) The charlson comorbidity index (CCI) as a mortality predictor after surgery in elderly patients. Am Surg 82:22–27. https://doi.org/10.1177/000313481608200113
Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CY et al (2013) Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 217:833-842.e3. https://doi.org/10.1016/j.jamcollsurg.2013.07.385
Kallini JR, Patel DC, Linaval N, Phillips EH, Van Allan RJ (2021) Comparing clinical outcomes of image-guided percutaneous transperitoneal and transhepatic cholecystostomy for acute cholecystitis. Acta Radiol 62:1142–1147. https://doi.org/10.1177/0284185120959829
Wang CH, Wu CY, Yang JCT, Lien WC, Wang HP, Liu KL et al (2016) Long-term outcomes of patients with acute cholecystitis after successful percutaneous cholecystostomy treatment and the risk factors for recurrence: a decade experience at a single center. PLoS One. https://doi.org/10.1371/journal.pone.0148017
Anderson JE, Inui T, Talamini MA, Chang DC (2014) Cholecystostomy offers no survival benefit in patients with acute acalculous cholecystitis and severe sepsis and shock. J Surg Res 190:517–521. https://doi.org/10.1016/j.jss.2014.02.043
Anderson JE, Chang DC, Talamini MA (2013) A nationwide examination of outcomes of percutaneous cholecystostomy compared with cholecystectomy for acute cholecystitis, 1998–2010. Surg Endosc 27:3406–3411. https://doi.org/10.1007/s00464-013-2924-5
Simorov A, Ranade A, Parcells J, Shaligram A, Shostrom V, Boilesen E et al (2013) Emergent cholecystostomy is superior to open cholecystectomy in extremely ill patients with acalculous cholecystitis: a large multicenter outcome study. Am J Surg 206:935–941. https://doi.org/10.1016/j.amjsurg.2013.08.019
Kirkegård J, Horn T, Christensen SD, Larsen LP, Knudsen AR, Mortensen FV (2015) Percutaneous cholecystostomy is an effective definitive treatment option for acute acalculous cholecystitis. Scand J Surg 104:238–243. https://doi.org/10.1177/1457496914564107
Boules M, Haskins IN, Farias-Kovac M, Guerron AD, Schechtman D, Samotowka M et al (2017) What is the fate of the cholecystostomy tube following percutaneous cholecystostomy? Surg Endosc 31:1707–1712. https://doi.org/10.1007/s00464-016-5161-x
Bhatt MN, Ghio M, Sadri L, Sarkar S, Kasotakis G, Narsule C et al (2018) Percutaneous cholecystostomy in acute cholecystitis—Predictors of recurrence and interval cholecystectomy. J Surg Res 232:539–546. https://doi.org/10.1016/j.jss.2018.06.051
Sinvani L, Kuriakose R, Tariq S, Kozikowski A, Patel V, Smilios C et al (2019) Using charlson comorbidity index to predict short-term clinical outcomes in hospitalized older adults. J Healthc Qual 41:146–153. https://doi.org/10.1097/JHQ.0000000000000153
Atherly A, Fink AS, Campbell DC, Mentzer RM, Henderson W, Khuri S et al (2004) Evaluating alternative risk-adjustment strategies for surgery. Am J Surg 188:566–570. https://doi.org/10.1016/j.amjsurg.2004.07.032
Noh SY, Gwon DI, Ko GY, Yoon HK, Sung KB (2018) Role of percutaneous cholecystostomy for acute acalculous cholecystitis: clinical outcomes of 271 patients. Eur Radiol 28:1449–55. https://doi.org/10.1007/s00330-017-5112-5
Loftus TJ, Brakenridge SC, Moore FA, Dessaigne CG, Sarosi GA, Zingarelli WJ et al (2017) Routine surveillance cholangiography after percutaneous cholecystostomy delays drain removal and cholecystectomy. J Trauma Acute Care Surg 82:351–355. https://doi.org/10.1097/TA.0000000000001315
Hung Y, Chen H, Fu C, Tsai C, Chong S, Wang S et al (2020) Surgical outcomes of patients with maintained or removed percutaneous cholecystostomy before intended laparoscopic cholecystectomy. J Hepatobiliary Pancreat Sci 27:461–469. https://doi.org/10.1002/jhbp.740
Colonna AL, Griffiths TM, Robison DC, Enniss TM, Young JB, McCrum ML et al (2019) Cholecystostomy: are we using it correctly? Am J Surg 217:1010–1015. https://doi.org/10.1016/j.amjsurg.2019.04.002
Byrne MF, Suhocki P, Mitchell RM, Pappas TN, Stiffler HL, Jowell PS et al (2003) Percutaneous cholecystostomy in patients with acute cholecystitis: experience of 45 patients at a US referral center. J Am Coll Surg 197:206–211. https://doi.org/10.1016/S1072-7515(03)00143-1
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to report and have received no financial support in relation to this manuscript.
Ethical approval
This study protocol was reviewed and approved by the Cedars-Sinai Medical Center Institutional Review Board (STUDY00000163).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The fourth sentence in the Results section of the abstract was corrected.
Rights and permissions
About this article
Cite this article
Chen, S.Y., Huang, R., Kallini, J. et al. Outcomes Following Percutaneous Cholecystostomy Tube Placement for Acalculous Versus Calculous Cholecystitis. World J Surg 46, 1886–1895 (2022). https://doi.org/10.1007/s00268-022-06566-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-022-06566-1