Abstract
Background
With the global pandemic of obesity and nonalcoholic fatty liver disease (NAFLD), the incidence of cirrhosis associated with nonalcoholic steatohepatitis (NASH) has greatly increased. This study aimed to evaluate the efficacy and safety of bariatric surgery in obese cirrhotic patients.
Methods
PubMed, EMBASE, and the Cochrane Library were searched for relevant studies. Effectiveness outcomes were weight loss, remission of comorbidities, and improvement in liver function. Safety outcomes were procedural complications and mortality.
Results
A total of 15 studies were included in this meta-analysis. Patients with compensated cirrhosis lost weight significantly after surgery, and the percentage of excess weight loss was 60.44 (95% CI, 44.34 to 76.55). Bariatric surgery resulted in remission of NAFLD in 57.9% (95% CI, 27.5% to 88.3%), T2DM in 58.4% (95% CI, 48.4% to 68.4%), hypertension in 53.1% (95% CI, 43% to 63.3%), dyslipidemia in 59.8% (95% CI, 41.1% to 78.5%) of patients with cirrhosis. Bariatric surgery reduced the levels of alanine aminotransferase and aspartate aminotransferase. The incidence of surgical complications in patients with cirrhosis was about 19.2% (95% CI, 11.7% to 26.6%), which was higher than that in patients without cirrhosis (OR 2.67 [95% CI, 1.26 to 5.67]). Patients with cirrhosis had an overall mortality rate of 1.3%, and the mortality rates for compensated cirrhosis and decompensated cirrhosis were 0.9% and 18.2%, respectively.
Conclusions
Bariatric surgery is effective for weight loss, remission of comorbidities, and reversal of liver damage. Although cirrhotic patients have a higher risk of complications and death, bariatric surgery is relatively safe for well-compensated cirrhosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is a major public health problem, accompanied by increasing healthcare and economic burden. The number of obese adults is projected to rise by 65 million in the USA and 11 million in the United Kingdom by 2030 [1]. Obesity is strongly associated with a plethora of chronic diseases, such as cardiovascular diseases, type 2 diabetes mellitus (T2DM), dyslipidemia, and nonalcoholic fatty liver disease (NAFLD). NAFLD is the most common liver disease with a global prevalence of 25% [2]. Nonalcoholic steatohepatitis (NASH) is present in 7–30% of patients with NAFLD [3]. An estimated 20% of patients with NASH will develop cirrhosis, and it is expected that NASH will become the main indication for liver transplantation in the future [4]. Further, patients with NASH are more likely to be obese or exhibit metabolic derangements than patients with only NAFLD or the general population.
As people become more aware of the efficacy of treating obesity and obesity-related comorbidities, bariatric surgery is increasingly performed worldwide. The prevalence of NAFLD among patients undergoing bariatric surgery exceeds 90% [4]. Several studies have shown that bariatric surgery improves liver function, histology, and alleviate the progression of NASH patients [5,6,7]. A recent meta-analysis indicated that NAFLD completely disappeared in obese patients after bariatric surgery [8].
The benefit and risks of bariatric surgery in obese patients with cirrhosis are unclear. García-Sesma et al. demonstrated that bariatric surgery is safe and effective for decompensated cirrhosis and improves candidacy in morbidly obese patients awaiting transplantation in a short time [9]. However, it is well known that the morbidity and mortality of abdominal surgery in patients with cirrhosis are higher [10]. The same holds true for bariatric surgery. Liver failure, kidney failure, and postoperative bleeding due to coagulation dysfunction all increase the risk of surgery. For a given obese cirrhotic patient, assessing the benefits and risks of bariatric surgery is extremely challenging. This systematic review and meta-analysis aimed to evaluate the efficacy and safety of bariatric surgery in obese patients with cirrhosis.
Methods
Data sources, search strategy, and inclusion criteria
We conduct and report the results of this meta-analysis following the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines 2020 [11, 12]. The protocol of this study was registered in the Prospective Register of Systematic Reviews before the start (ID: CRD42020184985). We searched the database (PUBMED, EMBASE, and Cochrane Library) for manuscripts published from the establishment to April 15, 2020, with the following keywords: “Roux-en-Y gastric bypass,” “laparoscopic adjustable gastric banding,” “sleeve gastrectomy,” “vertical banded gastroplasty,” “metabolic surgery,” “bariatric surgery,” and “cirrhosis.” We did not impose any language restriction on our searches. The references of identified publications were hand-searched for further relevant publications. Original articles were eligible for the present meta-analysis if they assessed the effectiveness and/or safety of bariatric surgery in obese cirrhotic patients. We included both single-arm studies (evaluating the effect before and after bariatric surgery and/or surgical safety in obese cirrhotic patients) or double-arm studies (comparing the outcome of bariatric surgery in obese patients with cirrhosis and non-cirrhosis). We excluded case reports, nonhuman studies, review articles, and those lacking sufficient data.
Data extraction and study quality assessment
Two investigators independently screened the titles and abstracts of studies identified by the above searches, eliminated duplicate studies, and then reviewed the full texts of articles according to inclusion and exclusion criteria. Appropriate data were extracted and subsequently assessed by another reviewer. The following basic information was extracted from each study: study characteristics (author, country, year, surgery type, study design, sample size, and follow-up duration), participant baseline characteristics (mean age, %female, weight, BMI, obese-related comorbidities, etiology of cirrhosis, portal hypertension, and Child–Pugh class), outcomes (weight loss, comorbidity remission rate, and improvement of liver function), complications, and mortality. If there were different extractions between the 2 reviewers, a third person would make a decision.
Both single-arm and double-arm studies were included, so two investigators independently appraised the quality of included articles by the Risk Of Bias In Non-randomized Studies-of Interventions (ROBINS-I) assessment tool [13]. Disagreement was resolved through consensus after discussion in the integrative session.
Outcomes
We evaluated the efficacy of bariatric surgery by changes and percentage changes in weight, remission rates of comorbidities, and improvements in liver function; we assessed the safety through the incidence of surgical complications and mortality. The criteria for comorbidities remission are shown in Table S1.
Data synthesis and statistical analysis
We preferentially extracted data about the mean differences (MDs; with 95% confidence intervals [CIs] or standard errors) in weight parameters before and after bariatric surgery or calculated MDs (with 95% CIs or standard errors). We also calculated the pooled proportion of patients with surgical complications and the remission of comorbidities after bariatric surgery.
All statistical analyses were conducted with STATA, version 14.0. Random effect model was used for calculating the pooled estimates. Inconsistency test (I2) was used to assess heterogeneity among the studies. In cases in which I2 ≥ 50%, we attempted to identify possible heterogeneity by subgroup analysis and meta-regression analysis. We also performed sensitivity analyses to evaluate the consistency and robustness of the results. Publication bias was assessed by Begg’s test and Egger’s test if needed. A P value < 0.05 was indicative of statistical significance.
Results
Study selection and characteristics
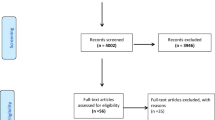
A total of 723 articles were obtained through the above database search. After excluding duplicates and articles that did not meet the inclusion criteria, we reviewed the full texts of 44 articles and excluded the following: 9 studies that did not include patients with cirrhosis, 1 study not for bariatric surgery, and 19 studies lacking the necessary data (Fig. 1). Finally, 15 articles met the eligibility criteria. Ten studies were single-arm studies that evaluated the effect of bariatric surgery on obese cirrhotic patients before and after surgery without comparators [9, 14,15,16,17,18,19,20,21,22]; 5 studies were double-arm studies comparing the effectiveness and/or safety of bariatric surgery for obese patients with cirrhosis and non-cirrhosis [23,24,25,26,27]. Besides, two studies included patients with compensated cirrhosis and decompensated cirrhosis [23, 27], while others included patients with well-compensated cirrhosis. Effective outcomes and complication analysis included only patients with compensated cirrhosis; mortality analysis included patients with compensated cirrhosis and decompensated cirrhosis.
The baseline characteristics of the studies are shown in Table 1. A total of 1,233,602 obese patients undergoing bariatric surgery were included, of which 7424 were cirrhotic patients and 1,226,178 were non-cirrhotic patients. The mean age of the patients was about 50 years old, and the proportion of women was 80.5% (992,542/1,233,300). At baseline, the patients' average weight and BMI were 116–149 kg and 39–54 kg/m2, respectively. More than half of the participants suffered from obesity comorbidities, including NAFLD/NASH, type 2 diabetes (T2DM), hypertension, dyslipidemia, and obstructive sleep apnea hypopnea syndrome (OSAHS) (Table S2). The most common cause of liver cirrhosis was NASH, followed by hepatitis C virus. Portal hypertension was present in 19.3% (33/171) of cirrhotic patients. The most common procedure is laparoscopic sleeve gastrectomy (LSG). The follow-up period ranged from hospitalization to 9 years. With the ROBINS-I tool, we found that the overall risk of bias for 3 studies was identified as “serious” and for 12 studies as “moderate” (Table S3). Except for complications, neither Begg’s test nor Egger’s test showed publication bias for the primary outcomes (Table S4).
Weight loss
In total, ten studies reported data regarding weight loss. For patients with compensated cirrhosis, bariatric surgery elicited the following changes in weight (MD −35.11 [95% CI, −45.42 to −24.79]; P = 0.000; I2 = 88%), and BMI (MD −12.93 [95% CI, −15.91 to −9.96]; P = 0.000; I2 = 76.6%), respectively (Fig. 2a, b). Specifically, the percentage of weight loss in patients with compensated cirrhosis was also significant after bariatric surgery. The results of percentage of total weight loss (%TWL) and percentage of excess weight loss (%EWL) were 26.45 (95% CI, 21.59 to 31.31; P = 0.000; I2 = 88.7%) and 60.44 (95% CI, 44.34 to 76.55; P = 0.000; I2 = 96.9%) (Fig. 2c, d). A case-matched study compared the weight change parameters of bariatric surgery for obese patients with cirrhosis and non-cirrhosis and showed similar changes in BMI, %TWL and %EWL in both groups after 12 months of follow-up (P > 0.05) [26].
Analysis of all weight change parameters showed significant heterogeneity among studies (I2 ≥ 50%); therefore, a random-effect model was used. We performed subgroup analysis and found that different follow-up duration may lead to heterogeneity (Fig. S1). However, neither meta-regression analysis nor sensitivity analysis identified the source of heterogeneity (data not shown).
Remission of comorbidities
Of the 15 studies included, 5 provided data on NAFLD remission [14, 17,18,19, 22]. Our results indicated that 57.9% (95% CI, 27.5% to 88.3%; P = 0.000; I2 = 95.1%) obese patients with cirrhosis achieved NAFLD remission (Fig. 3a). Further, a meta-analysis of the proportions showed that 58.4% of patients with cirrhosis had remission of T2DM (95% CI, 48.4% to 68.4%; P = 0.000; I2 = 0.0%), 53.1% had remission of hypertension (95% CI, 40.6% to 65.0%; P = 0.000; I2 = 26.8%), and 59.8% had remission of dyslipidemia (95% CI, 41.1% to 78.5%; P = 0.000; I2 = 56.3%) (Fig. 3b, d). Only one study reported OSAHS remission data, of which 83.3% (10/12) showed OSAHS remission after bariatric surgery [16]. Because of the small number of studies included in each analysis, we did not conduct subgroup analyses. Sensitivity analyses did not find any single study that altered the consistency and robustness of the results.
Liver function improvement
Seven studies were included in the analysis of liver function improvement [14,15,16,17,18,19, 22]. Bariatric surgery elicited the following decreases in alanine aminotransferase (ALT) (MD −17.68 [95% CI, −24.11 to −11.24]; P = 0.000; I2 = 67.9%), and aspartate aminotransferase (AST) (MD −10.38 [95% CI, −15.44 to −5.32]; P = 0.000; I2 = 64.1%) for patients with compensated cirrhosis, respectively. Sensitivity analyses did not identify any single study to change the consistency and robustness of any results.
Complications and mortality
In this systematic review, 12 articles discussed surgical complications. As shown in Fig. 4, the incidence of surgical complications in obese patients with compensated cirrhosis is 19.2% (95% CI, 11.7% to 26.6%; P = 0.000; I2 = 65.0%). Sensitivity analysis suggested that the results may be unstable due to Pestana’s study [17]. After discarding this article, the pooled complication rate was 20.8%, and heterogeneity dropped (95% CI, 14.9% to 26.7%; P = 0.000; I2 = 28.1%) (Fig. S2). Three studies reported the complications of bariatric surgery for obese patients with and without cirrhosis [24,25,26]. The meta-analysis revealed that cirrhotic patients had a higher complication rate than non-cirrhotic patients (OR 2.67 [95% CI, 1.26 to 5.67]; P = 0.011; I2 = 0%).
The mortality analysis included 12 articles. Mortality and causes of death are summarized in Table 2. The overall mortality rate was 1.3% (94/7360) in obese cirrhotic patients, with 0.9% (62/7195) in compensated cirrhosis and 18.2% (30/165) in decompensated cirrhosis, respectively. As shown in Table 2, causes of death included multiple organ failure caused by infectious colitis, hepatocellular carcinoma, esophageal cancer, postoperative pulmonary embolism, and hepatic failure [15, 18, 19, 21, 22].
Postoperative complications and mortality for the different procedures are shown in Table S5. The complication rate was 17.1% (22/129), 33.9% (21/62), 20% (3/15), and 14.3% (2/14) with sleeve gastrectomy (SG), Roux-en-Y gastric bypass (RYGB), adjustable gastric banding (AGB), and biliopancreatic diversion (BPD), respectively. In addition, the SG group had the lowest rate of Clavien–Dindo grade ≥ III complications at 3.9% (5/129). Surgery-related mortality was seen in SG, RYGB, AGB and BPD group at 0.8% (1/129), 1.6% (1/62), 6.7% (1/15), and 21.4% (3/14), respectively.
Discussion
This comprehensive meta-analysis suggests that for obese patients with compensated cirrhosis, bariatric surgery can significantly reduce weight and improve obesity comorbidities and liver function. Although patients with cirrhosis, especially those with decompensated cirrhosis, have increased postoperative complications and mortality. In the present review, a relatively satisfactory safety profile after bariatric surgery is observed in patients with compensated cirrhosis. To our knowledge, this is the first meta-analysis to report the effectiveness and safety of bariatric surgery for obese patients with cirrhosis.
As bariatric surgery is increasingly performed worldwide, bariatric surgeons are likely to encounter patients with cirrhosis, especially NASH-related cirrhosis. A systematic review showed that about 1.0–4.0% of patients undergoing bariatric surgery were diagnosed with incidental cirrhosis [28]. Therefore, both patients with cirrhosis and surgeons often face the dilemma of whether to perform surgery or not. Our review of 15 studies provides evidence that bariatric surgery is effective in reducing the weight of patients with compensated cirrhosis. After bariatric surgery, cirrhotic patients had obvious weight loss and reduction in BMI and achieved significant TWL% and EWL%. And one of the included studies by Rebibo reported that bariatric surgery had no difference in weight loss between cirrhotic and non-cirrhotic patients [26]. Our results demonstrate significant heterogeneity between studies. Such heterogeneity may be due to differences in follow-up periods between studies. Other factors that may explain the observed heterogeneity include the diversity of surgical procedures and differences in population baseline characteristics, such as the severity of cirrhosis and comorbidities.
Obesity-related comorbidities are well known to increase long-term medication usage, cardiovascular risk, and the rate of psychosocial disabilities. Our systematic review shows that cirrhosis may not be an adverse factor for the remission of comorbidities after bariatric surgery. We found remission of NAFLD in 57.9%, T2DM in 58.4%, hypertension in 53.1%, dyslipidemia in 59.8%, and OSAHS in 83.3% of patients with cirrhosis after bariatric surgery. These data are similar to the previously reported remission rates of obesity comorbidities with metabolic surgery (regardless of whether the patient is cirrhotic). [29, 30].
In this meta-analysis, bariatric surgery shows the potential to halt or even reverse liver damage. The sustained and stable decline in transaminases and remission of NAFLD all suggest that bariatric surgery has a protective effect on the liver. Younus et al. found a significant improvement in Model for End-Stage Liver Disease (MELD) score of patients with cirrhosis [24]. A 16-month follow-up cohort of cirrhotic patients revealed that decompensated cirrhosis-related complications such as ascites, encephalopathy, bleeding, infections, or documented progression of liver dysfunction did not occur after bariatric surgery [21]. Two of these studies reported that bariatric surgery also improved eligibility and candidacy in morbidly obese patients awaiting liver transplantation [9, 20]. In contrast, Miñambres et al. demonstrated that 20% of Child–Pugh A patients showed a deterioration [16]. This may be due to higher BMI and bilirubin at baseline and during follow-up, which exacerbates the negative impact of obesity on the prognosis of cirrhosis. The study also showed that during the 5 years of follow-up, the MELD score progressed slowly (P > 0.05), and only 5 patients developed decompensated cirrhosis, suggesting that bariatric surgery slowed down the progression of liver disease [16]. Except for the biliopancreatic diversion and Roux-en-Y gastric bypass, there is less evidence of liver harm after other bariatric procedures [31]. However, bariatric surgery is associated with varying degrees of nutritional deficiency, of which protein deficiency is a major issue [32]. Importantly, patients with cirrhosis often suffer from malnutrition, sarcopenia, and impaired protein metabolism [33]. The long-term effects of bariatric surgery on protein metabolism and nutrition status in patients with cirrhosis need further exploration. Furthermore, there are little data on the efficacy of bariatric surgery on patients with decompensated cirrhosis. Therefore, this study does not discuss the benefits of bariatric surgery for patients with decompensated cirrhosis.
It is known that patients with cirrhosis have an increased risk of abdominal and non-abdominal surgery. Therefore, the risk of undergoing bariatric surgery in the cirrhotic population is of great concern. Our results confirm the higher morbidity and mortality of cirrhotic patients, especially in those with decompensated cirrhosis. In this review, the overall incidence of complications in patients with cirrhosis is about 19%, which is 2.67 times that of patients without cirrhosis. Moreover, the total mortality of patients with cirrhosis is 1.3%; the mortality rates of patients with compensated cirrhosis and compensated cirrhosis were 0.9% and 18.2%, respectively. Two factors could explain these results. The first one is the increased risk and technical difficulties associated with cirrhosis-related complications (including portal hypertension), such as ascites, malnutrition, abnormal blood coagulation, and liver failure [24, 31]. The second reason is malnutrition in cirrhotic patients, which is significantly related to the increased risk of postoperative morbidity and mortality after abdominal surgery [23, 24]. Large sample studies based on the NIS database also showed that the in-hospital mortality rate of patients with compensated cirrhosis is significantly lower than that of patients with decompensated cirrhosis, comparable to that in non-cirrhotic patients (decompensated cirrhosis: 16.3%–19.4%, compensated cirrhosis: 0.6%-0.9%, no cirrhosis: 0.1%-0.3%), and the mortality rate is low in centers that perform large amounts of bariatric surgery [23, 27]. This suggested that bariatric surgery is relatively safe for patients with well-compensated Child–Pugh class A cirrhosis. Now, the rapid development and evolution of surgical procedures have greatly improved the safety of bariatric surgery. Overall, better outcomes are observed with laparoscopic surgery over open surgery [31]. And compared with other surgical procedures, sleeve gastrectomy had the lowest complication rate, Clavien–Dindo grade ≥ III complication rate, and mortality, suggesting that sleeve gastrectomy is the safest for obese patients with cirrhosis. Multidisciplinary cooperation and appropriate perioperative care can further guarantee surgical safety.
The key strength of our review is a comprehensive assessment of the effectiveness and risks of bariatric surgery in patients with liver cirrhosis, including weight loss, remission of comorbidities, improvement in liver function, complications, and mortality. This review also compares the effect of bariatric surgery in patients with cirrhosis and non-cirrhosis, focusing on the outcomes commonly described in individual studies. However, our systematic review has some limitations. First, there was obvious heterogeneity in some of the results. We conducted subgroup analyses, sensitivity analyses, and meta-regression analyses to identify the sources of heterogeneity and found that a wide range of follow-up duration may contribute to heterogeneity. Other factors, such as diversity in surgical procedures and different baseline characteristics among populations, may also lead to heterogeneity. Second, the sample size varied greatly. Of these 15 articles, 3 reported on less than 100 cirrhotic patients who underwent bariatric surgery, whereas the other two database-based studies included thousands of cirrhotic patients. Third, most studies were retrospective observational design, ultimately leading to relatively low certainty in the body of evidence. As blinded RCTs face ethical issues related to equipoise and sham procedures, to date there are no RCTs available for this research question. Forth, due to the high prevalence of NAFLD and NASH, the cirrhosis of most patients included in this study is attributed to NAFLD/NASH. The safety and effectiveness of surgery in patients with liver cirrhosis caused by other reasons need to be further explored.
Conclusions
Bariatric surgery is effective for weight loss, alleviating comorbidities, and reversing liver damage. Bariatric surgery is relatively safe for compensated cirrhosis, although the risk of complications and death is higher in patients with cirrhosis. The long-term effects of bariatric surgery in obese cirrhotic patients need to be further explored.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and its additional files.
References
Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M (2011) Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 378(9793):815–825
Zobair MY, Aaron BK, Dinan A, Yousef F, Linda H, Mark W (2016) Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. https://doi.org/10.1002/hep.28431-28484
Cotter TG, Rinella M (2020) Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology 158(7):1851–1864
Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S (2020) Nonalcoholic steatohepatitis: a review. JAMA 323(12):1175–1183
Wirth KM, Sheka AC, Kizy S, Irey R, Benner A, Sieger G, Simon G, Ma S, Lake J, Aliferis C, Leslie D, Marmor S, Ikramuddin S. Bariatric Surgery is Associated With Decreased Progression of Nonalcoholic Fatty Liver Disease to Cirrhosis: A Retrospective Cohort Analysis. Ann Surg 2020.
Cherla DV, Rodriguez NA, Vangoitsenhoven R, Singh T, Mehta N, McCullough AJ, Brethauer SA, Schauer PR, Aminian A (2019) Impact of sleeve gastrectomy and Roux-en-Y gastric bypass on biopsy-proven non-alcoholic fatty liver disease. Surg Endosc 34:2266–2272
Nikai H, Ishida K, Umemura A, Baba S, Nitta H, Sugai T, Sasaki A (2020) Effects of laparoscopic sleeve gastrectomy on non-alcoholic steatohepatitis and liver fibrosis in japanese patients with severe obesity. Obes Surg 30:2579–2587
Lee Y, Doumouras AG, Yu J, Brar K, Banfield L, Gmora S, Anvari M, Hong D (2019) Complete resolution of nonalcoholic fatty liver disease after bariatric surgery: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 17(6):1040-1060.e1011
García-Sesma A, Calvo J, Manrique A, Cambra F, Justo I, Caso O, Marcacuzco A, Loinaz C, Jiménez C (2019) Morbidly obese patients awaiting liver transplantation-sleeve gastrectomy: safety and efficacy from a liver transplant unit experience. Transplant Proc 51(1):33–37
Kay MJ, Kira LN, Pamela KG, Kristin B, Paul BC, Peter W, Lauren AB, Kamal I, Alex HSH, Patrick SK, George NI (2019) Incidence and risk factors of postoperative mortality and morbidity after elective versus emergent abdominal surgery in a national sample of 8193 patients with cirrhosis. Ann Surg. https://doi.org/10.1097/SLA.0000000000003674
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, McKenzie JE (2021) PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 372:n160
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919
Salman MA, Mikhail HMS, Nafea MA, Sultan A, Elshafey HE, Tourky M, Awad A, Abouelregal TE, Ahmed RA, Ashoush O, AbdelAal AA, Shaaban HE, Atallah M, Yousef M, Salman AA (2020) Impact of laparoscopic sleeve gastrectomy on fibrosis stage in patients with child-A NASH-related cirrhosis. Surg Endosc 35:1269–1277
Hanipah ZN, Punchai S, McCullough A, Dasarathy S, Brethauer SA, Aminian A, Schauer PR (2018) Bariatric surgery in patients with cirrhosis and portal hypertension. Obes Surg 28(11):3431–3438
Miñambres I, Rubio MA, de Hollanda A, Breton I, Vilarrasa N, Pellitero S, Bueno M, Lecube A, Marcuello C, Goday A, Ballesteros MD, Soriano G, Caixàs A (2019) Outcomes of bariatric surgery in patients with cirrhosis. Obes Surg 29(2):585–592
Pestana L, Swain J, Dierkhising R, Kendrick ML, Kamath PS, Watt KD (2015) Bariatric surgery in patients with cirrhosis with and without portal hypertension: a single-center experience. Mayo Clin Proc 90(2):209–215
Woodford RM, Burton PR, O’Brien PE, Laurie C, Brown WA (2015) Laparoscopic adjustable gastric banding in patients with unexpected cirrhosis: safety and outcomes. Obes Surg 25(10):1858–1862
Shimizu H, Phuong V, Maia M, Kroh M, Chand B, Schauer PR, Brethauer SA (2013) Bariatric surgery in patients with liver cirrhosis. Surg Obes Relat Dis 9(1):1–6
Takata MC, Campos GM, Ciovica R, Rabl C, Rogers SJ, Cello JP, Ascher NL, Posselt AM (2008) Laparoscopic bariatric surgery improves candidacy in morbidly obese patients awaiting transplantation. Surg Obes Relat Dis 4(2):159–164 (Discussion 164-155)
Dallal RM, Mattar SG, Lord JL, Watson AR, Cottam DR, Eid GM, Hamad G, Rabinovitz M, Schauer PR (2004) Results of laparoscopic gastric bypass in patients with cirrhosis. Obes Surg 14(1):47–53
Kral JG, Thung SN, Biron S, Hould FS, Lebel S, Marceau S, Simard S, Marceau P (2004) Effects of surgical treatment of the metabolic syndrome on liver fibrosis and cirrhosis. Surgery 135(1):48–58
Mumtaz K, Lipshultz H, Jalil S, Porter K, Li N, Kelly SG, Conteh LF, Michaels A, Hanje J, Black S, Hussan H (2020) Bariatric surgery in patients with cirrhosis: careful patient and surgery-type selection is key to improving outcomes. Obes Surg 30:3444–3452
Younus H, Sharma A, Miquel R, Quaglia A, Kanchustambam SR, Carswell KA, Patel AG (2020) Bariatric surgery in cirrhotic patients: is it safe? Obes Surg 30(4):1241–1248
Wolter S, Duprée A, Coelius C, El Gammal A, Kluwe J, Sauer N, Mann O (2017) Influence of liver disease on perioperative outcome after bariatric surgery in a northern German cohort. Obes Surg 27(1):90–95
Rebibo L, Gerin O, Verhaeghe P, Dhahri A, Cosse C, Regimbeau JM (2014) Laparoscopic sleeve gastrectomy in patients with NASH-related cirrhosis: a case-matched study. Surg Obes Relat Dis 10(3):405–410
Mosko JD, Nguyen GC (2011) Increased perioperative mortality following bariatric surgery among patients with cirrhosis. Clin Gastroenterol Hepatol 9(10):897–901
Jan A, Narwaria M, Mahawar KK (2015) A systematic review of bariatric surgery in patients with liver cirrhosis. Obes Surg 25(8):1518–1526
English WJ, Williams DB (2018) Metabolic and bariatric surgery: an effective treatment option for obesity and cardiovascular disease. Prog Cardiovasc Dis 61(2):253–269
Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K (2004) Bariatric surgery: a systematic review and meta-analysis. JAMA 292(14):1724–1737
Goh GB, Schauer PR, McCullough AJ (2018) Considerations for bariatric surgery in patients with cirrhosis. World J Gastroenterol 24(28):3112–3119
Ledoux S, Flamant M, Calabrese D, Bogard C, Sami O, Coupaye M (2020) What are the micronutrient deficiencies responsible for the most common nutritional symptoms after bariatric surgery? Obes Surg 30(5):1891–1897
Stirnimann J, Stirnimann G (2019) Nutritional challenges in patients with advanced liver cirrhosis. J Clin Med 8(11):1926
Funding
This study was supported by Beijing Municipal Science and Technology Project (No.Z171100002217070), National Key R&D Program of China (No.2017YFA0103000), National Science and Technology Key Project on “Major Infectious Diseases such as HIV/AIDS, Viral Hepatitis Prevention and Treatment” (NO. 2012ZX10002004-006, No.2017ZX10203201-005, 2017ZX10201201, No.2017ZX10202203-006–001 and No.2017ZX10302201-004–002), “Beijing Municipal Administration of Hospitals” Ascent Plan (No. DFL20151601), Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (No.ZYLX201806 and No. ZYLX202125), the Digestive Medical Coordinated Development Center of Beijing Municipal Administration of Hospitals (No.XXZ0503), the You An fund for liver diseases and AIDS (YNKTTS201801189), the Basic-Clinical Cooperation Project of Capital Medical University (17JL47).
Author information
Authors and Affiliations
Contributions
JB and ZJ contributed to conception and design. JB and ZJ contributed to systematic literature search and data extraction. JB contributed to data analysis. JB and ZJ contributed to manuscript draft. YC, YL, SZ, and ZD contributed to editing and revision. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All the authors listed report nothing to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bai, J., Jia, Z., Chen, Y. et al. Bariatric Surgery is Effective and Safe for Obese Patients with Compensated Cirrhosis: A Systematic Review and Meta-Analysis. World J Surg 46, 1122–1133 (2022). https://doi.org/10.1007/s00268-021-06382-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-021-06382-z