Abstract
Background
Tumours involving the supra-renal segment of IVC have dismal prognosis if left untreated. Currently, aggressive surgical management is the only potentially curative treatment but is associated with relatively high morbidity and mortality. This study aims to evaluate perioperative factors, associated with adverse postoperative outcomes, based on the perioperative characteristics and type of IVC reconstruction.
Methods
We identified 44 consecutive patients, who underwent supra-renal IVC resection with a mean age of 57.3 years. Isolated resection of IVC was performed in four patients, concomitant liver resection was performed in 27 patients and other associated resection in 13 patients. Total vascular exclusion was applied in 21 patients, isolated IVC occlusion in 11 patients. Neither venovenous bypass (VVB) nor hypothermic perfusion was used in any of the cases.
Results
The mean operative time was 205 min (150–324 min) and the mean estimated blood loss was 755 ml (230–4500 ml). Overall morbidity was 59% and major complications (Dindo-Clavien ≥ III) occurred in 11 patients (25%). The 90-day mortality was 11% (5pts). Intraoperative haemotransfusion was significantly associated with postoperative general complications (p < 0,001). With a mean follow-up of 26.2 months, the actuarial 1-, 3- and 5-year survival is 69%, 34%, and 16%, respectively.
Conclusions
IVC resection and reconstruction in the aspect of aggressive surgical management of malignant disease confers a survival advantage in patients, often considered unresectable. When performed in experienced centres it is associated with acceptable morbidity and mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The portion of the inferior vena cava (IVC) extending from the renal veins to the diaphragm is intimately related to the liver. Miscellaneous tumours, both intra- and extrahepatic, may engage the IVC, either by direct invasion or tumour thrombus, protruding into the caval lumen. Involvement of this retrohepatic segment or the hepatic veins was previously considered a contraindication to surgery, due to the risks of uncontrollable haemorrhage or air embolism. However, if left untreated, those tumours have universally poor prognosis with survival rarely exceeding 12 months [1,2,3]. Nowadays there are a lot of treatment modalities, that allow for palliation of disease, but surgery offers the only potential for cure. The methods of vascular control [4,5,6,7,8] and the experience, related to liver transplantation, as well as the advances in anaesthesia and intensive care, have overcome many of the technical limitations, previously associated with IVC resection. There has been an accumulation of reports, establishing the safety of the resection and reconstruction of the supra-renal vena cava, combined with liver resection [9,10,11,12,13,14,15,16], or not [3, 17,18,19]. Nevertheless, it is still associated with relatively high morbidity, and mortality exceeding 10% in some series [14, 20, 21]. Such complex procedures are seldomly performed and are restricted mainly to highly specialized centres. Moreover, the diverse patient population with various tumours and stages of progression complicates data interpretation and renders the conduction of RCTs highly impractical. Therefore, this study aims to assess the relationship between early postoperative outcomes and complications, based on the perioperative characteristics and type of IVC reconstruction. Hereby we share our updated experience [22] and insights in IVC resection and methods of reconstruction.
Methods
Data accumulation
We searched our units’ database for patients who underwent supra-renal IVC resection. After approval of the local ethics committee, data were collected retrospectively, through a comprehensive review of the patient charts. Survival information was obtained from the national registry, but detailed follow-up about disease progression was missing for most patients. Postoperative complications were graded according to Dindo-Clavien classification [23]. They were separated into three categories–general, surgical, and thrombotic. As thrombotic complications were regarded the presence of partial or complete graft thrombosis, postoperative Budd-Chiari syndrome, or pulmonary thromboembolism. Major complications were considered as Dindo-Clavien III or higher. Statistical analysis was performed on IBM SPSS, version 25.0 (IBM Corp, Armonk, NY).
From January 2005 to January 2018, we identified 44 patients, requiring resection of IVC, of whom 20 were male (46%). The mean age was 57 years (range: 22–76, SD ± 12.07). The indications for surgery were particularly diverse and are shown in Table 1.
Preoperative evaluation
Preoperative chest, abdominal and pelvic contrast-enhanced computed tomography (CT) was obtained for every patient. Since 2014, we acquired an additional whole-body 18F-FDG-PET/CT for evaluation of distant spread. Ascending cavography was performed in seven patients. Transoesophageal echocardiography was performed in five patients, in whom intracardiac extension of IVC thrombus was suspected. Every patient underwent evaluation of cardio-circulatory and respiratory status and was discussed at a multi-disciplinary meeting. Extrahepatic metastatic disease was considered a contraindication to surgery.
Surgical technique and vascular control
Surgery was performed through an inverted L-shape incision or bilateral subcostal incision with midline extension. An additional sternotomy with pericardiotomy was used in two cases for tumours, extending to the right atrium. Some form of vascular control was utilized in all patients. Side clamp of IVC was applied in 12 patients. Isolated occlusion of vena cava was performed in 11 patients. Total vascular exclusion [6, 7] (TVE) was performed in 21 patients (47%) for a mean duration of 23.9 min (SD ± 14.8 min). TVE did not exceed 60 min in any of the patients (6–57 min). In four of these patients, selective isolation of the hepatic veins was employed, with preservation of caval flow. Two-step vascular exclusion, as described by Azoulay et al. [13] was performed in 4 cases. Transabdominal pericardiotomy was performed in three patients and the IVC was controlled above the diaphragm. Two patients–one with renal cell carcinoma and thrombus in the IVC, extending to the right atrium, and one with primary vena cava sarcoma were placed on cardio-pulmonary bypass. Venovenous bypass (VVB) or hypothermic perfusion were not applied in any of the cases.
The decision on the type of IVC reconstruction was based on the intraoperative assessment of the extent of circumferential involvement. When less than 60 degrees of the circumference was compromised, a tangential resection was performed. IVC was sutured longitudinally, with continuous 4–0 monofilament polypropylene suture. Larger defects required patch repair or segmental resection with tube-graft replacement. As patch material, we have used preserved donor iliac vein allograft, Dacron or ePTFE prostheses. For segmental IVC-replacement, our current material of choice is 20 mm ringed expanded polytetrafluoroethylene graft—ePTFE (Gore-Tex®; W. L. Gore & Associates Inc., Flagstaff, AZ, USA).
Results
Most of the patients were asymptomatic, at the time of presentation. The most common symptom was pain in the upper abdomen (16 patients, 36%). Oedema of lower extremities was observed in three patients (6%) and one patient presented with Budd-Chiari syndrome, with hepatomegaly, ascites, and jaundice.
The main intraoperative characteristics are summarized in Table 2. Four patients underwent an isolated IVC resection. In one of the cases with IVC sarcoma, the tumour extended to the right atrium, which necessitated vena cava to be replaced with ePTFE graft under cardio-pulmonary bypass. The others underwent patch reconstruction (Dacron patch, n = 2 and donor iliac vein allograft, n = 1), Fig. 1.
Resection of IVC, associated with hepatectomy was performed in 27 patients (Table 3). Major hepatectomy (resection of more than three continuous segments) was performed in 21 patients. Liver segment 1 was removed in 11 patients. In one case the middle hepatic vein was re-implanted, as part of the IVC-excision, combined with thrombectomy. In the patients without hepatectomy, surgery of retrohepatic IVC was combined with removal of retroperitoneal tumours, n = 5; adrenal gland, n = 1; kidney, n = 3; pancreaticoduodenectomy, n = 4 (Table 4). Sixteen patients (36%) required multi-visceral resection. The final pathologic analysis revealed true vena cava invasion in 28 patients (64%). Tumour clearance (1 mm tumour-cell free margin, regarded as R0-resection) was achieved in 43 patients.
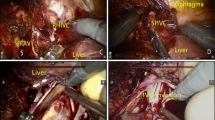
Whole segment of IVC was removed in seven patients (Fig. 2). Two of them had chronic thrombosis, engaging the infrarenal portion. Because of the rich collateral circulation, the vein was not reconstructed and was ligated above the renal vein confluence. In five patients, vena cava was replaced with an ePTFE tube-graft, mean length 8.2 cm (3–14 cm). One suffered post-operative death. One patient developed graft thrombosis 6 months after surgery (Fig. 3), related to disease progression and succumbed 2 months later. The other three patients are still alive with patent grafts 21, 23 and 42 months after surgery.
Segmental IVC–replacement with ePTFE tube-graft. Patient No 37 (primary IVC sarcoma)-preoperative CT (a), PET (e), intraoperative IVC reconstruction (c) and follow-up CT (g); Patient No 41 (liver metastasis from adrenocortical carcinoma)–preoperative CT (b), PET (f), intraoperative reconstruction (d), follow-up CT (h)
Patient with colorectal liver metastases. Resection of liver segment 1 and 4B, metastasectomies form segment 6 and 8, and IVC replacement with ePTFE-graft. a Intraoperative photograph; b and c: Follow-up CT 6 months later showing disease progression causing compression of the ePTFE graft with thrombosis
Operative time was not associated with postoperative surgical (p = 0.959) or clinical complications (p = 0.697). Intraoperative blood transfusion was performed in 23 patients, median 2 units (1–6). Data analysis showed no correlation between the haemotransfusion and consequent surgical or thrombotic complications, as well as perioperative mortality. However, it was associated with postoperative general complications p < 0.001. A trend toward shorter overall survival (OS) was observed in cases with intraoperative hemotransfusion (33.3 vs. 23.87 months, p = 0.360). The type of vascular control or the type of IVC-reconstruction did not influence the postoperative outcome. There was no difference in postoperative complications or perioperative mortality.
Morbidity and mortality
The mean ICU and hospital stay were 3.4 (1–11) and 12.6 days (3–49), respectively. Overall, 58 complications were recorded in 26 patients (59%). Twenty-two major complications (Dindo-Clavien ≥ III) occurred in 11 patients (25%). Cardiac and pulmonary adverse events were managed conservatively. One patient developed acute upper GI-bleed due to duodenal ulcer, which was managed endoscopically. Two patients developed mild lower limb oedema. Transient post-hepatectomy liver failure was recorded in 4 patients, which was resolved with supportive measures. The surgical complications were as follows: Two patients developed postoperative haemorrhage during heparin infusion. Intraabdominal abscess was recorded in three patients. Bile leaks or biloma formation was seen in six patients and wound infection in two patients. Six patients required reoperation. Thrombotic complications occurred in four patients. One patient developed pulmonary thromboembolism; one patient developed partial graft thrombosis which was managed conservatively. The other two patients developed acute Budd-Chiari syndrome and right atrial thrombosis with fatal outcome. The 90-day postoperative mortality was 11% (five patients), shown in Table 5.
Survival
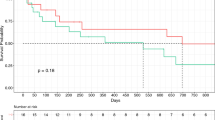
Survival data are available for 43 of 44 patients, with a mean follow-up of 26.2 months (range, 5 – 80 months). The 1-, 3- and 5-year actuarial survival is 69%, 34% and 16%, respectively. Because of the wide variety of diagnoses and the small number of patients, detailed analysis of postoperative outcomes was unfeasible. No difference in postoperative morbidity and mortality between major and minor hepatectomy, associated with IVC-resection was found, as well as no difference in OS. Thirteen of the patients with combined liver and IVC resection had colorectal liver metastasis. For this group of patients, the actuarial 1-, 3- and 5-year survival was 46%, 23% and 0%, respectively. Kaplan–Meier survival analysis (Fig. 4) showed no difference in overall survival in patients with synchronous (n = 6) or metachronous (n = 7) CRLM (Log-Rank test p = 0,730). These results were compared with the overall survival of patient with liver resection for colorectal metastases in our centre–48.28 months for metachronous lesions and 46.5 months for synchronous lesions [24]. These results imply that IVC-involvement is poor prognostic factor, but definitive conclusions cannot be made at this point.
Discussion
Surgery of the supra-renal IVC emerged as a treatment option primarily due to progress related to liver transplantation. It is associated with great technical complexity and requires excellent communication between the surgical and anaesthesiologic teams. Even today it is mainly restricted to specialized HPB and liver transplant units [10, 13,14,15]. Evidence in the literature is scarce, as most of the data come from case reports and small case studies. In our unit, which serves as tertiary referral HPB-centre, of more than 1000 radical liver resections, reconstruction of the retrohepatic portion of IVC was performed in 44 cases. This number is comparable with the results from other series [12,13,14,15].
Patients with involvement of the IVC often present at an advanced stage and are universally considered poor candidates for surgical management. If left untreated, however, the median survival is less than 12 months [1,2,3]. Chemotherapy alone does not offer a curative option with few 5-year survivors reported [25]. The aggressive surgical approach offers a chance for extended survival in selected patients, who will otherwise have dismal prognosis [9, 17].
True invasion of IVC cannot be determined by any diagnostic modality [11, 26, 27]. The final decision to perform an IVC resection should be made in the operating room. Using blunt dissection, an attempt to detach the tumour from the IVC wall should be made. If the separation of these structures is difficult or too risky, or if there is strong suspicion for venous invasion, concomitant IVC resection should be performed. Careful dissection of the structures should enable the size of the resected IVC to be minimized [27]. The reported percentage of histologic invasion in the resected IVC specimen ranges from 23 to 100% [9, 11,12,13,14,15, 26, 27]. In our series, 28 patients had proven histologic invasion (63%).
Appropriate vascular control is of key importance for limiting blood loss and safe dissection. The technique of total vascular exclusion (TVE) was first reported by Heaney [5] and later developed by Huguet [6] and Bismuth [7]. The main drawbacks of TVE are the low hepatic tolerance to warm ischemia, the splanchnic venous congestion and the hemodynamic instability, based on the marked reduction of venous return and decrease of cardiac output [28]. TVE could be safely applied for up to 90 min [6] in healthy livers and up to 60 min in cirrhotic livers [29]. According to the literature, TVE is not tolerated in around 15% of cases, despite adequate fluid loading [30]. However, up until now, we have not encountered this issue. There are variations to the standard TVE, like the two-step vascular exclusion and vascular exclusion without caval clamping, that avoid some of the drawbacks of TVE, but their application is not always possible [13].
Hypothermic perfusion of the liver [31] diminishes the ischemia–reperfusion injury and allows for extension of the TVE, for more than 60 min [32]. The VVB, on the other hand, enables the surgery to be conducted under stable haemodynamics. It was introduced into practice in relation to liver transplantation, although its use today is not routine [33]. It is associated with increased operative time, and risk of some potentially lethal complications [34]. However, some of the largest series report no major morbidity, related to the use of VVB [9, 14, 15, 17, 35]. In a recent study, Soubrane et al. [20] compared perioperative outcomes of patients, undergoing liver resection, according to the use of VVB. The operative time in the VVB-group was longer, but with less blood loss. The postoperative morbidity and mortality between the two groups were comparable, although TVE without VVB was associated with significantly higher rate of respiratory complications (64% vs 15%).
Nevertheless, it is our opinion that the increased technical complexity of VVB and its infrequent application in the present time may account for increased rate of complications, especially in the newer generation of surgeons. In our series, we have never applied cold perfusion or VVB. Our approach consists of maximal dissection of the liver from IVC as possible. Liver transection is initiated under intermittent Pringle manoeuvre. As much of the liver parenchyma is transected as possible, before initiating TVE. This approach is similar to others, reported in the literature [16, 36,37,38]. Supplimentary Table 6 summarizes some of the largest series of hepatectomy with IVC reconstruction. Our results, without the use of VVB or cold perfusion, fit well within the range of reported morbidity and mortality. Appropriate planning during the dissection phase enables reduction of clamping times. The use of the two-surgeon technique is helpful in further reducing the time for liver transection [39]. In our series, the maximal duration of TVE was 57 min. Based on this experience, TVE could be maintained under 60 min in most of the cases and thus, executed safely, without the use of VVB or hypothermic perfusion.
Some small IVC defects can be repaired using lateral venorrhaphy or transverse suture, as described by Machado [40]. Larger defects require the use of patches or even replacement of IVC. Various materials are described in the literature, such as allografts, autologous grafts [41], Dacron [10] or ePTFE [42]. Cold-stored vein allografts have been described mainly in portal vein reconstruction [21]. We have used preserved deceased donor iliac veins for IVC reconstruction in two patients – one of which included in the present series. Peritoneal patches seem appealing for reconstruction of caval defects, mainly because they are available in the same operative field, they are cost-effective, the peritoneum is more resistant to infection, compared to synthetic grafts and its mesothelial lining is nonthrombogenic [43, 44]. A recent report from the Beaujon group described a series of six patients with anterolateral IVC resection and reconstruction with peritoneal patch [45]. We have used similar technique for portal vein reconstruction in pancreatoduodenectomy, but we have never applied it for reconstruction of caval defects. Currently, our material of choice for IVC replacement is 20 mm ring-reinforced ePTFE-graft. The main concerns for synthetic grafts are the long-term patency and the risk of infection. Surprisingly, most of the series have not reported on graft infection and during our search, we managed to identify only single cases [17, 46].
There is no consensus, regarding postoperative and long-term anticoagulation. Some advocate continuous heparin infusion [12, 13], while others use low-molecular weight heparins (LMWH) [14, 15]. Techniques to increase the long-term patency, such as construction of arteriovenous fistula or using smaller graft diameter have also been described [47]. Initially, when we performed patch or tube-graft IVC reconstruction, we used heparin infusion, maintaining partial thromboplastin time 1.5–2 × the normal range. After the fourth day, we substituted with LMWH, up to 30 days after discharge. In our opinion, the continuous heparin infusion increases the risk of postoperative bleeding, without any evidence of reducing the thrombotic rate. This is especially true in the context of deranged coagulation, accompanying major liver resections. We had to interrupt the infusion in two patients, because of postoperative bleeding. While in the first patient it was managed conservatively, the second required surgical haemostasis. Currently, we are administering LMWH 1.5–2 times the prophylactic dose for the patient bodyweight and on discharge, we are substituting with oral factor Xa-inhibitors.
There is an accumulation of reports in the literature, regarding the feasibility of retrohepatic IVC resection and reconstruction [9,10,11,12,13,14,15,16,17]. Our data confirm tumour clearance could be achieved with acceptable morbidity and mortality, comparable with other series. It is important to stress that patients undergoing this surgery have advanced oncologic disease and are not optimal candidates for surgical management. Selection of the patients may explain the difference in long-term survival in different series, more than perioperative management, but such comparison is beyond the scope of this article. Shortcomings of this study include its retrospective nature, the incomplete long-term follow-up and the wide variety of patients, that do not allow more detailed analysis.
References
Cady B (1983) Natural history of primary and secondary tumors of the liver. Semin Oncol 10(2):127–134
Rougier P, Milan C, Lazorthes F et al (1995) Prospective study of prognostic factors in patients with unresected hepatic metastases from colorectal cancer Fondation Francaise de Cancerologie Digestive. Br J Surg 82(10):1397–400
Mingoli A, Cavallaro A, Sapienza P et al (1996) International registry of inferior vena cava leiomyosarcoma: analysis of a world series on 218 patients. Anticancer Res 16(5b):3201–3205
Pringle JH (1908) Notes on the arrest of hepatic hemorrhage due to trauma. Ann Surg 48(4):541–9
Heaney JP, Stanton WK, Halbert DS et al (1966) An improved technic for vascular isolation of the liver: experimental study and case reports. Ann Surg 163(2):237–241
Huguet C, Nordlinger B, Galopin JJ et al (1978) Normothermic hepatic vascular exclusion for extensive hepatectomy. Surg Gynecol Obstet 147(5):689–693
Bismuth H, Castaing D, Garden OJ (1989) Major hepatic resection under total vascular exclusion. Ann Surg 210(1):13–19
Hannoun L, Panis Y, Balladur P et al (1991) Ex-situ in-vivo liver surgery. Lancet 337(8757):1616–1617
Miyazaki M, Ito H, Nakagawa K et al (1999) Aggressive surgical resection for hepatic metastases involving the inferior vena cava. Am J Surg 177(4):294–298
Madariaga JR, Fung J, Gutierrez J et al (2000) Liver resection combined with excision of vena cava. J Am Coll Surg 191(3):244–250
Okada Y, Nagino M, Kamiya J et al (2003) Diagnosis and treatment of inferior vena caval invasion by hepatic cancer. World J Surg 27(6):689–694
Arii S, Teramoto K, Kawamura T et al (2003) Significance of hepatic resection combined with inferior vena cava resection and its reconstruction with expanded polytetrafluoroethylene for treatment of liver tumors. J Am Coll Surg 196(2):243–249
Azoulay D, Andreani P, Maggi U et al (2006) Combined liver resection and reconstruction of the supra-renal vena cava: the Paul Brousse experience. Ann Surg 244(1):80–88
Malde DJ, Khan A, Prasad KR et al (2011) Inferior vena cava resection with hepatectomy: challenging but justified. HPB (Oxford) 13(11):802–810
Hemming AW, Mekeel KL, Zendejas I et al (2013) Resection of the liver and inferior vena cava for hepatic malignancy. J Am Coll Surg 217(1):115–24 (Discussion 124-5)
Nardo B, Ercolani G, Montalti R et al (2005) Hepatic resection for primary or secondary malignancies with involvement of the inferior vena cava: is this operation safe or hazardous? J Am Coll Surg 201(5):671–679
Bower TC, Nagorney DM, Cherry KJ Jr et al (2000) Replacement of the inferior vena cava for malignancy: an update. J Vasc Surg 31(2):270–281
Fiore M, Colombo C, Locati P et al (2012) Surgical technique, morbidity, and outcome of primary retroperitoneal sarcoma involving inferior vena cava. Ann Surg Oncol 19(2):511–518
Sweeney P, Wood CG, Pisters LL et al (2003) Surgical management of renal cell carcinoma associated with complex inferior vena caval thrombi. Urol Oncol 21(5):327–333
Navez J, Cauchy F, Dokmak S et al (2019) Complex liver resection under hepatic vascular exclusion and hypothermic perfusion with versus without veno-venous bypass: a comparative study. HPB (Oxford) 21(9):1131–1138
Azoulay D, Pascal G, Salloum C et al (2013) Vascular reconstruction combined with liver resection for malignant tumours. Br J Surg 100(13):1764–1775
Vladov NN, Mihaylov VI, Belev NV et al (2012) Resection and reconstruction of the inferior vena cava for neoplasms. World J Gastrointest Surg 4(4):96–101
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Vasilevski I, Takorov I, Mihaylov V et al (2019) Surgical outcomes and prognostic factors for survival for colorectal liver metastases: a 10-years single center experience. HPB 21:S800–S801
Isenberg J, Fischbach R, Kruger I et al (1996) Treatment of liver metastases from colorectal cancer. Anticancer Res 16(3a):1291–1295
Maeba T, Okano K, Mori S et al (2000) Extent of pathologic invasion of the inferior vena cava in resected liver cancer compared with possible caval invasion diagnosed by preoperative images. J Hepatobiliary Pancreat Surg 7(3):299–305
Hashimoto T, Minagawa M, Aoki T et al (2008) Caval invasion by liver tumor is limited. J Am Coll Surg 207(3):383–392
Eyraud D, Richard O, Borie DC et al (2002) Hemodynamic and hormonal responses to the sudden interruption of caval flow: insights from a prospective study of hepatic vascular exclusion during major liver resections. Anesth Analg 95(5):1173–8 (Table of contents)
Hannoun L, Delriviere L, Gibbs P et al (1996) Major extended hepatic resections in diseased livers using hypothermic protection: preliminary results from the first 12 patients treated with this new technique. J Am Coll Surg 183(6):597–605
Delva E, Barberousse JP, Nordlinger B et al (1984) Hemodynamic and biochemical monitoring during major liver resection with use of hepatic vascular exclusion. Surgery 95(3):309–318
Fortner JG, Shiu MH, Kinne DW et al (1974) Major hepatic resection using vascular isolation and hypothermic perfusion. Ann Surg 180(4):644–652
Azoulay D, Eshkenazy R, Andreani P et al (2005) In situ hypothermic perfusion of the liver versus standard total vascular exclusion for complex liver resection. Ann Surg 241(2):277–285
Hoffmann K, Weigand MA, Hillebrand N et al (2009) Is veno-venous bypass still needed during liver transplantation? A review of the literature. Clin Transplant 23(1):1–8
Budd JM, Isaac JL, Bennett J et al (2001) Morbidity and mortality associated with large-bore percutaneous venovenous bypass cannulation for 312 orthotopic liver transplantations. Liver Transpl 7(4):359–362
Azoulay D, Lim C, Salloum C et al (2015) Complex Liver Resection Using Standard Total Vascular Exclusion, Venovenous Bypass, and In Situ Hypothermic Portal Perfusion: An Audit of 77 Consecutive Cases. Ann Surg 262(1):93–104
Stattner S, Yip V, Jones RP et al (2014) Liver resection with concomitant inferior vena cava resection: experiences without veno-venous bypass. Surg Today 44(6):1063–1071
Torzilli G, Makuuchi M, Midorikawa Y et al (2001) Liver resection without total vascular exclusion: hazardous or beneficial? An analysis of our experience. Ann Surg 233(2):167–175
Oldhafer F, Ringe KI, Timrott K et al (2018) Modified ante situm liver resection without use of cold perfusion nor veno-venous bypass for treatment of hepatic lesions infiltrating the hepatocaval confluence. Langenbecks Arch Surg 403(3):379–386
Cauchy F, Brustia R, Perdigao F et al (2016) In Situ Hypothermic Perfusion of the Liver for Complex Hepatic Resection: Surgical Refinements. World J Surg 40(6):1448–1453
Machado MA, Herman P, Bacchella T et al (2007) Resection and reconstruction of retrohepatic vena cava without venous graft during major hepatectomies. J Surg Oncol 96(1):73–76
Miller CM, Schwartz ME, Nishizaki T (1991) Combined hepatic and vena caval resection with autogenous caval graft replacement. Arch Surg 126(1):106–108
Dale WA, Harris J, Terry RB (1984) Polytetrafluoroethylene reconstruction of the inferior vena cava. Surgery 95(5):625–630
Dokmak S, Aussilhou B, Sauvanet A et al (2015) Parietal Peritoneum as an Autologous Substitute for Venous Reconstruction in Hepatopancreatobiliary Surgery. Ann Surg 262(2):366–371
Pulitano C, Crawford M, Ho P et al (2013) Autogenous peritoneo-fascial graft: a versatile and inexpensive technique for repair of inferior vena cava. J Surg Oncol 107(8):871–872
Hobeika C, Cauchy F, Soubrane O (2020) Case series of extended liver resection associated with inferior vena cava reconstruction using peritoneal patch. Int J Surg 80:6–11
Addeo P, Rosso E, Oussoultzoglou E et al (2012) Inferior vena cava graft-enteric fistula after extended hepatectomy with caval replacement. J Vasc Surg 55(1):226–229
Gloviczki P, Hollier LH, Dewanjee MK et al (1984) Experimental replacement of the inferior vena cava: factors affecting patency. Surgery 95(6):657–666
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Nikola Vladov was involved in study concept and design; Maria Yakova, Tsvetan Trichkov contributed to acquisition of data ; Radoslav Kostadinov, Vassil Mihaylov, Evelina Odisseeva were involved in analysis and interpretation of data; Radoslav Kostadinov, Ivelin Takorov contributed to drafting of manuscript; Tsonka Lukanova, Ventsislav Mutafchiyski, Nikola Vladov were involved in critical revision of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical approval was waived by the local Ethics Committee of Military Medical Academy—Sofia given the retrospective nature of the study and all the procedures being performed were part of the routine care.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vladov, N., Kostadinov, R., Mihaylov, V. et al. Single-Centre Experience of Supra-Renal Vena Cava Resection and Reconstruction. World J Surg 45, 2270–2279 (2021). https://doi.org/10.1007/s00268-021-06048-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-021-06048-w