Abstract
Background
Omentectomy is considered an essential part of curative gastrectomy for locally advanced gastric cancer (GC), albeit without solid evidence. We conducted a randomized phase II trial (the TOP-G trial) comparing omentectomy and omentum preservation for gastric cancer. This report describes the short-term findings regarding the trial’s secondary endpoints.
Methods
The trial protocol was submitted to the University Hospital Medical Information Network Clinical Trials Registry (http://www.umin.ac.jp/ctr/: UMIN000005421). The key eligibility criteria were histologically confirmed cT2–4a and N0–2 gastric adenocarcinoma. Short-term surgical outcomes, including morbidity and mortality, were compared between the omentectomy group (group A, control arm) and the omentum-preserving surgery group (group B, test arm). All procedures were performed via an open approach. Based on a non-inferiority margin of 7%, statistical power of 0.7, and type I error of 0.2, the sample size was set to 250 patients.
Results
A total of 251 patients were eligible and randomized (group A: 125 patients, group B: 126 patients) between April 2011 and October 2018. After excluding patients who had peritoneal metastasis or laparotomy history, safety outcomes were analyzed for 247 patients. Group A had a significantly longer median operation time (225 min vs. 204 min, p = 0.022) and tended to have greater median blood loss (260 mL vs. 210 mL p = 0.073). The incidences of morbidity were similar and < 10% in both groups (8% vs. 9%, p = 1.000). There was no mortality in either group.
Conclusions
Operative risk was generally similar between omentectomy and omentum-preserving surgery for locally advanced gastric cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is a leading cause of cancer-related death [1], and surgical treatment is key to curing localized GC. Radical surgery for gastrointestinal cancer has two theoretical goals: en bloc resection of the mesothelium, including the vessels and regional lymph nodes, and complete resection of the localized tumor. However, the stomach has unique mesothelial characteristics. For example, the stomach rotates during its embryological formation, the greater curvature becomes prolonged with the visceral mesothelium, and the omentum and bursa omentalis are formed. The omentum consists of three parts. The first part is the membranes close to the stomach’s greater curvature, including the right and left epiploic vessels and lymph nodes. The second part is the membranes adjacent to the first part that hang over the transverse colon, which are filled with fat tissues and blood/lymphatic vessels. This second part is often narrowly referred to as the omentum. The third part is the membrane covering the bursa omentalis. All three parts should theoretically be resected to achieve en bloc resection and cure GC. This is because the omentum is a common site of peritoneal metastasis, and animal models have indicated that cancer cells implanted into the abdominal cavity aggregate to openings that connect the omental lymphatic system to the peritoneal cavity [2, 3]. Furthermore, omental micrometastasis can occasionally be detected in patients with GC using special staining or reverse transcription polymerase chain reaction [3, 4]. Therefore, if peritoneal metastasis is localized to the omentum as micrometastasis in some patients, it may be important to resect all three parts of the omentum. The control of peritoneal metastasis is important for survival in advanced gastric cancer treatment [5, 6].
Based on these concepts, surgeons have completely resected the omentum in locally advanced GC cases [7]. However, a recent large phase III trial (JCOG1001) confirmed that bursectomy, which involves complete resection of the membrane covering the bursa omentalis (i.e., the third omentum part), did not improve survival relative to non-bursectomy [8]. Thus, en bloc resection of the entire gastric mesothelium was not considered necessary. Nevertheless, resection of the first omentum part (near the greater curvature) is essential for dissecting lymph nodes closed to the stomach, although it remains unclear whether the second part should be resected (the membranes that hang over the transverse colon). For the purpose of this report, we define resection of the second part as “omentectomy” and preservation of the second part as “omentum preservation.” It is unlikely that peritoneal metastasis would be limited to the second omentum part, as peritoneal metastasis is a systemic disease. Furthermore, some retrospective studies have demonstrated that omentectomy increased the operation time, blood loss, and surgical morbidity, relative to preservation of the second omentum part [9,10,11,12], although retrospective studies involve significant bias. Moreover, accurate estimation of risks and benefits is essential for calculating the sample size for a large phase III study to compare omentectomy and omentum-preserving surgery for GC. Therefore, we conducted a multicenter screening randomized phase II trial (the TOP-G trial) to compare omentectomy and omentum preservation [13]. This report describes the short-term findings regarding the trial’s secondary endpoints.
Materials and methods
Ethical considerations
The study protocol was approved by the institutional review boards at all participating institutions. The study procedures complied with the recommendations in the Declaration of Helsinki, the Ethical Guideline for Medical and Health Research Involving Human Subjects, and the guidelines of the responsible Japanese governmental agency. All patients were required to provide written informed before enrollment. The trial protocol was registered with the University Hospital Medical Information Network Clinical Trials Registry (http://www.umin.ac.jp/ctr/: UMIN000005421).
Study design and endpoints
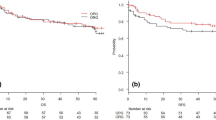
This phase II randomized controlled trial was designed to compare two surgical techniques: gastrectomy with omentectomy and omentum-preserving gastrectomy. Both procedures were performed via laparotomy, and the extent of nodal dissection was determined according to the Japanese Gastric Cancer Treatment Guidelines [14]. The primary endpoint was relapse-free survival (RFS), and the secondary endpoints were overall survival, blood loss, operation time, and surgical morbidity. Randomization and data handling for this study were performed by the Yokohama City University Center for Novel and Exploratory Clinical Trials (Y-NEXT).
Eligibility
The first preoperative eligibility criteria were histologically confirmed adenocarcinoma of stomach; clinical stage T2(MP)–T4a(SE), N0–N2, and M0 according to the Japanese Classification of Gastric Carcinoma [15]; an indication for R0 surgery; preoperative macroscopic type 0–3 or 5 disease; age of ≥ 20 years, Eastern Cooperative Oncology Group performance status of 0–2; and sufficient organ functions. The exclusion criteria were previous chemotherapy for GC, history of abdominal surgery except appendectomy and laparoscopic cholecystectomy, unstable angina or myocardial infarction within 6 months of registration, uncontrolled diabetes mellitus, respiratory disease requiring oxygen therapy, history of allergy or hypersensitivity to any drug, presence of acute inflammation, pregnancy, synchronous or metachronous (within 5 years) malignancy, and psychiatric disease that required treatment. After laparotomy and before initiating gastrectomy, the surgeons evaluated whether the patient fulfilled the second inclusion criteria: T2(MP)–T4a(SE) disease, possible macroscopic curative resection including R1 surgery based on positive peritoneal lavage cytology (CY1), possibility of achieving total omentectomy, and not requiring thoracotomy.
Randomization
After confirming the patient fulfilled the second eligibility criteria, the surgeons communicated this fact to the Y-NEXT via telephone, and the Y-NEXT immediately provided a randomized assignment (1:1) to the omentectomy group (group A) or the omentum preservation group (group B). The groups were balanced according to institution, T status (T2–3 vs. T4a), and type of gastrectomy (distal vs. total). Interventions were not masked for the patients or investigators.
Surgical methods
The operative approach was limited to open surgery and laparoscopic surgery was not allowed.
Group A (total omentectomy) was treated using gastrectomy, D2 lymphadenectomy, and total omentectomy without bursectomy. Depending on the primary tumor’s location, the surgeon performed total or distal gastrectomy. The spleen was removed to dissect the splenic hilar lymph nodes as part of total gastrectomy according to the Japanese gastric cancer treatment guidelines (version 3, 2010) from when the study was designed [14]. However, after publication of the results from the phase III JCOG0110 trial, which evaluated splenectomy during total gastrectomy, the spleen was preserved for upper GC that did not invade the greater curvature [16].
Group B (omentum preservation) was treated using gastrectomy and D2 lymphadenectomy. The first part of the omentum was dissected at 3 cm below the right and left gastroepiploic arteries, with the preservation of the second part of the omentum.
The type of reconstruction was not specified in the study protocol, and the protocol treatment was considered completed when the surgery was finished. The protocol treatment was also terminated when apparent distant metastasis (M1) or direct invasion of the adjacent organs (T4b) was detected after enrollment. Any patients who were diagnosed with pathological stage II/III disease (except T1N2–3 or T3N0) were recommended to receive oral S-1 for 12 months as adjuvant therapy. To ensure surgical quality, only surgeons who had performed ≥ 50 gastrectomies were allowed to participate. Compliance with the assigned omentectomy procedure was validated based on case report forms.
Surgical outcomes
Operative techniques and pathology results were recorded according to the Japanese Gastric Cancer Treatment Guidelines and the Japanese Classification of Gastric Carcinoma (14th edition) [14, 15]. Postoperative complications that occurred during the hospitalization or within 30 days after surgery were evaluated according to the Clavien–Dindo classification. Morbidities that were considered grade ≥ 3 were noted on the case report form [17]. The operation time, blood loss, and blood transfusion requirement values were also recorded.
Sample size
The sample size was calculated based on a hypothesis that the expected 3-year RFS rate was 70% for both groups. Using a non-inferiority margin of 7% for the 3-year RFS rate (the rate for group B being 7% below that for group A), the total sample size to provide statistical power of 0.7 and type I error of 0.2 was calculated to be 245 patients. However, given that a few cases would inevitably be considered ineligible for registration, the number of patients was set to 250.
Statistical analysis
Morbidity and mortality rates were evaluated in the safety cohort, which was created by excluding ineligible cases from the intention-to-treatment cohort. Differences in those outcomes were evaluated using Fisher’s exact test. Differences in length of hospital stay and blood loss were evaluated using the Wilcoxon test. All P-values were two-sided, and the statistical analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC).
Results
Patients
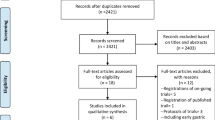
Recruitment opened in April 2011 and closed in October 2018. The CONSORT diagram is shown in Fig. 1. At the first registration, 275 patients were enrolled although 8 patients were initially deemed ineligible and 16 patients were excluded because they did not fulfill the second eligibility criteria (generally because T4b tumors or peritoneal metastasis were found during surgery). Thus, 251 patients were enrolled at the second registration, although 4 patients were judged to be ineligible after the second registration. One patient had peritoneal metastasis that was found during gastrectomy and 3 patients had severe adhesions, which were possibly related to an unidentified history of laparotomy. The safety cohort included 247 patients (group A: 122 patients and group B: 125 patients). The patient and tumor characteristics were generally well balanced between the two groups, with the exception of age (Table 1).
Surgery
The operative details are shown in Table 2. Violation of the protocol treatment was not identified in either group. Although the proportion of total gastrectomy was almost balanced between the two groups, splenectomy was slightly more common in group B. Relative to group B, group A had a significantly longer median operation time (+ 21 min) and tended to have greater median blood loss (+ 50 mL). The number of retrieved nodes was similar in both groups.
Operative morbidity and mortality
One patient in group B experienced an intraoperative spleen injury that resulted in unplanned splenectomy. The postoperative morbidity rates were similar and < 10% in both groups (Table 3). Anastomotic leakage tended to be more common in group B than in group A (5% vs. 2%). Pancreatic fistula only occurred in group A (2%). Two patients in group B experienced postoperative bleeding, which involved the gastroduodenal artery in one case and an unidentified source of bleeding in the second case. Mortality was not identified in either group.
Pathology findings
There were no apparent differences in the pathology findings for the two groups (Table 4).
Discussion
To the best of our knowledge, this is the first randomized study to compare omentectomy and omentum preservation for locally advanced gastric cancer. Our hypothesis was that overall survival would be similar, but operative risk would be reduced by omentum preservation. However, we only identified small differences in operation time and blood loss, with similar overall morbidities in the two groups. These results suggested that operative risk was fairly similar between omentum-preserving surgery and omentectomy.
We observed that omentum preservation was associated with approximately 20 min shorter operation times and 50 mL less blood loss, although it was also associated with a slightly higher rate of splenectomy. The differences in operation time and blood loss might be emphasized if the splenectomy rates were similar in the two arms. Hasegawa et al. have reported that omentum preservation was associated with approximately 80 min shorter operation times and 170 mL less blood loss, based on a retrospective propensity score-matched analysis [10]. Ri et al. also reported that omentum preservation was associated with 38 mL less blood loss, albeit with similar operation times [12]. This may be because the omentum contains numerous blood and lymphatic vessels that are covered with fat tissue, which forces the surgeon to carefully dissect these tissues if they wish to avoid bleeding while preserving the omentum. A manual dissection may be time-consuming, although surgeon workload and operation time might be improved by using new vessel sealing devices or ultrasonic cutting and coagulation devices. Previous studies have indicated that these devices are useful for dissecting the tissues while minimizing related bleeding [18, 19]. In contrast, omentectomy does not involve cutting large vessels but does require the surgeon to carefully dissect the membrane’s attachment to the transverse colon. Thus, the new devices may not be preferable to traditional scissors or an electric scalpel. Nevertheless, care is still needed, as sharp dissection may induce bleeding from small regional blood vessels.
The present study revealed that omentectomy and omentum-preserving surgery had similar morbidity rates, although splenectomy was more common in the omentum preservation group. Although splenectomy is a well-known risk factor for morbidity, especially for pancreatic fistula, we observed that pancreatic fistula and abdominal abscess were more common in the omentectomy group. Thus, preserving the omentum may prevent pancreatic fistula or abdominal abscess, as the omentum theoretically prevents the spread of local inflammation to the whole abdomen. A multicenter cohort study has also indicated that the rates of pancreatic fistula and abdominal abscess were 5.7% among patients who underwent omentectomy but only 3.0% among patients who underwent omentum-preserving surgery [12]. Anastomotic leakage was slightly more common in the omentum preservation group, although the rates were < 5% in both groups and not significantly different. The preserved omentum might interfere with movement of the ante-colic jejunum to meet the esophagus, and surgeons must also avoid placing excessive tension on the jejunum in order to minimize the risk of anastomotic leakage. Nevertheless, it is unclear whether the increased rate of anastomotic leakage was related to chance or the omentum preservation.
The morbidity rate was 8% in our omentectomy group, which is slightly lower than the rate from the phase III JCOG1001 trial (bursectomy vs. non-bursectomy). In that trial, the non-bursectomy arm underwent the same procedure as our omentectomy group and the incidence of grade 3–4 morbidities was 11% [8]. However, the non-bursectomy arm of the JCOG1001 trial had a higher rate of splenectomy (27%) than in our trial (6% in group A) [8]. Our morbidity rate for omentectomy is also slightly lower than the rate from the phase III JCOG0110 trial (splenectomy vs. spleen-preserving surgery). The morbidity rate was 16.7% in the spleen-preservation arm of that trial [16], although all of the patients in the JCOG0110 trial underwent total gastrectomy. Thus, our overall morbidity rate of 8% after omentectomy seems reasonable.
Open surgery remains the standard approach for advanced GC. However, omentum preservation would be useful during laparoscopic surgery, where omentectomy can be complicated, time-consuming, and hazardous. A previous report comparing omentectomy and omentum preservation during laparoscopic surgery revealed that omentum preservation shortened the mean operation time by 15 min, and only omentectomy was associated with organ injury [11].
Our study has two important limitations. First, a small phase II trial cannot definitively confirm the safety and efficacy of omentum preservation. Our findings suggest that the risks of omentectomy and omentum preservation were generally similar, although the slight imbalance in the splenectomy rates might lead to underestimation of the risk associated with omentum preservation. Second, we did not consider long-term morbidities, such as bowel obstruction, although omentum preservation would theoretically reduce intestinal adhesions that could lead to bowel obstruction.
Conclusions
The present study revealed that the operative risks were generally similar between omentum-preserving surgery and omentectomy for locally advanced GC.
References
Ferlay J, Soerjomataram I, Dikshit R et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–E586
Oosterling SJ, van der Bij GJ, Bogels M et al (2006) Insufficient ability of omental milky spots to prevent peritoneal tumor outgrowth supports omentectomy in minimal residual disease. Cancer Immunol Immunother 55:1043–1051
Hagiwara A, Takahashi T, Sawai K et al (1993) Milky spots as the implantation site for malignant cells in peritoneal dissemination in mice. Cancer Res 53:687–692
Kodera Y, Nakanishi H, Ito S et al (2002) Quantitative detection of disseminated cancer cells in the greater omentum of gastric carcinoma patients with real-time RT-PCR: a comparison with peritoneal lavage cytology. Gastric Cancer 5:69–76
Hagiwara A, Sawai K, Sakakura C et al (1998) Complete omentectomy and extensive lymphadenectomy with gastrectomy improves the survival of gastric cancer patients with metastases in the adjacent peritoneum. Hepatogastroenterology 45:1922–1929
Etoh T, Sasako M, Ishikawa K et al (2006) Extranodal metastasis is an indicator of poor prognosis in patients with gastric carcinoma. Br J Surg 93:369–373
Groves EW (1910) On the radical operation for cancer of the pylorus: with especial reference to the advantages of the two-stage operation and to the question of the removal of the associated lymphatics. Br Med J 1:366–370
Kurokawa Y, Doki Y, Mizusawa J et al (2018) Bursectomy versus omentectomy alone for resectable gastric cancer (JCOG1001): a phase 3, open-label, randomised controlled trial. Lancet Gastroenterol Hepatol 3:460–468
Ha TK, An JY, Youn HG et al (2008) Omentum-preserving gastrectomy for early gastric cancer. World J Surg 32:1703–1708
Hasegawa S, Kunisaki C, Ono H et al (2013) Omentum-preserving gastrectomy for advanced gastric cancer: a propensity-matched retrospective cohort study. Gastric Cancer 16:383–388
Kim DJ, Lee JH, Kim W (2014) A comparison of total versus partial omentectomy for advanced gastric cancer in laparoscopic gastrectomy. World J Surg Oncol 12:64
Ri M, Nunobe S, Honda M et al (2020) Gastrectomy with or without omentectomy for cT3-4 gastric cancer: a multicentre cohort study. Br J Surg. https://doi.org/10.1002/bjs.11702
Hasegawa S, Yamamoto Y, Taguri M et al (2013) A randomized phase II trial of omentum-preserving gastrectomy for advanced gastric cancer. Jpn J Clin Oncol 43:214–216
Japanese Gastric Cancer Association (2017) Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 20:1–19
Japanese Gastric Cancer Association (2011) Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14: 101–112
Sano T, Sasako M, Mizusawa J et al (2017) Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma. Ann Surg 265:277–283
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Inoue K, Nakane Y, Michiura T et al (2012) Ultrasonic scalpel for gastric cancer surgery: a prospective randomized study. J Gastrointest Surg 16:1840–1846
Cheng H, Hsiao CW, Clymer JW et al (2015) Gastrectomy and D2 lymphadenectomy for gastric cancer: a meta-analysis comparing the harmonic scalpel to conventional techniques. Int J Surg Oncol 2015:397260
Acknowledgements
We thank all of the patients, their families, the investigators, and medical staff members who participated in this study.
Author information
Authors and Affiliations
Contributions
SH, TY, and TY conceived of and designed the trial. HM and TY were the principal investigators. MT, HM, TY, and TY were responsible for the data analysis. All authors contributed to the data acquisition and interpretation. HM, TY, and TY draft the article. All authors contributed to the writing of the final manuscript and approved the final version. All authors agree to be accountable for all aspects of the work and to ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding authors
Ethics declarations
Conflict of interest
Dr. Yamada reports personal fees from ONO Pharmaceutical and personal fees from Bristol-Myers Squibb, outside the submitted work. Dr. Yamanaka reports grants and personal fees from Takeda, grants and personal fees from Chugai, grants and personal fees from Boehringer Ingelheim, grants and personal fees from Taiho, grants and personal fees from Daiichi-Sankyo, grants from Ono, grants and personal fees from Bayer, grants from Merck Serono, grants from Astellas, grants from Eli Lilly, personal fees from Pfizer, personal fees from Sysmex, personal fees from Huya Biosciences, and personal fees from Gilead Sciences, outside the submitted work. Dr. Rino reports personal fees from Taiho pharmaceutical, personal fees from Abbott, personal fees from Asahi Kasei, personal fees from Daiichi-Sankyo, personal fees from Tsumura & Co, personal fees from Covidien, personal fees from Zeria pharmaceutical, personal fees from EA Pharma, personal fees from Johnson and Johnson, personal fees from Otsuka, other from Lilly, other from Bristol-Myers Squibb, other from Daiichi-Sankyo, other from Johnson and Johnson, other from Otsuka, and other from Taiho pharmaceutical, outside the submitted work. Dr. Oshima reports grants from Taiho pharmaceutical Co., Ltd, grants from Chugai pharmaceutical Co., Ltd, grants from Ono pharmaceutical Co., Ltd, grants from Daiichi-Sankyo pharmaceutical, grants from Nippon Kayaku Co., Ltd, grants from Eli Lilly Japan K. K., personal fees from Nippon Kayaku Co., Ltd, personal fees from Ono pharmaceutical Co., Ltd, personal fees from Bristol-Myers Squibb K. K., personal fees from Taiho pharmaceutical Co., Ltd, personal fees from Chugai pharmaceutical Co., Ltd, and personal fees from Eli Lilly Japan K. K., outside the submitted work. Dr. Yoshikawa reports personal fees from MSD, personal fees from BMS, personal fees from ONO, personal fees from Taiho, personal fees from Chugai, personal fees from Nihon Kayaku, grants and personal fees from Lilly, personal fees from Pfizer, personal fees from TERUMO, personal fees from Johnson and Johnson, and personal fees from Covidien, outside the submitted work; All the remaining authors have no conflicts of interest to declare.
Ethical approval
This trial was registered with the University Hospital Medical Information Network Clinical Trials Registry (http://www.umin.ac.jp/ctr/: UMIN000005421) and was approved by the ethical review boards of the participating institutions. All patients provided informed consent before enrollment.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Murakami, H., Yamada, T., Taguri, M. et al. Short-Term Outcomes from a Randomized Screening Phase II Non-inferiority Trial Comparing Omentectomy and Omentum Preservation for Locally Advanced Gastric Cancer: the TOP-G Trial. World J Surg 45, 1803–1811 (2021). https://doi.org/10.1007/s00268-021-05988-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-021-05988-7