Abstract
Background
Extended-spectrum β-lactamase-producing Enterobacteriaceae (ESBL-PE) are increasing in globally. The aim of this study was to compare community-acquired infections (CAIs) and hospital-acquired infections (HAIs) and determine the rate of third-generation cephalosporin resistance and ESBL-PE at a tertiary referral hospital in Rwanda.
Methods
This was a cross-sectional study of Rwandan acute care surgery patients with infection. Samples were processed for culture and susceptibility patterns using Kirby-Bauer disk diffusion method. Third-generation cephalosporin resistance and ESBL-PE were compared in patients with CAI versus HAI.
Results
Over 14 months, 220 samples were collected from 191 patients: 116 (62%) patients had CAI, 59 (32%) had HAI, and 12 (6%) had both CAI and HAI. Most (n = 178, 94%) patients were started on antibiotics with third-generation cephalosporins (ceftriaxone n = 109, 57%; cefotaxime n = 52, 27%) and metronidazole (n = 155, 81%) commonly given. Commonly isolated organisms included Escherichia coli (n = 62, 42%), Staphylococcus aureus (n = 27, 18%), and Klebsiella spp. (n = 22, 15%). Overall, 67 of 113 isolates tested had resistance to third-generation cephalosporins, with higher resistance seen in HAI compared with CAI (74% vs 46%, p value = 0.002). Overall, 47 of 89 (53%) isolates were ESBL-PE with higher rates in HAI compared with CAI (73% vs 38%, p value = 0.001).
Conclusions
There is broad and prolonged use of third-generation cephalosporins despite high resistance rates. ESBL-PE are high in Rwandan surgical patients with higher rates in HAI compared with CAIs. Infection prevention practices and antibiotic stewardship are critical to reduce infection rates with resistant organisms in a low-resource setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Surgical infections include both infectious conditions requiring surgery (e.g., appendicitis) and infectious complications of surgical procedures (e.g., surgical site infections) [1]. Surgical infections are managed by a combination of surgery and antibiotics. Gram-negative pathogens account for a substantial burden of surgical infections globally [1]. Common gram-negative pathogens implicated in surgical infections include Enterobacteriaceae such as Escherichia coli and Klebsiella spp.
Community-acquired infections (CAIs) are any infection occurring before hospital admission, while hospital-acquired infections (HAIs) are infections that develop during the hospitalization, often defined as occurring after 48 h of hospitalization. The most common HAI globally is surgical site infection [2]. Mortality rate associated with HAI is higher than with community-acquired infection with HAI hospital mortality rate of 19% compared with mortality rate in CAI of 11% [3, 4].
The incidence of HAI is increasing worldwide, especially in low- and middle-income countries (LMICs) with an average prevalence of 15.5% [5]. Rates of HAIs in intensive care units (ICU) are higher, with a prevalence of 35.2% in LMIC ICUs [5]. In the USA, there are an estimated 1.7 million cases of HAI with a mortality of 99,000 deaths annually [6]. HAI increases mortality rate and length of hospital stay and is a financial burden to patients and families [7].
Antimicrobial resistance (AMR) is a burden worldwide as it increases rate of morbidity and mortality and cost of treatment in patient with infection [8]. AMR is particularly challenging in LMICs where there are limited antibiotic options and minimal antibiotic stewardship. Factors contributing to AMR in LMICs are multifactorial, including misuse of antibiotics, poor antimicrobial stewardship, and environmental exposure to antibiotics [9, 10]. Gram-negative bacteria that produce extended-spectrum β-lactamase (ESBL)-enzymes are increasing in both CAI and HAI [11]. In a hospital-wide antibiogram in Rwanda, gram-negative pathogens were commonly isolated (89%) with a high rate of resistance to ceftriaxone (76%) and 72% were ESBL producers [12]. Despite the high rates of AMR, most patients are treated with third-generation cephalosporins and metronidazole [13].
Rwanda is a low-income country located in East Africa with a high density population [14]. Infections are common in surgical patients [15, 16]. However, there are limited data on prevalence of AMR in community- versus hospital-acquired infections [13]. The aim of this study was to compare CAI and HAI and determine the rate of third-generation cephalosporin resistance and ESBL production in surgery patients at a tertiary referral hospital in Rwanda.
Materials and methods
This study was conducted at University Teaching Hospital of Kigali (Centre Hospitalier Universitaire de Kigali, CHUK). CHUK is a tertiary referral hospital in Kigali, Rwanda, with a referral base of 6 million which has an acute care surgery (ACS) firm that treats emergency trauma and non-trauma surgical conditions [17].
This study was a prospective observational study of 14-month duration (August 2018–October 2019). Trained data collectors enrolled all ACS patients with a suspected infection and followed them until hospital discharge. Data were obtained from patients or caretakers, patient files, and electronic laboratory records. Patients were contacted via telephone at 30 days post-discharge for follow-up.
Samples were collected based on the suspected source of infection and included blood, urine, wound swabs. Wound cultures included intraoperative samples as well as samples collected on the surgical wards. For operative procedures, povidone is routinely used for preoperative skin disinfectant. For clean and clean-contaminated procedures, wounds are usually closed with interrupted nylon sutures and covered with a gauze dressing. For contaminated or infected procedures, wounds are commonly left open or packed with wet-to-dry gauze.
Specimens were processed in the hospital microbiology laboratory for culture and sensitivity per local hospital protocol, in accordance with Clinical and Laboratory Standards Institute (CLSI) guidelines [18]. Gram-positive specimens were plated on mannitol salt agar and blood agar. Gram-negative specimens were plated on MacConkey agar and xylose-lysine-deoxycholate agar. To process gram-positive specimens, we used catalase and coagulase tests. To process gram-negative specimens, we used colony morphology. To identify Enterobacteriaceae, we conducted biochemical testing with triple sugar iron, motility indole urea, and citrate tests.
For all isolated specimens, we determined the AMR patterns using standard Kirby–Bauer disk diffusion methods. To prepare a suspension from growth on a solid media plate, we added bacterial colonies to sterile distilled water until the turbidity approximated MacFarland turbidity standard 0.5. Then, we plated the suspension on Mueller–Hinton agar and added the appropriate antibiotic disks. We incubated the plates at 37 °C for 18–24 h. The following antibiotic disks were used: ampicillin, 10 μg; amikacin, 30 μg; amoxicillin/clavulanic acid, 20/10 μg; ceftazidime, 30 μg; cefotaxime, 30 μg; ceftriaxone, 30 μg; cefalothin, 30 μg; cefuroxime, 30 μg; ciprofloxacin, 5 μg; clindamycin, 2 μg; cloxacillin, 1 μg; erythromycin, 10 μg; gentamicin, 10 μg; imipenem, 10 μg; meropenem, 10 μg; penicillin, 10 units; piperacillin, 100 μg; tetracycline, 30 μg; chloramphenicol, 30 μg; trimethoprim/sulfamethoxazole, 1.25/23.75 μg; and vancomycin, 30 μg. Interpretation of the diameter of inhibition was done according to CLSI guidelines [18]. ESBL producers were identified based on diameters of inhibition using amoxicillin/clavulanic acid 20/20 g disks and ceftazidime 30 g and cefotaxime 30 g disks.
Data were collected on demographics, vital signs at admission, sepsis or septic shock, laboratory studies on hospital admission, prior surgery in 30 days, antibiotic use prior 30 days, duration of symptoms, comorbidities, surgeon, diagnosis, operation, duration of surgery, American Society of Anesthesiologist (ASA) score, intraoperative transfusion, microbiology, antibiotic management, surgical site infection, death, length of hospital stay, and 30-day follow-up post-discharge. Sepsis and septic shock were defined according to sepsis-3 definitions [19]. Antibiotic details included initial choice of antibiotic, duration, and any change to antibiotics.
Community-acquired infection (CAI) was defined as any sample collected within 48 h of hospital admission, while hospital-acquired infection (HAI) was defined as any sample collected more than 48 h after hospital admission. Third-generation cephalosporin resistance was defined as resistance to any one of the following: ceftriaxone, cefotaxime, or ceftazidime.
Data were entered in REDCAP and analyzed using STATA (version 13.0, College Station, TX) [20]. Categorical data were reported as frequencies and percentages, and continuous data were reported as median and interquartile range (IQR). Analysis of categorical variables was performed using Chi-square or Fisher’s exact test. Analysis of continuous variables was performed using Kruskal–Wallis rank test.
This study was approved by the University of Rwanda College of Medicine and Health Sciences institutional review board (IRB) (333/CMHS IRB/2017), CHUK Ethics Committee (EC/CHUK/484/2017), and University of Minnesota IRB (STUDY00001252). All patients provided informed consent prior to study enrollment.
Results
Patients
Over a period of 14 months, 220 samples were collected from 191 patients. Median age was 34 years (IQR: 24, 55), and most patients were male (n = 123, 64%) (Table 1). Most patients (n = 169, 88%) were transferred from another hospital. In the past 30 days, 43 (23%) of patients had taken antibiotics with the most common antibiotics being metronidazole (n = 24, 56%) and third-generation cephalosporins (n = 20, 47%). In the past 30 days, 11 (6%) patients had undergone surgery which included incision and drainage (n = 3), appendectomy (n = 2), hernia repair (n = 1), laparoscopy (n = 1), laparotomy (n = 1), Caesarean section (n = 1), hysterectomy (n = 1), and amputation (n = 1). Common diagnoses on hospital admission included soft tissue infection (n = 38, 20%) and appendicitis (n = 26, 14%). Most (n = 184, 96%) patients underwent an operation during hospitalization at CHUK.
Overall, 116 (62%) patients had CAI, 59 (32%) patients had HAI, and 12 (6%) had both CAI and HAI (Table 2). Demographic and clinical features were similar between patients with CAI and HAI. Common diagnoses in patients with CAI were soft tissue infection (n = 10, 17%), appendicitis (n = 20, 17%), abscess (n = 19, 16%), and peptic ulcer disease perforation (n = 17, 15%). Common diagnoses with patients with HAI were soft tissue infection (n = 20, 34%), blunt trauma (n = 9, 15%), abscess (n = 6, 10%), and other (n = 6, 10%). Median length of hospital stay differed between CAI and HAI (CAI: 8 days; HAI: 26 days; both: 43 days; p < 0.001).
Overall, 55 of 96 tested patients had an isolate with third-generation cephalosporin resistance. Median length of hospital stay in patients with third-generation cephalosporin resistance was longer than those without resistance (22 days IQR 11, 32 vs 14.5 days, IQR: 6, 41, p value = 0.145), but this was not statistically significant. There was no association between antibiotics in the past 30 days and development of third-generation cephalosporin resistance (p = 0.500). There was no association between having a cephalosporin in the past 30 days and third-generation cephalosporin resistance (p = 0.292).
Overall, 42 of 79 tested patients had isolates that were ESBL producers. The median length of hospital stay in patients with ESBL isolates was longer than in patients with non-ESBL isolates (24.5 days, IQR: 12, 37 vs 10 days, IQR: 6, 43; p value 0.092), but this was not statistically significant.
Most (n = 178, 94%) patients were started on antibiotics with third-generation cephalosporins (ceftriaxone n = 109, 57%; cefotaxime n = 52, 27%) and metronidazole (n = 155, 81%) commonly given (Table 3). Median duration of antibiotics was 6 days (IQR: 4, 7) with duration varying with diagnosis. Median duration of antibiotics in patients was 7 days (IQR: 4, 10) in patients with SSI compared with 5 days (IQR: 4, 7) in patients without SSI (p value = 0.011).
Antibiotics were changed in 71 (40%) patients with the antibiotics most common being changed to: ciprofloxacin (n = 22, 29%), third-generation cephalosporins (n = 20, 27%), metronidazole (n = 18, 24%), and cloxacillin (n = 16, 21%). The reasons for changing antibiotics were not recorded but included AMR resistance patterns, newly diagnosed infection, or antibiotics availability. Patients with SSI were more likely to have antibiotics changed compared to patients without SSI (67% vs 30%, p < 0.001). Change of antibiotics was not associated with third-generation cephalosporin resistance (p = 0.413) or ESBL status (p = 0.084).
Samples
Overall, 220 samples were collected from wound (n = 192, 87%), urine (n = 19, 9%), and blood (n = 9, 4%) (Table 4). In total, 128 (58%) specimens collected were CAI and 93 (42%) were HAI. Overall, 148 (67%) samples were positive for culture growth, with no difference in culture growth between CAI (n = 80, 63%) and HAI (n = 68, 73%) (p value = 0.114).
The most common isolated organisms were E. coli (n = 62, 42%), Staphylococcus aureus (n = 27, 18%), and Klebsiella spp. (n = 22, 15%) (Table 4). S. aureus was more common in CAI compared with HAI (24% vs 12%), whereas Acinetobacter (0% vs 9%), Proteus (3% vs 8%), and Pseudomonas (3% vs 11%) isolates were more common in HAI.
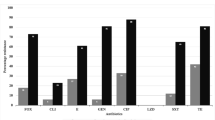
Overall, 67 of 113 isolates tested had resistance to third-generation cephalosporins, with higher resistance seen in HAI compared with CAI (74% vs 46%, p value = 0.002) (Table 5). ESBL-producing isolates were more common in HAI compared with CAI (73% vs 38%, p value = 0.001).
Among E. coli isolates, 36 of 62 (58%) were resistant to third-generation cephalosporins and 28 of 54 (52%) were ESBL producers (Table 6). In Klebsiella isolates, 14 of 21 (67%) were resistant to third-generation cephalosporins and 8 of 21 (38%) were ESBL producers. Rates of carbapenem resistance were relatively low in E. coli and Klebsiella isolates. Resistance to amikacin was identified in 2 of 47 (4%) E. coli isolates and 5 of 19 (26%) Klebsiella isolates. Acinetobacter isolates were resistant to all third-generation cephalosporins, and 2 of 4 (50%) Acinetobacter isolates were resistant to cefepime (fourth-generation cephalosporin). Acinetobacter isolates remained susceptible to amikacin and carbapenems.
Of 24 S. aureus isolates, 22 (92%) were resistant to penicillin. Of 9 S. aureus isolated tested, 7 (78%) were resistant to ampicillin. Of 27 S. aureus isolates tested, 1 (4%) was resistant to vancomycin.
Discussion
The high rate of AMR is a global burden and will worsen if actions are not taken to control AMR. There is lack of data on national AMR in around 40% of African countries [21]. Challenges of AMR control range from inadequate resources and weak supply chains for consumables for microbiological laboratory procedures, lack of human resources well trained in AMR surveillance, and poor or lack of commitment by facility management to embrace AMR as a healthcare issue [22].
Our study showed that AMR is high in our acute care surgery patients, with high rates of third-generation cephalosporin resistance and high rates of ESBL production. This is consistent with other studies [23]. Prior studies have shown that 46–69% of gram-negative microorganisms are resistant to third-generation cephalosporins [20]. A prior study at CHUK showed that 31.4% and 58.7% of E. coli and Klebsiella isolates were ESBL producers [24]. These results are consistent with other studies highlighting high rate of ESBL-producing microorganisms in African health facilities and the community [25].
When compared to a prior hospital-wide antibiogram study at the same hospital [12], AMR patterns seen in this current study are lower. This is likely due to the fact that samples were taken from all patients suspected to have infection, with many samples taken from intraoperative sources. As such, there was more CAI samples, which have lower rates of AMR compared with HAI.
The rates of AMR were higher in HAI compared with CAI. This is consistent with other studies globally [23]. However, AMR in CAI remained relatively high in this study, with rates of third-generation cephalosporin resistance and ESBL production at 46% and 38%, respectively. This may be due to the tiered structure of the Rwandan healthcare system. Patients initially present to a health center, then a district hospital, before being referred to the tertiary referral hospital. As such, many patients are exposed to antibiotics prior to presentation at the tertiary referral hospital. There is a limited spectrum of available antibiotics in the country, suggesting that patients could be exposed to the same antibiotics multiple times. In addition, antibiotics are commonly available in pharmacies without a prescription which may lead to misuse.
There is a variety in types of microorganisms in surgical infections. In this study, E. coli and Klebsiella were frequently isolated pathogens in surgical patients with a high resistance to commonly used antibiotics, similar to previous studies [10, 11]. Mitha et al. [6] found that S. aureus and E. coli were common hospital-acquired pathogens in rural Gabon and 40% of identified E. coli and Klebsiella species were ESBL producers [26]. In Tanzania, common pathogens isolated from surgical site infections included S. aureus (37%), E. coli (11%), and Enterococcus species (5%) [27]. A study in Rwanda found that S. aureus accounted 62.5% of post-Caesarean section surgical site infection [28]. While E. coli and Klebsiella isolates were common overall, Acinetobacter, Proteus, and Pseudomonas isolates were predominately seen in HAI.
Our study found longer hospital stay in HAI versus CAI. In addition, length of hospital stay was longer in ESBL producers compared to non-ESBL producers, though this was not statistically significant. This is consistent with other studies which show that, with higher resistance rates to commonly available and used antimicrobial agents, there are prolonged longer hospital stays with subsequently increased hospital bills as well as morbidity and mortality [3, 18].
Antimicrobial stewardship is vital as antibiotic agent misuse is associated with worse patient outcomes and contributes to AMR [13]. Most patients during our study received metronidazole and third-generation cephalosporins despite high rates of third-generation cephalosporin resistance [13]. This may be due to limited antibiotic options and poor knowledge of antibiotic alternatives. In addition, misuse and overuse of broad spectrum of antibiotics is a challenge contributing to high rates of AMR. Stewardship of antibiotic use, availability of antimicrobial susceptibility testing, and measures of infection control are necessary to prevent antibiotic misuse and AMR.
Conclusion
Rates of cephalosporin resistance and ESBL production are relatively high in Rwandan surgical patients with higher rates notes in HAIs compared with CAIs. Infection prevention practices and antibiotic stewardship are critical to reduce infection rates with resistant organisms in a low-resource setting.
References
Rickard J, Beilman G, Forrester J, Sawyer R, Stephen A, Weiser TG et al (2019) Surgical infections in low- and middle-income countries: a global assessment of the burden and management needs. Surg Infect (Larchmt). https://doi.org/10.1089/sur.2019.142
Bhangu A, Ademuyiwa AO, Aguilera ML, Alexander P, Al-Saqqa SW, Borda-Luque G et al (2018) Surgical site infection after gastrointestinal surgery in high-income, middle-income, and low-income countries: a prospective, international, multicentre cohort study. Lancet Infect Dis 18(5):516–525
Jung Y, Lee MJ, Sin HY, Kim NH, Hwang JH, Park J et al (2012) Differences in characteristics between healthcare-associated and community-acquired infection in community-onset Klebsiella pneumoniae bloodstream infection in Korea. BMC Infect Dis. 12:239
Cardoso T, Ribeiro O, Aragão IC, Costa-Pereira A, Sarmento AE (2012) Additional risk factors for infection by multidrug-resistant pathogens in healthcare-associated infection: a large cohort study. BMC Infect Dis 12:375
Phu VD, Wertheim HFL, Larsson M, Nadjm B, Dinh QD, Nilsson LE et al (2016) Burden of hospital acquired infections and antimicrobial use in Vietnamese adult intensive care units. PLoS ONE 11(1):1–15
Mitha M, Furuya EY, Larson E (2014) Risk of healthcare associated infections in HIV positive patients. J Infect Prev 15(6):214–220
Siegel JD, Rhinehart E, Jackson M, Chiarello L (2007) Management of multidrug-resistant organisms in health care settings, 2006. Am J Infect Control 35(10 Suppl 2):165–193
WHO (2014) Antimicrobial resistance. global report on surveillance (Internet). World Health Organization. Available from: https://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2536104&tool=pmcentrez&rendertype=abstract
Cars O, Högberg LD, Murray M, Nordberg O, Sivaraman S, Lundborg CS et al (2008) Meeting the challenge of antibiotic resistance. BMJ 337:a1438
Mendelson M, Røttingen JA, Gopinathan U, Hamer DH, Wertheim H, Basnyat B et al (2016) Maximising access to achieve appropriate human antimicrobial use in low-income and middle-income countries. Lancet 387:188–198
Harris PNA, Tambyah PA, Lye DC, Mo Y, Lee TH, Yilmaz M et al (2018) Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E. coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance. JAMA J Am Med Assoc 320(10):984–994
Sutherland T, Mpirimbanyi C, Nziyomaze E, Niyomugabo JP, Niyonsenga Z, Muvunyi CM et al (2019) Widespread antimicrobial resistance among bacterial infections in a Rwandan referral hospital. PLoS ONE 14(8):1–15
Rickard J, Ngarambe C, Ndayizeye L, Smart B, Riviello R, Majyambere JP et al (2018) Antibiotic use and antimicrobial resistance of surgical patients with peritonitis at a tertiary referral hospital in Rwanda. Surg Infect (Larchmt) 19(4):382–387
National Institute of Statistics of Rwanda (2016), Ministry of Finance and Economic Planning, Ministry of Health, DHS Program II. Rwanda demographic and health survey, 2014–2015
Ndayizeye L, Ngarambe C, Smart B, Riviello R, Majyambere JP, Rickard J (2016) Peritonitis in Rwanda: Epidemiology and risk factors for morbidity and mortality. Surgery (United States) 160(6):1645–1656
Mpirimbanyi C, Rickard J, Furaha C, Ntirenganya F (2018) Necrotizing soft tissue infections at a tertiary referral hospital in Rwanda: epidemiology and risk factors for mortality. World J Surg 42(8):2314–2320. https://doi.org/10.1007/s00268-018-4515-z
Abahuje E, Sibomana I, Rwagahirima E, Urimubabo C, Munyaneza R, Rickard J (2019) Development of an acute care surgery service in Rwanda. Trauma Surg Acute Care Open 4(1):1–6
CLSI standards & guidelines (Internet). (cited 2020 Jan 21). Available from: https://clsi.org
Singer M, Deutschman CS, Seymour C, Shankar-Hari M, Annane D, Bauer M et al (2016) The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA J Am Med Assoc 315:801–810
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42(2):377–381
Tadesse BT, Ashley EA, Ongarello S, Havumaki J, Wijegoonewardena M, González IJ et al (2017) Antimicrobial resistance in Africa: a systematic review. BMC Infect Dis 17(1):1–17
Kariuki S, Keddy KH, Antonio M, Okeke IN (2018) Antimicrobial resistance surveillance in Africa: successes, gaps and a roadmap for the future. Afr J Lab Med 7(2):1–2
Ashley EA, Lubell Y, White NJ, Turner P (2011) Antimicrobial susceptibility of bacterial isolates from community acquired infections in Sub-Saharan Africa and Asian low and middle income countries. Trop Med Int Heal 16(9):1167–1179
Ntirenganya C, Manzi O, Muvunyi CM, Ogbuagu O (2015) High prevalence of antimicrobial resistance among common bacterial isolates in a tertiary healthcare facility in Rwanda. Am J Trop Med Hyg 92(4):865–870
Storberg V (2014) ESBL-producing Enterobacteriaceae in Africa: a non-systematic literature review of research published 2008–2012. Infect Ecol Epidemiol 4:20342
Scherbaum M, Kösters K, Mürbeth RE, Ngoa UA, Kremsner PG, Lell B et al (2014) Incidence, pathogens and resistance patterns of nosocomial infections at a rural hospital in Gabon. BMC Infect Dis 14(1):13–15
Fehr J, Hatz C, Soka I, Kibatala P, Urassa H, Smith T et al (2006) Risk factors for surgical site infection in a Tanzanian district hospital: a challenge for the traditional national nosocomial infections surveillance system index. Infect Control Hosp Epidemiol 27(12):1401–1404
Bizimana K, Ndoli J, Bayingana C, Baluhe I, Gilson GJ, Habimana E (2016) Prevalence and risk factors for post cesarean delivery surgical site infection in a teaching hospital setting in rural Rwanda: a prospective cross sectional study. Int J Curr Microbiol Appl Sci 5(6):631–641
Acknowledgements
The authors would like to acknowledge the staff of the operating room and surgical wards who assisted with sample collection. The authors acknowledge the laboratory staff who assisted with specimen processing. The authors also acknowledge the acute care surgery team and surgical residents who identified patients and assisted in sample collection.
Funding
This research was supported by the National Institutes of Health’s National Center for Advancing Translational Sciences, grant UL1TR002494. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health’s National Center for Advancing Translational Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Muvunyi, V., Mpirimbanyi, C., Katabogama, J.B. et al. Community- and Hospital-Acquired Infections in Surgical patients at a Tertiary Referral Hospital in Rwanda. World J Surg 44, 3290–3298 (2020). https://doi.org/10.1007/s00268-020-05634-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-020-05634-8