Abstract
Background
Current American Thyroid Association (ATA) guidelines state that patients with intermediate-risk papillary thyroid cancer (PTC) may benefit from remnant ablation. One criterion for intermediate-risk classification is >5 positive lymph nodes (LNs). We investigate whether performing step-sectioning of LNs increases the metastatic detection rate, thereby influencing ATA risk of recurrence (ROR) classification.
Methods
A retrospective review was conducted of cases in which ≥ 5 LNs were removed during thyroidectomy and ≤5 LNs were found positive for PTC. Step-sectioning was performed on the original tissue blocks. All slides were re-reviewed by a senior pathologist.
Results
Twenty patients met study criteria. Step-sectioning significantly increased LN yield compared to standard sectioning. In total, we found 12 new positive lymph nodes; seven (58%) were in totally new lymph nodes, while five (42%) were in lymph nodes previously read as negative. All newly discovered metastases were classified as micrometastases (≤2 mm). Of the 15 patients originally classified as low-risk, the step-sectioning protocol impacted two patients (13%), increasing ROR stratification.
Conclusion
Intensive step-sectioning reveals additional micrometastases. More detailed analysis did not identify clinically significant nodal disease likely to impact the clinical course of patients in this study. Our study supports current standards of pathology specimen handling related to LN assessment and the impact on ATA ROR classification. Nonetheless, it is important for clinicians to understand their institution’s sectioning protocol utilized to report positive and total LN counts, which could impact ATA risk stratification and denote the comprehensive nature of the LN dissection that was performed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
American Joint Committee on Cancer (AJCC) staging for well-differentiated thyroid cancer is not affected by nodal metastatic burden, and the stage of disease according to the TNM system is intended to predict disease-specific survival. The American Thyroid Association (ATA) guidelines are dynamic throughout a patient’s disease-course, but the initial assessment of a patient’s disease is intended to provide insight into a patient’s prognosis with respect to disease-free survival, or risk of recurrence (ROR). Evidence suggests that ROR/disease-free survival may be impacted by nodal metastatic burden [1,2,3].
As noted above, nodal metastatic burden is an important variable in the ATA ROR guidelines (Fig. 1) [4]. Any single lymph node greater than 3 cm classifies a patient as intermediate-ROR (around 30%). Metastases to ≥6 lymph nodes classify a patient as intermediate-ROR (around 19%). If all positive lymph nodes (no specified number) are micrometastatic (≤2 mm), the patient is classified as low-ROR (around 5%). Finally, if there are metastases in ≤5 lymph nodes (≤3 cm and at least >2 mm), this also classifies a patient as low-ROR (around 5%).
Intermediate-risk stratification often leads to the recommendation of radioactive iodine (RAI) treatment. The risks of RAI are not negligible and include miscarriage, secondary malignancy, salivary gland damage, nasolacrimal duct obstruction, and dental caries [4]. Therefore, clinicians are advised to be highly selective in their recommendations for RAI [5].
The reported rates of positive lymph nodes within neck dissections vary significantly, between 15 and 83% [6]. The dependencies contributing to this variability include inherent tumor biology, the extent of the surgical procedure, and the diligence of pathologic specimen processing. The standard for pathology departments is to section lymph nodes from papillary thyroid cancer (PTC) patients by usual sectioning protocols based on Association of Directors of Anatomic and Surgical Pathology (ADASP) recommendations [7]. Routine lymph nodes are usually sectioned at 3µ thickness to produce a single ribbon of 4–5 connected tissue sections for examination. Here, we query the total and positive lymph node count boundary between five and six in a patient group with clinically suspicious lymph nodes treated with central ± lateral neck dissections. How often would more rigorous pathologic examination impact the total and positive lymph node counts? [8,9,10] Our aim was to examine the potential impact to ATA ROR classification and association with outcome as well as the cost and time of more detailed lymph node sectioning.
Materials and methods
Patient selection
A retrospective review was conducted for patients undergoing total thyroidectomy for PTC from January 2010 to August 2017 and ≥ 5 resected lymph nodes, but ≤5 reported positive lymph nodes, regardless of size. Exclusionary criteria included ≥6 positive nodes, ≤ 5 total number of nodes harvested, and unavailable tissue blocks. The following histopathological data were collected: number of positive lymph nodes, nodal size, lymph node compartment dissected, pTNM stage, tumor multifocality, age, gender, recurrence status, histological subtype, as well as invasive properties (extrathyroidal extension, perineural or lymphovascular invasion). The study was planned and executed with permission from the Mount Sinai Institutional Review Board.
Pathology examination
The standard lymph node sectioning at 3µ was performed, as part of routine surgical pathologic processing. The original lymph node specimen blocks were obtained for study patients. A “step-sectioning” protocol was followed on all study tissue blocks. This technique involves the creation of a “ribbon” of multiple tissue sections, 3µ thick. A slide is created from this ribbon. The block is then “trimmed” 10–15µ and that tissue discarded. Another ribbon is produced, and another slide is created. This procedure was continued until the tissues were exhausted from the paraffin block (Fig. 2). By comparison, a serial sectioning protocol is a more intensive method, which does not involve discarding of any tissue. A senior thyroid pathologist (MBW) reviewed the slides and recorded the total lymph nodes identified, lymph nodes positive for micrometastasis, and their respective sizes.
Statistical analysis
For comparison of the results of sectioning protocols, a paired t test was performed using Microsoft Excel. A p value of <0.05 was defined as statistically significant.
Results
Twenty patients met study criteria: 18 females and 2 males, age 12–70 years (mean 45 years). All cases included central compartment neck dissections, and five cases additionally included a lateral compartment neck dissection for primary treatment of papillary thyroid cancer. Fifteen of the 20 patients had positive central compartment lymph nodes, and five patients had positive lymph nodes in both central and lateral compartments.
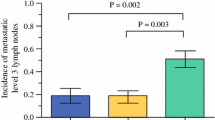
Step-sectioning was performed on the original specimen blocks (~ 200 blocks) to yield ~ 1920 slides. New additional lymph nodes were identified after the step-sectioning protocol in 7/20 patients (35%). Step-sectioning significantly increased lymph node yield compared to standard sectioning (18.4 lymph nodes ± 14.4 SD vs. 17.4 lymph nodes ± 13.8 SD, respectively; p = 0.02, paired t test). In total, we found 12 new positive lymph nodes; seven (58%) were in totally new lymph nodes, while five (42%) were in lymph nodes previously read as negative (Table 1). For one patient, the total number of positive nodes decreased from three to two after re-review determined misdiagnosis in one lymph node. The new metastatic foci were significantly smaller than foci within previously identified positive lymph nodes (1.1 mm ± 0.5 SD vs. 7.1 mm ± 7.2 SD, respectively; p = 0.017) (Fig. 3). All of the newly identified positive lymph nodes harbored micrometastases (≤2 mm).
For three patients, the revised total number of positive lymph nodes was greater than five (4–6, 3–6, and 5–8 positive lymph nodes, Table 1, Fig. 4). One of these patients was already classified as intermediate-risk based on an initial finding of extrathyroidal extension. Two patients were up-classified from low- to intermediate-ROR according to ATA guidelines. Thus, of the 15/20 patients originally classified as low-risk, the step-sectioning protocol impacted 2/15, or 13% of patients, increasing ROR stratification. Neither of the two patients reclassified as intermediate-ROR have developed recurrences based on 1-year follow up.
Lymph node step-sectioning revealing a papillary thyroid carcinoma metastasis. a Low-power view of occult metastatic papillary thyroid carcinoma near center of this small lymph node seen only after step-sectioning (bar = 0.5 mm). The arrow indicates metastatic cells. b Higher-power view of papillary thyroid carcinoma with usual papillary type architecture (bar = 0.05 mm)
Discussion
Historically, cN0 occult metastatic PTC has been considered a disease of limited oncologic significance, as no significant differences in survival and recurrence rates are reported for patients undergoing prophylactic central dissection compared to those not undergoing this procedure [11, 12]. Here, we do not focus on primary occult metastatic disease, as our cohort was composed of patients with clinically suspicious lymph nodes, which already triggered the decision to perform central ± lateral neck dissections. After comprehensive step-sectioning, we reexamined the number of positive lymph nodes and the impact on ATA ROR classification. From the viewpoint of surgical pathology, our results are hardly surprising. It is known that detection rates for micrometastases vary between laboratories, protocols, and pathologists. This variability can impact published retrospective data and clinical practice, as well as the interpretation of individual patient pathologic reporting. ADASP recommends that more than one tissue section be examined from each block [7]. Generating one tissue ribbon from each block satisfies that recommendation. The College of American Pathologists recommends a more intensive step-sectioning protocol be reserved for sentinel lymph nodes in breast carcinoma and melanoma, which enhances detection of micrometastases [8,9,10, 13].
Haglund et al. [14] examined the impact of step-sectioning for pN0 PTC from a staging point of view. After intensive lymph node sectioning, 16% of patients were upstaged from N0 to N1. Here, we look at patients with positive lymph nodes and find, not surprisingly, that 35% (7/20 patients) had more lymph nodes identified, and 12 new positive lymph nodes were found (Fig. 4). Not all new foci were found in newly identified lymph nodes; five of 12 lymph nodes were previously deemed benign. The mean size of the new foci (1.1 mm ± 0.5 SD) was similar to that found by Haglund et al. (1.0 mm, range 0.1–2.0 mm; 55% ≤1 mm) [14]. As expected, these results show that conventional sectioning protocols may not reflect the true rate of positive lymph nodes. Importantly, all newly identified metastases were micrometastatic. They are not expected to impact clinical outcome, yet could increase risk stratification in a small percentage of PTC patients.
While an intensified, step-sectioning protocol impacts lymph node findings, a major disadvantage is the cost of time and resources. The time to process one block, including block retrieval, sectioning, and staining, translates into roughly 2–3 h, compared to 1.5 h for the standard sectioning protocol. Costs can be divided into technical labor compensation ($80–120 per case) versus material costs. The material costs of the processing paraffin blocks (i.e., re-cutting, staining and cover-slipping) are $15 for the first slide and $10 for each slide thereafter at this institution. This study generated 15–20 additional slides per block, which may cost $165–215 per block, for an estimated material cost of $40,000 for this study, or an average of $2000 per patient. There is no billable professional component for step-sectioning.
Pathologic processing is not the only variable impacting risk classification. The quality of preoperative imaging has a major impact on the surgeon’s decision to perform therapeutic neck dissection. The surgeon’s experience also affects the detection of “clinically evident” central compartment nodes, which impacts the decision to perform central compartment node dissection. Finally, the degree of surgical dissection and clearing of a cervical compartment impacts the final lymph node counts. All of the above factors contribute to the total number of detected nodes as well as the reported positive lymph node counts. The fact that all new metastatic nodal deposits were less than 2 mm provides reassurance that the impact on our understanding of a patient’s disease biology and ATA ROR is small and does not justify the cost in time and money for performing this additional pathologic assessment.
Every primary pathology report does become “etched in stone” to serve as the ultimate basis for AJCC staging and ROR stratification. It becomes the starting milepost in a patient’s thyroid cancer journey. We can conclude that current pathology practices are “good enough” in detecting clinically relevant positive lymph node counts; more, here, is not better.
Two of our patients, theoretically, could have been upstaged to ATA ROR—intermediate-risk, and might have received unwarranted RAI. It appears that step-sectioning could potentially impact patients not classified as “micrometastases only.” Five study patients were classified as “micrometastases only” (≤2 mm) based on the largest metastatic deposit (Patients 1, 5, 6, 8, 10). Step-sectioning did not increase their lymph node counts to ≥ 6 positive nodes. As step-sectioning is not the standard of care for any patient with metastatic papillary thyroid carcinoma, routine ATA ROR upstaging is an unlikely scenario. It remains very important for clinicians caring for thyroid cancer patients to understand the factors that could influence pathology reporting.
While the retrospective nature and small size (20 patients) are limiting features of this study, the cost ($40,000), effort, and conservative nature of our conclusions (current pathology practices are “good enough”) would not warrant larger additional studies.
Conclusion
This study supports current lymph node sectioning standards for papillary thyroid carcinoma patients as per ADASP. Step-sectioning could impact the ATA risk stratification in a small percentage of patients, even though the newly positive lymph nodes are micrometastases and are unlikely to significantly impact clinical outcome.
References
Ito Y, Jikuzono T, Higashiyama T et al (2006) Clinical significance of lymph node metastasis of thyroid papillary carcinoma located in one lobe. World J Surg 30:1821–1828. https://doi.org/10.1007/s00268-006-0211-5
Randolph GW, Duh QY, Heller KS et al (2012) The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid 22:1144–1152
Sugitani I, Kasai N, Fujimoto Y et al (2004) A novel classification system for patients with PTC: addition of the new variables of large (3 cm or greater) nodal metastases and reclassification during the follow-up period. Surgery 135:139–148
Haugen BR, Alexander EK, Bible KC et al (2016) 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 26:1–133
Hong YR, Lee SH, Lim DJ et al (2017) The stratification of patient risk depending on the size and ratio of metastatic lymph nodes in papillary thyroid carcinoma. World J Surg Oncol 15:74
Cranshaw IM, Carnaille B (2008) Micrometastases in thyroid cancer. An important finding? Surg Oncol 17:253–258
Pathology AoDoAS (2001) Recommendations for processing and reporting of lymph node specimens submitted for evaluation of metastatic disease. Am J Clin Pathol 115:799–801
Calhoun BC, Chambers K, Flippo-Morton T et al (2014) Breast cancer detection in axillary sentinel lymph nodes: the impact of the method of pathologic examination. Hum Pathol 45:2497–2501
Maguire A, Brogi E (2016) Sentinel lymph nodes for breast carcinoma: a paradigm shift. Arch Pathol Lab Med 140:791–798
Weaver DL (2010) Pathology evaluation of sentinel lymph nodes in breast cancer: protocol recommendations and rationale. Mod Pathol 23(Suppl 2):S26–S32
Dobrinja C, Troian M, Cipolat Mis T et al (2017) Rationality in prophylactic central neck dissection in clinically node-negative (cN0) papillary thyroid carcinoma: is there anything more to say? A decade experience in a single-center. Int J Surg 41(Suppl 1):S40–S47
Giordano D, Frasoldati A, Gabrielli E et al (2017) Long-term outcomes of central neck dissection for cN0 papillary thyroid carcinoma. Am J Otolaryngol 38:576–581
Riber-Hansen R, Hastrup N, Clemmensen O et al (2012) Treatment influencing down-staging in EORTC Melanoma Group sentinel node histological protocol compared with complete step-sectioning: a national multicentre study. Eur J Cancer 48:347–352
Haglund F, Garvin S, Ihre-Lundgren C et al (2016) Detailed lymph node sectioning of papillary thyroid carcinoma specimen increases the number of pN1a patients. Endocr Pathol 27:346–351
Acknowledgements
The authors wish to thank Jill Gregory for the artwork and the Mount Sinai Health System for its generous support of this research project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Urken is the Medical Advisor of the THANC Foundation. No other potential conflict of interest relevant to this article exists.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Disclaimer Initial results of this paper were presented at the American Thyroid Association (ATA) Conference in Victoria, BC, Canada from October 18-22, 2017.
Rights and permissions
About this article
Cite this article
Griffin, M.J., Baik, F.M., Brandwein-Weber, M. et al. Positive Lymph Node Counts in American Thyroid Association Low-Risk Papillary Thyroid Carcinoma Patients. World J Surg 44, 1892–1897 (2020). https://doi.org/10.1007/s00268-020-05399-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-020-05399-0