Abstract
Background
Neoadjuvant chemotherapy (NAC) can improve cosmesis by reducing resection volume. Breast-conserving surgery (BCS) aims to achieve clear excision margins while optimizing cosmesis. However, the influence of NAC on margin re-excision after BCS is unclear. This study examines the rate and determinants of margin re-excision in patients undergoing BCS following NAC in our institution.
Methods
From 2011–2015, all patients treated with NAC prior to BCS were identified from a prospectively maintained database. Mann–Whitney and Fisher’s exact test tests were used to compare variables in patients who did and did not require re-excision. Patients undergoing primary surgical treatment in 2015 comprised an unmatched comparison group.
Results
Of 211 patients treated with NAC, 69 initially underwent BCS. The re-excision rate was 32% (n = 22) compared to 17% in the primary operable group (38 of 221, p = 0.02). Re-excision rates were lowest in triple-negative and HER2+ tumors (0% and 10%, respectively). Lobular carcinoma and ER+ tumors had a significantly higher rate of re-excision (100% and 42%, respectively). Of 22 patients undergoing re-excision, 9 had further BCS and 13 had a mastectomy.
Conclusion
The re-excision rate following NAC is almost twice that of patients who underwent primary operative management. Her2+ and triple-negative tumors have lower re-excision rates and may represent a selected cohort most suitable for BCS. Patients with invasive lobular carcinoma or ER+ disease have significantly higher rates of margin positivity, and these patients should be considered for a cavity shave during primary surgery to reduce the rates of re-excision.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neoadjuvant chemotherapy (NAC), while initially developed for advanced breast cancer, is now increasingly administered to patients with primary operable disease. Although NAC does not result in survival advantage or disadvantage compared with postoperative chemotherapy [1], it can reduce resection volumes and extent of breast and axillary surgery, thereby improving cosmesis and reducing complications [2, 3]. The tumor response to NAC can help guide prognosis, especially in the subgroup with a pathologic complete response, as these patients have significantly increased disease-free and overall survival [1]. NAC also has the benefit of enabling assessment of tissue, blood samples and imaging before and after chemotherapy for research purposes.
Despite these advantages, the influence of NAC on re-excision of margins following breast-conserving surgery (BCS) is unclear. While BCS is desirable following NAC, the rate of margin positivity and subsequent re-operation is variable in previous publications [4]. Although re-operation in patients with positive margins does not affect survival [5, 6], it compromises cosmesis, increases costs and causes undue anxiety for patients.
This study aims to examine the rate and determinants of margin re-excision in patients undergoing BCS following NAC in our institution.
Methods
Patient population
All patients diagnosed with breast cancer in our institution are discussed at a weekly multidisciplinary team meeting. Patients from this prospectively maintained database were included if they were treated with neoadjuvant chemotherapy and breast-conserving surgery during the 5-year study period (2011–2015). Patients undergoing primary surgical treatment in 2015 were used as an unmatched comparison group.
Clinical, radiologic and pathologic data
Tumor size was determined from pre-treatment imaging. All patients had a mammogram and ultrasound, and most had an MRI at diagnosis and following chemotherapy. The chemotherapy used was taxane based. Estrogen receptor (ER) and human epidermal growth factor receptor 2 (HER2) status was determined from diagnostic biopsy before treatment. Pathologic complete response was defined as no residual invasive carcinoma in breast or axillary tissue. A positive margin was defined as invasive tumor or DCIS < 1 mm from the resection margin.
Surgical technique
A clip was inserted in all tumors prior to commencement of neoadjuvant chemotherapy. A preoperative wire was inserted to guide excision of the clip and tumor, and an intra-operative specimen x-ray was performed to confirm excision of both. Cavity shaving was not routinely performed.
Statistical analysis
Mann–Whitney test and Fisher’s exact test were used to compare variables in patients who did and did not require re-excision. Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS, Version 23; IBM) to calculate odds ratio (OR) and significance.
Results
During the 5-year study period, 211 patients were treated with NAC. Of these, 69 underwent subsequent BCS and comprise our study population. The comparison group of patients who underwent primary surgical treatment with BCS during 2015 consists of 221 patients. Table 1 shows the tumor characteristics of both groups. Patients treated with NAC had larger tumors and were more likely to be HER2 positive.
The overall margin positivity rate was 38% in the NAC group compared with 20% in those treated with primary surgery. Tumor on ink was found in 22% and 8%, respectively. A pathologic complete response was found in 15 (22%) of the NAC group. Table 2 shows details of resection margin status.
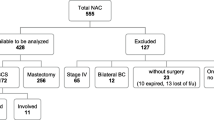
Re-excision of margins was performed in 32% (n = 22) of the NAC group compared with 17% (n = 38) of the primary surgery group. At final operation, 81.2% of patients in the NAC study had a successful BCS and 18.8% underwent mastectomy (Fig. 1).
In the NAC group, larger tumors, lobular cancers (OR = 17) and ER+ HER2− tumors (OR = 16.6) had significantly higher re-excision rates. Patients with HER2-positive tumors had a significantly lower rate of margin re-excision (OR = 0.1). No triple-negative tumor required re-excision; however, this was not statistically significant as numbers were small (n = 4). Determinants of re-excision are shown in Table 3.
Discussion
Currently, there are no guidelines defining acceptable resection margins for patients treated with NAC and BCS as there are no data from prospective trials on this subject. Breast surgeons follow criteria based on evidence for primary BCS which differ by country and organization. Therefore, when interpreting studies on re-excision rates following BCS, it is important to be cognizant of the definition of positive margins, the criteria for re-excision and the guidelines followed.
Our data show an almost twofold increase in rates of re-excision (32% vs 17%) and margin positivity (38% vs 20%) following BCS after NAC compared to primary BCS. However, these groups are unmatched and therefore have patient and tumor cofounders other than NAC. The re-excision rate in the primary surgery group is in agreement with that reported from other breast cancer centers in Great Britain and Ireland of 17.2% [7].
Data from over 9000 patients in a nationwide network and registry of histology and cytopathology in the Netherlands (PALGA) showed an increased re-excision rate of 9.1% in patients treated with NAC and BCS compared with 5.3% in the primary operable group [8]. Despite lower re-excision rates than our study, the rates of margin positivity were higher (45% following NAC and 34% following primary surgery) when defined as invasive or in situ disease < 1 mm from the resection margin, as in our study. The authors acknowledge the limitations of using nationwide databases and postulate the re-excision rate is an underestimation based on the high rate of margin positivity in both groups.
Another database review from over 70,000 patients in the National Cancer Database in the USA showed a significantly decreased re-operation rate in patients undergoing BCS following NAC compared with those treated with BCS and adjuvant chemotherapy (11.4 vs 20.3%) [9]. This difference persisted even after propensity score matching between groups to control for patient and tumor characteristics. However, neither the indication for re-operation nor the rate or definition of margin positivity was presented in the study.
A recent review on BCS following NAC found 10 comparative studies reporting on margin status in this cohort of patients compared with those treated with primary surgery [4]. All studies were low level 3 or 4 evidence. The definition of a positive margin and the indication for re-excision varied widely between these studies. Positive margins ranged from 5 to 39.8% after NAC versus 13.1–46% without NAC, leading to re-operation in 0–45.4% versus 0–76.5%, respectively.
A major influence on the rate of re-excision is tumor subtype. Unsurprisingly, tumors that were HER2+ or triple negative have the lowest rate of margin positivity as these tumors are more sensitive to chemotherapy. Patients with invasive lobular carcinoma (ILC) or ER+/HER2− tumors had a significantly higher rate or re-excision in our study. This has also been reported in other studies [8,9,10,11,12] and is likely due to the reduced response to chemotherapy in these tumors. Due to the high rate of re-excision in patients with these tumors, intra-operative cavity shaving should be considered as this has been shown to reduce margin positivity in a randomized controlled trial [13].
The pattern of tumor response to NAC is another important area to consider when planning surgery. Where there is no response, or a patchy response, as if often seen in HR-positive ILC, the original surgical plan should be maintained, and the same original footprint should be excised. However, where there appears to be a complete pathologic response or a concentric reduction in the tumor size, risk-adapted conservation can be safely attempted. Guidelines from the American college of radiologists, surgeons and pathologists as well as the society of surgical oncology recommend that where there is a patchy response and ‘if viable tumor is present throughout the specimen even if it does not extend to the margin, a further re-excision should be considered’ [14].
Overall 81.2% of patients planned to have BCS had successful excision and avoided a mastectomy in this study. Preoperative patient counseling should include discussion based on their tumor subtype and response to chemotherapy, with those more likely to require re-excision informed as such.
This study is limited by the number of patients included and the retrospective nature of analysis. The primary operable group serves as an unmatched comparison group and so the re-excision rate may be affected by tumor and patient cofounders.
Conclusion
The re-excision rate following NAC is almost twice that of patients who underwent primary operative management. Her2+ and triple-negative tumors have lower re-excision rates and may represent a selected cohort most suitable for BCS. Patients with invasive lobular carcinoma or ER-positive disease have significantly higher rates of margin positivity, and these patients should be considered for a cavity shave during primary surgery to reduce the rates of re-excision.
References
Rastogi P, Anderson SJ, Bear HD et al (2008) Preoperative chemotherapy: updates of national surgical adjuvant breast and bowel project protocols B-18 and B-27. J Clin Oncol 26:778–785
Mieog JS, van der Hage JA, van de Velde CJ (2007) Preoperative chemotherapy for women with operable breast cancer. Cochrane Database Syst Rev 18(2):Cd005002
King TA, Morrow M (2015) Surgical issues in patients with breast cancer receiving neoadjuvant chemotherapy. Nat Rev Clin Oncol 12:335–343
Volders JH, Negenborn VL, Spronk PE et al (2018) Breast-conserving surgery following neoadjuvant therapy-a systematic review on surgical outcomes. Breast Cancer Res Treat 168:1–12
Vos EL, Jager A, Verhoef C et al (2015) Overall survival in patients with a re-excision following breast conserving surgery compared to those without in a large population-based cohort. Eur J Cancer 51:282–291
Fisher S, Yasui Y, Dabbs K et al (2018) Re-excision and survival following breast conserving surgery in early stage breast cancer patients: a population-based study. BMC Health Serv Res 18:94
Tang SS, Kaptanis S, Haddow JB et al (2017) Current margin practice and effect on re-excision rates following the publication of the SSO–ASTRO consensus and ABS consensus guidelines: a national prospective study of 2858 women undergoing breast-conserving therapy in the UK and Ireland. Eur J Cancer 84:315–324
Volders JH, Haloua MH, Krekel NM et al (2016) Neoadjuvant chemotherapy in breast-conserving surgery—consequences on margin status and excision volumes: a nationwide pathology study. Eur J Surg Oncol 42:986–993
Landercasper J, Bennie B, Parsons BM et al (2017) Fewer reoperations after lumpectomy for breast cancer with neoadjuvant rather than adjuvant chemotherapy: a report from the national cancer database. Ann Surg Oncol 24:1507–1515
Truin W, Vugts G, Roumen RM et al (2016) Differences in response and surgical management with neoadjuvant chemotherapy in invasive lobular versus ductal breast cancer. Ann Surg Oncol 23:51–57
Bouzon A, Acea B, Garcia A et al (2016) Risk factors for positive margins in conservative surgery for breast cancer after neoadjuvant chemotherapy. Cir Esp 94:379–384
Soucy G, Belanger J, Leblanc G et al (2008) Surgical margins in breast-conservation operations for invasive carcinoma: does neoadjuvant chemotherapy have an impact? J Am Coll Surg 206:1116–1121
Chagpar AB, Killelea BK, Tsangaris TN et al (2015) A randomized, controlled trial of cavity shave margins in breast cancer. N Engl J Med 373:503–510
Morrow M, Strom EA, Bassett LW et al (2002) Standard for breast conservation therapy in the management of invasive breast carcinoma. CA Cancer J Clin 52:277–300
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Devane, L.A., Baban, C.K., O’Doherty, A. et al. The Impact of Neoadjuvant Chemotherapy on Margin Re-excision in Breast-Conserving Surgery. World J Surg 44, 1547–1551 (2020). https://doi.org/10.1007/s00268-020-05383-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-020-05383-8