Abstract
Background
Negative pressure wound therapy (NPWT) is a promising advance in the management of closed surgical incisions. NPWT application induces several effects locally within the wound including reduced lateral tension and improving lymphatic drainage. As a result, NPWT may improve wound healing and reduce surgical site complications. We aim to evaluate the efficacy of prophylactic application of NPWT in preventing surgical site complications for closed incisions in breast surgery.
Methods
This systematic review was reported according to PRISMA guidelines. The protocol was published in PROSPERO (CRD42018114625). Medline, Embase, CINAHL and Cochrane Library databases were searched for studies which compare the efficacy of NPWT versus non-NPWT dressings for closed incisions in breast surgery. Specific outcomes of interest were total wound complications, surgical site infection (SSI), seroma, haematoma, wound dehiscence and necrosis.
Results
Seven studies (1500 breast incisions in 904 patients) met the inclusion criteria. NPWT was associated with a significantly lower rate of total wound complications [odds ratio (OR) 0.36; 95% CI 0.19–069; P = 0.002], SSI (OR 0.45; 95% CI 0.24–0.86; P = 0.015), seroma (OR 0.28; 95% CI 0.13–0.59; P = 0.001), wound dehiscence (OR 0.49; 95% CI 0.32–0.72; P < 0.001) and wound necrosis (OR 0.38; 95% CI 0.19–0.78; P = 0.008). There was no significant difference in haematoma rate (OR 0.8; 95% CI 0.19–3.2; P = 0.75). Statistically significant heterogeneity existed for total wound complications, but no other outcomes.
Conclusion
Compared with conventional non-NPWT dressings, prophylactic application of NPWT is associated with significantly fewer surgical site complications including SSI, seroma, wound dehiscence and wound necrosis for closed breast incisions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wound healing complications following surgery are a major cause of morbidity for patients and incur a significant cost burden for healthcare providers [1,2,3,4,5]. Frequently occurring complications include surgical site infection (SSI), wound dehiscence, skin necrosis, haematoma and seroma formation. An estimated 20–36% of nosocomial infections occurring in the USA each year are SSI-related [6]. Zimlichman et al. estimated that healthcare-associated infections in the USA cost $9.8 billion dollars annually with SSI making up 33.7% of this total cost [6].
Prophylactic negative pressure wound therapy (NPWT) has recently emerged as a promising advance in the prevention of surgical site complications [7,8,9,10]. There is a growing body of evidence demonstrating a significant reduction in surgical site complications when NPWT is compared to conventional dressings. This effect appears to be uniform across a range of surgical disciplines involving both clean and contaminated wounds [11,12,13,14,15]. There is also evidence to suggest that prophylactic use of NPWT may be a cost-saving intervention when compared to standard dressings particularly in the higher-risk patient [16, 17].
The incidence of SSI in patients undergoing breast surgery varies depending on the type of procedure being undertaken [18]. In their retrospective analysis of 18,696 mastectomies, Olsen et al. reported an SSI rate of 5% in patients undergoing mastectomy alone rising to 10.3% in patients undergoing mastectomy plus implant [19]. In a separate study, the same authors calculated the cost of SSI per patient undergoing breast surgery to be $4,091 after adjusting for type of surgical procedure and other variables [3]. These figures suggest a need for further infection control interventions in order to improve both patient outcomes and treatment-associated costs. This is of particular relevance in breast cancer patients as surgical site complications can delay the initiation of adjuvant treatment and may impact negatively both recurrence risk and overall survival [20, 21].
NPWT consists of the continuous delivery of negative pressure to the wound bed via a vacuum device. Commercially available devices at present have the capability of generating −80 mm Hg to −150 mm Hg of negative pressure, depending on the device, which is then applied to the wound. As a result, the negative pressure environment leads to a reduction in lateral wound tension, improved lymphatic drainage and propagation of local wound factors required for wound bed granulation [14]. It was initially utilised to expedite the healing of open or chronic wounds, but its indications have expanded in recent times to encompass the prevention of wound healing complications in closed surgical incisions [9, 22, 23].
While the body of evidence continues to grow regarding NPWT, its overall efficacy for closed incisions in breast surgery when compared with standard dressings remains unclear. Therefore, the aim of this systematic review and meta-analysis is to assess the efficacy of prophylactic NPWT versus non-NPWT dressings in closed breast incisions.
Methods
This systematic review and meta-analysis was reported according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines (https://www.prisma-statement.org/) (Appendix 1).
Eligibility criteria
Any study which met all of the following inclusion criteria was included in the analysis: (1) published full-text studies in English language (either randomised or non-randomised) which directly compared NPWT with non-NPWT dressings; (2) studies involving only closed incisions in breast surgery; and (3) studies which report any of the following outcomes (total wound complications, surgical site infection (SSI), seroma, haematoma, wound dehiscence and necrosis).
Studies which examined the effect of NPWT on closed axillary or autologous donor site incisions were excluded.
Search strategy
PubMed, Embase, CINAHL and Cochrane Library databases were searched without any language restrictions, using the following combination of medical subject heading terms: “breast surgery” OR “breast reconstruction” OR “breast reduction” OR “mastectomy” OR “breast augmentation” AND “PICO” OR “VAC” OR “PREVENA” OR “negative pressure wound therapy” OR “negative pressure dressings” (Appendix 2). The search was performed from 1 to 31 October 2018. All potentially relevant titles and abstracts found were individually reviewed by two investigators (DC and LS), and full texts of relevant studies were examined. Any disagreement regarding publications was resolved by discussion, and if the question remained unsettled, the opinion of a third investigator (POL) was sought. Reference tracking from retrieved studies was further searched for additional studies which meet the inclusion criteria.
Data analysis
The following data were extracted from the included studies—authors, journal, year of publication, sample size, type of NPWT, duration of treatment, SSI rate, seroma rate, dehiscence rate, haematoma rate, wound necrosis rate, time to drain removal and length of follow-up.
Meta-analysis was performed if there were three or more studies providing the outcome data. The unit of analysis is the breast itself, and not the individual participant. The pooled relative risks were calculated using a Mantel–Haenszel random effects model (DerSimonian and Laird method) [24]. A random effects model was used in expectation of clinical heterogeneity, irrespective of statistical heterogeneity. Pooled results were expressed according to odds ratios (OR) with the associated 95% confidence intervals (CIs). The absolute risk reduction (ARR)/absolute risk increase (ARI)/absolute risk difference and the associated number needed to treat (NNT) will be calculated if the OR was statistically significant. The ARR or ARI are weighted estimates of the difference in event rates [24]. Heterogeneity assessment was assessed using the I2 index test. In the event of significant heterogeneity for an outcome, data were re-analysed following exclusion of relevant trial(s). The risk of bias within studies was assessed using the Cochrane Collaboration tool. Review authors’ judgements about each risk of bias item were assessed and presented as overall summary and percentages across all included studies [25]. A two-sided P value of <0.05 was considered as statistically significant. Calculations were performed using RevMan version 5.3 and STATA version 14.2.
Results
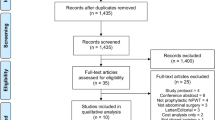
A total of seven studies, which included 904 patients with 1500 closed breast incisions were analysed. There were five prospective and two retrospective studies with a total of 681 and 819 incisions in the NPWT and non-NPWT dressing groups, respectively. A flow diagram of the selection process is summarised in Appendix 3.
All patients included in the analysis were female. The mean age of participants from those studies which provided this information was 43.7 years. Ferrando et al. [26] did not report the mean age of their cohort. All included studies were published between 2014 and 2018 (Table 1).
Two of the included studies were performed comparing NPWT to standard non-NPWT dressing [27, 28] by randomising either right or left breast to NPWT (Table 1). Tanaydin et al. [28] compared a single-use PICO™ (Smith and Nephew) NPWT dressing set at −80 mm Hg for up to 7 days with fixation strips (Steri-Strip™ (3 M)). Galiano et al. [27] also used the PICO™ set to −80 mm Hg, but it could be used for up to 14 days. In their case series of twenty-four patients undergoing oncoplastic procedures, Holt et al. [29] also utilised the PICO™ dressing set to −80 mm Hg for 6 days, but made no mention of their comparator. The study by Pellino et al. [30] included a mixture of colorectal (50%) and breast (50%) procedures. The investigators applied a PICO™ dressing at −80 mm Hg to the incisions in the NPWT group. Only the results from the breast group in this study were included in our meta-analysis. Gabriel et al. [31] utilised the PREVENA™ (KCI) NPWT system set to −125 mm Hg for 7 days in their retrospective study. In their prospective study, Ferrando et al. [26] also used the PREVENA™ system set to −125 mm Hg for 7 days.
Apart from the study by Tanaydin et al.[28], data on total wound complications were available in all included studies (1,436 incisions). NPWT was associated with a statistically significant lower rate of total wound complications compared to non-NPWT dressings [pooled odds ratio (OR) 0.36; 95% CI 0.19–069; P = 0.002] (Fig. 1). The number needed to treat (NNT) to prevent one wound complication was 6. Heterogeneity amongst included studies was statistically significant (τ23 = 0.35; P = 0.0006; I2 = 69%) (Fig. 1).
Four studies provided data on SSI (1,341 incisions) (Fig. 2). NPWT was associated with a statistically significant lower rate of SSI compared to non-NPWT dressings (pooled OR 0.45; 95% CI 0.24–0.86; P = 0.015, NNT = 50) (Fig. 2).
NPWT was associated with a statistically significantly lower rate of seroma formation compared to non-NPWT dressings in the four studies for which data were included (990 incisions) (pooled OR 0.28; 95% CI 0.13–0.59; P = 0.001, NNT = 20) (Fig. 3).
Data on wound dehiscence were included in four studies (1,175 incisions), and there was a statistically significant difference in favour of NPWT (pooled OR 0.49; 95% CI 0.32–0.72; P = 0.000, NNT = 13) (Fig. 4). Three studies provided data on wound necrosis (940 incisions). NPWT was associated with a statistically significant lower rate of necrosis compared to non-NPWT dressings (pooled OR 0.38; 95% CI 0.19–0.78; P = 0.008, NNT = 9) (Fig. 5).
Data on haematoma were included in three studies (940 incisions), but we found there to be no statistically significant difference between NPWT and non-NPWT dressings (pooled OR 0.8; 95% CI 0.19–3.2; P = 0.75) (Fig. 6).
There was no significant statistical evidence of heterogeneity for all secondary outcomes. Test for funnel plot asymmetry was not performed because its power is too low to distinguish chance from real asymmetry, since there were less than ten studies with available data for analysis.
Discussion
This study demonstrates that prophylactic use of NPWT for closed incisions in breast surgery is associated with a reduced risk of total wound complications, SSI, wound dehiscence, wound necrosis and seroma formation when compared to conventional non-NPWT dressings. There was no significant difference in rates of haematoma between the two groups. Overall, the prophylactic use of NPWT was associated with improved wound outcomes in patients with closed incisions undergoing breast surgery.
The use of NPWT on surgical wounds remains controversial. A 2014 Cochrane review concluded that there was no clear benefit from using NPWT in closed incisions [32]. This review included nine randomised control trials, three of which included patients undergoing split skin grafting. Of the six trials that looked at closed surgical incisions, four used the VAC® (KCI) negative pressure vacuum‐assisted closure device, one used the PREVENA™ system and the other used a homemade negative pressure device. None of those trials included patients undergoing breast surgery. The availability of newer devices specifically designed for closed surgical incisions such as PICO™ has prompted further interval research which lays the foundation for this study. In 2016, the World Health Organization published their Global Guidelines for the Prevention of SSI [33]. In the development of these guidelines, De Vries et al. [12] conducted a meta-analysis which showed that NPWT caused a significant reduction in SSI, but concluded that the overall quality of evidence was low. However, high-quality evidence continues to emerge that shows that wound complications can be prevented in both clean and clean–contaminated wounds with prophylactic application of NPWT [11, 34]. At present, the evidence for NPWT in breast surgery largely consists of small- to moderate-sized observational studies. The results of our study provide support for NPWT in the management of closed surgical incisions on the breast. Further research should be performed to determine which patients are likely to benefit most from these interventions.
Our understanding of NPWT and the role it can play in the management of both closed and open wounds is continually growing. Animal studies have shown demonstratable changes in microvascular blood flow around wounds that is dependent on the pressure applied, the distance from the wound edge and the tissue type [35]. There is uncertainty as to the optimum level of negative pressure to enhance this phenomenon, but it appears to be inhibited at values below −400 mm Hg with two studies by Kairinos et al., demonstrating that lower levels of negative pressure may reduce tissue perfusion and compromise vascularity. These findings suggest that NPWT application to already ischaemic tissue may further compromise their blood supply particularly in cases when it is applied circumferentially [36, 37]. Further studies have also shown increased rates of granulation tissue formation and reduced tissue bacterial counts with the application of NPWT [38]. There also appears to be a reduction in the level of tissue oedema which likely relates to improved lymphatic drainage, thus further enhancing the conditions for wound healing [10, 39, 40]. At a cellular level, this appears to translate into a modulation of cytokines to an anti-inflammatory profile with increased expression of signal proteins such as vascular endothelial growth factor, platelet‐derived growth factor and fibroblast growth factor 2, leading to angiogenesis, extracellular matrix remodelling and deposition of granulation tissue [41].
NPWT devices such as PICO™ are now available as a single-use battery-powered device and an easy-to-apply wound dressing with or without a small portable canister to collect the absorbed fluid. Patients can be easily taught about the device and be discharged home with it in place. Cost–benefit was not reported by any of our included studies; therefore, we have made no attempt to address it in this review. Nherera et al. suggest that the reduction in surgical site complications brought about by NPWT makes it a suitably cost-effective alternative to conventional dressings [16, 17]. Heard et al. [42] estimated that a 15% reduction in SSI would make NPWT cost-effective. Our results suggest that SSI can be reduced by more than 50% in breast incisions with NPWT use. This is a fast-moving and exciting development in wound management, and further studies regarding mechanism of action and cost-effectiveness will only provide further support for its widespread adaptation in clinical practice.
There are some potential limitations to our review. Due to an underreporting of patient co-morbidities in the included studies, we were unable to perform a meaningful subgroup analysis to assess NPWT efficacy in higher- versus lower-risk patients undergoing breast surgery. Similarly, there was significant heterogeneity in the types of surgical procedures being undertaken between the included studies ranging from simple mastectomy to implant-based reconstruction. We did not perform a subgroup analysis of different surgical procedures as there was not three or more studies assessing the effect of NPWT in any one procedure. Most of the included studies are non-randomised and therefore subject to selection bias. Within-patient randomisation was performed by Tanaydin et al. and Galiano et al., but this is not without limitations [27, 28]. Given the visible nature of the treatment, it is not possible to blind patients or investigators, thereby further predisposing our results to performance and detection bias. There was also significant clinical heterogeneity. Three studies made no mention of prophylactic antibiotic use [28, 29, 31] despite two of those studies including patients undergoing implant reconstruction. Two studies only provided antibiotics at induction [27, 30], while the remaining studies continued antibiotics until at least drain removal [26, 43]. Similarly, differences were evident regarding the use of surgical drains. Three studies did not mention whether drains were utilised [28,29,30]. Galiano et al. [27] used drains at the discretion of the operating surgeon, and the remaining three studies all used surgical drains [26, 31, 43]. The NPWT device utilised also varied amongst the included studies along with the applied negative pressure setting and length of treatment. While Steri-Strip™ were the most commonly investigated comparator, they were not utilised in all studies, thereby potentially further reducing the effect of our results. Despite this, our meta-analysis did not demonstrate any statistically significant heterogeneity for included studies apart from total wound complications.
Conclusions
Prophylactic negative pressure dressings applied to closed incisions in breast surgery are associated with a significant reduction in the total wound complications, SSI, seroma, wound dehiscence and wound necrosis. Widespread adaptation of NPWT in clinical practice is limited by its higher cost in comparison with conventional dressings. Further research evaluating the effect of NPWT on length of hospital stay and need for readmission or re-intervention in the event of surgical site complication will serve as a basis for calculating the long-term cost-saving potential of this technology.
References
Shepard J, Ward W, Milstone A, Carlson T, Frederick J, Hadhazy E et al (2013) Financial impact of surgical site infections on hospitals: the hospital management perspective. JAMA Surg 148:907–914
Cima R, Dankbar E, Lovely J, Pendlimari R, Aronhalt K, Nehring S et al (2013) Colorectal surgery surgical site infection reduction program: a national surgical quality improvement program-driven multidisciplinary single-institution experience. J Am Coll Surg. 216(1):23–33
Olsen MA, Chu-Ongsakul S, Brandt KE, Dietz JR, Mayfield J, Fraser VJ (2008) Hospital-associated costs due to surgical site infection after breast surgery. Arch Surg 43(1):53–60 (discussion 1)
Umscheid CA, Mitchell MD, Doshi JA, Agarwal R, Williams K, Brennan PJ (2011) Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect Control Hosp Epidemiol 32(2):101–114
de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB (2009) Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control 37(5):387–397
Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK et al (2013) Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med 173(22):2039–2046
Wilkes RP, Kilpad DV, Zhao Y, Kazala R, McNulty A (2012) Closed incision management with negative pressure wound therapy (CIM): biomechanics. Surg Innov 19(1):67–75
Meeker J, Weinhold P, Dahners L (2011) Negative pressure therapy on primarily closed wounds improves wound healing parameters at 3 days in a porcine model. J Orthop Trauma 25(12):756–761
Argenta LC, Morykwas MJ (1997) Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 38(6):563–576 (discussion 77)
Kilpadi DV, Cunningham MR (2011) Evaluation of closed incision management with negative pressure wound therapy (CIM): hematoma/seroma and involvement of the lymphatic system. Wound Repair Regener 19(5):588–596
Sahebally SM, McKevitt K, Stephens I, Fitzpatrick F, Deasy J, Burke JP, et al (2018) Negative pressure wound therapy for closed laparotomy incisions in general and colorectal surgery: a systematic review and meta-analysis. JAMA Surg 153:e183467
De Vries FE, Wallert ED, Solomkin JS, Allegranzi B, Egger M, Dellinger EP et al (2016) A systematic review and meta-analysis including GRADE qualification of the risk of surgical site infections after prophylactic negative pressure wound therapy compared with conventional dressings in clean and contaminated surgery. Medicine (Baltimore) 95(36):e4673
Strugala V, Martin R (2017) Meta-analysis of comparative trials evaluating a prophylactic single-use negative pressure wound therapy system for the prevention of surgical site complications. Surg Infect (Larchmt) 18(7):810–819
Karlakki S, Brem M, Giannini S, Khanduja V, Stannard J, Martin R (2013) Negative pressure wound therapy for managementof the surgical incision in orthopaedic surgery: a review of evidence and mechanisms for an emerging indication. Bone Joint Res 2(12):276–284
Hyldig N, Birke-Sorensen H, Kruse M, Vinter C, Joergensen JS, Sorensen JA et al (2016) Meta-analysis of negative-pressure wound therapy for closed surgical incisions. Br J Surg 103(5):477–486
Nherera LM, Trueman P, Karlakki SL (2017) Cost-effectiveness analysis of single-use negative pressure wound therapy dressings (sNPWT) to reduce surgical site complications (SSC) in routine primary hip and knee replacements. Wound Repair Regener 25(3):474–482
Nherera LM, Trueman P, Schmoeckel M, Fatoye FA (2018) Cost-effectiveness analysis of single use negative pressure wound therapy dressings (sNPWT) compared to standard of care in reducing surgical site complications (SSC) in patients undergoing coronary artery bypass grafting surgery. J Cardiothorac Surg 13(1):103
Berrios-Torres SI, Umscheid CA, Bratzler DW, Leas B, Stone EC, Kelz RR et al (2017) Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg 152(8):784–791
Olsen MA, Nickel KB, Fox IK, Margenthaler JA, Ball KE, Mines D et al (2015) Incidence of surgical site infection following mastectomy with and without immediate reconstruction using private insurer claims data. Infect Control Hosp Epidemiol 36(8):907–914
Buchholz TA, Austin-Seymour MM, Moe RE, Ellis GK, Livingston RB, Pelton JG et al (1993) Effect of delay in radiation in the combined modality treatment of breast cancer. Int J Radiat Oncol Biol Phys 26(1):23–35
Hershman DL, Wang X, McBride R, Jacobson JS, Grann VR, Neugut AI (2006) Delay of adjuvant chemotherapy initiation following breast cancer surgery among elderly women. Breast Cancer Res Treat 99(3):313–321
Vikatmaa P, Juutilainen V, Kuukasjärvi P, Malmivaara A (2008) Negative pressure wound therapy: a systematic review on effectiveness and safety. Eur J Vasc Endovasc Surg 36(4):438–448
Peinemann F, Sauerland S (2011) Negative-pressure wound therapy: systematic review of randomized controlled trials. Deutsches Arzteblatt Int 108(22):381–389
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD et al (2011) The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343:d5928
Ferrando PM, Ala A, Bussone R, Bergamasco L, Actis Perinetti F, Malan F (2018) Closed incision negative pressure therapy in oncological breast surgery: comparison with standard care dressings. Plast Reconstr Surg Glob Open 6(6):e1732
Galiano RD, Hudson D, Shin J, van der Hulst R, Tanaydin V, Djohan R et al (2018) Incisional negative pressure wound therapy for prevention of wound healing complications following reduction mammaplasty. Plast Reconstr Surg Glob Open 6(1):e1560
Tanaydin V, Beugels J, Andriessen A, Sawor JH, van der Hulst RRWJ (2018) Randomized controlled study comparing disposable negative-pressure wound therapy with standard care in bilateral breast reduction mammoplasty evaluating surgical site complications and scar quality. Aesthetic Plast Surg 42(4):927–935
Holt R, Murphy J (2015) PICO™ incision closure in oncoplastic breast surgery: a case series. Br J Hosp Med (Lond) 76(4):217–223
Pellino G, Sciaudone G, Candilio G, De Fatico GS, Landino I, Della Corte A et al (2014) Preventive NPWT over closed incisions in general surgery: does age matter? Int J Surg. 12:S64–S68
Gabriel A, Sigalove S, Sigalove N, Storm-Dickerson T, Rice J, Maxwell P et al (2018) The impact of closed incision negative pressure therapy on postoperative breast reconstruction outcomes. Plast Reconstr Surg Glob Open 6(8):e1880
Webster J, Scuffham P, Stankiewicz M, Chaboyer WP (2014) Negative pressure wound therapy for skin grafts and surgical wounds healing by primary intention. Cochrane Database Syst Rev 10:Cd009261
WHO Guidelines Approved by the Guidelines Review Committee (2016) Global guidelines for the prevention of surgical site infection. Geneva: World Health Organization. Copyright (c) World Health Organization
O'Leary DP, Peirce C, Anglim B, Burton M, Concannon E, Carter M, et al (2017) Prophylactic negative pressure dressing use in closed laparotomy wounds following abdominal operations: a randomized, controlled, open-label trial: the P.I.C.O. trial. Ann Surg 265(6):1082–1086
Wackenfors A1, Sjögren J, Gustafsson R, Algotsson L, Ingemansson R, Malmsjö M (2004) Effects of vacuum-assisted closure therapy on inguinal wound edge microvascular blood flow. Wound Repair Regen 12(6):600–606
Kairinos N, Solomons M, Hudson DA (2009) Negative-pressure wound therapy I: the paradox of negative-pressure wound therapy. Plast Reconstr Surg 123(2):589–598 (discussion 99–600)
Kairinos N, Voogd AM, Botha PH, Kotze T, Kahn D, Hudson DA, et al (2009) Negative-pressure wound therapy II: negative-pressure wound therapy and increased perfusion. Just an illusion? Plast Reconstr Surg 123(2):601–612
Morykwas MJ, Argenta LC, Shelton-Brown EI, McGuirt W (1997) Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 38(6):553–562
Kamolz LP, Andel H, Haslik W, Winter W, Meissl G, Frey M (2004) Use of subatmospheric pressure therapy to prevent burn wound progression in human: first experiences. Burns 30(3):253–258
Eisenhardt SU, Schmidt Y, Thiele JR, Iblher N, Penna V, Torio-Padron N et al (2012) Negative pressure wound therapy reduces the ischaemia/reperfusion-associated inflammatory response in free muscle flaps. J Plast Reconstr Aesthet Surg 65(5):640–649
Glass GE, Murphy GF, Esmaeili A, Lai LM, Nanchahal J (2014) Systematic review of molecular mechanism of action of negative-pressure wound therapy. Br J Surg 101(13):1627–1636
Heard C, Chaboyer W, Anderson V, Gillespie BM, Whitty JA (2017) Cost-effectiveness analysis alongside a pilot study of prophylactic negative pressure wound therapy. J Tissue Viability 26(1):79–84
Kim DY, Park SJ, Bang SI, Mun GH, Pyon JK (2016) Does the use of incisional negative-pressure wound therapy prevent mastectomy flap necrosis in immediate expander-based breast reconstruction? Plast Reconstr Surg 138(3):558–566
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1
See Table
2.
Appendix 2. PubMed search strategy
(((((negative[All Fields] AND ("pressure"[MeSH Terms] OR "pressure"[All Fields])) OR ("negative-pressure wound therapy"[MeSH Terms] OR ("negative-pressure"[All Fields] AND "wound"[All Fields] AND "therapy"[All Fields]) OR "negative-pressure wound therapy"[All Fields] OR ("negative"[All Fields] AND "pressure"[All Fields] AND "dressing"[All Fields]) OR "negative pressure dressing"[All Fields])) OR VAC[All Fields]) OR PICO[All Fields]) OR ("negative-pressure wound therapy"[MeSH Terms] OR ("negative-pressure"[All Fields] AND "wound"[All Fields] AND "therapy"[All Fields]) OR "negative-pressure wound therapy"[All Fields] OR ("negative"[All Fields] AND "pressure"[All Fields] AND "wound"[All Fields] AND "therapy"[All Fields]) OR "negative pressure wound therapy"[All Fields])) AND (((((("breast"[MeSH Terms] OR "breast"[All Fields]) AND ("surgery"[Subheading] OR "surgery"[All Fields] OR "surgical procedures, operative"[MeSH Terms] OR ("surgical"[All Fields] AND "procedures"[All Fields] AND "operative"[All Fields]) OR "operative surgical procedures"[All Fields] OR "surgery"[All Fields] OR "general surgery"[MeSH Terms] OR ("general"[All Fields] AND "surgery"[All Fields]) OR "general surgery"[All Fields])) OR ("mammaplasty"[MeSH Terms] OR "mammaplasty"[All Fields] OR ("breast"[All Fields] AND "reconstruction"[All Fields]) OR "breast reconstruction"[All Fields])) OR (("breast"[MeSH Terms] OR "breast"[All Fields]) AND augmentation[All Fields])) OR (("breast"[MeSH Terms] OR "breast"[All Fields]) AND reduction[All Fields])) OR ("mastectomy, simple"[MeSH Terms] OR ("mastectomy"[All Fields] AND "simple"[All Fields]) OR "simple mastectomy"[All Fields] OR "mastectomy"[All Fields] OR "mastectomy"[MeSH Terms])).
Appendix 3
See Fig.
7.
Rights and permissions
About this article
Cite this article
Cagney, D., Simmons, L., O’Leary, D.P. et al. The Efficacy of Prophylactic Negative Pressure Wound Therapy for Closed Incisions in Breast Surgery: A Systematic Review and Meta-Analysis. World J Surg 44, 1526–1537 (2020). https://doi.org/10.1007/s00268-019-05335-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-019-05335-x