Abstract
Background
Hepatectomy remains an important curative treatment for hepatocellular carcinoma (HCC). Intermittent Pringle maneuver (IPM) is commonly applied during hepatectomy for control of bleeding. Whether the ischemia/reperfusion injury brought by IPM adversely affects the operative outcomes is controversial. This study aims to examine whether the application of IPM during hepatectomy affects the long-term outcomes.
Methods
Two randomized controlled trials (RCT) have been carried out previously to evaluate the short-term outcomes of IPM. The present study represented a post hoc analysis on the HCC patients from the first RCT and all patients from the second RCT, and the long-term outcomes were evaluated.
Results
There were 88 patients each in the IPM group and the no-Pringle-maneuver (NPM) group. The patient demographics, type and extent of liver resection and histopathological findings were comparable between the two groups. The 1-, 3-, 5-year overall survival in the IPM and NPM groups was 92.0%, 82.0%, 72.1% and 93.2%, 68.8%, 58.1%, respectively (P = 0.030). The 1-, 3-, 5-year disease-free survival in the IPM and NPM groups was 73.6%, 56.2%, 49.7% and 71.6%, 49.4%, 40.3%, respectively (P = 0.366). On multivariable analysis, IPM was a favorable factor for overall survival (P = 0.035). Subgroup analysis showed that a clamp time of 16–30 min (P = 0.024) and cirrhotic patients with IPM (P = 0.009) had better overall survival.
Conclusion
IPM provided a better overall survival after hepatectomy for patients with HCC. Such survival benefit was noted in cirrhotic patients, and the beneficial duration of clamp was 16–30 min.
Trial registration
NCT00730743 and NCT01759901 (http://www.clinicaltrials.gov).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatectomy remains the most effective curative treatment for hepatocellular carcinoma (HCC) [1]. Hepatic pedicle clamping or Pringle maneuver (PM) is commonly applied to reduce blood loss during liver resection [2,3,4]. Among various ways of applying PM, the commonly used method is to apply clamping of hepatoduodenal ligament for 15 min followed by unclamping for 5 min or the intermittent Pringle maneuver (IPM) [5]. Controversy still exists in regard to the efficacy of IPM to reduce blood loss and possible damage to the liver due to ischemia/reperfusion injury [6,7,8].
Two randomized controlled trials on IPM were carried out in our center previously [9, 10]. The first one was done in the period 2008–2011 to assess the efficacy of IPM to reduce blood loss during hepatectomy for various indications [9]. The second one was done in the period 2013–2016 to assess the postoperative complication rate with or without applying IPM during hepatectomy for HCC [10]. The results of both studies have been reported previously.
Another controversial issue on PM is its long-term effect on tumor recurrence and survival. While some studies showed that PM might increase tumor recurrence and jeopardize the long-term survival [11, 12], other studies revealed no adverse effect of PM at all [13, 14]. The pattern of survival curves from our second study [10] suggested that there might be some difference in survival between IPM and NPM groups. In order to address the relationship between IPM and survival, the current study represented a post hoc analysis of HCC patients from the first trial and all patients from the second trial.
Methods
The present study combined two previous prospective randomized studies on IPM from our group. The first trial included 63 patients each in the IPM and the no-Pringle-maneuver (NPM) group. Thirty-eight patients from each group had histologically confirmed HCC [9]. In the second trial, there were 50 patients each in the IPM and NPM groups, all with histological confirmed HCC [10]. By combining all HCC patients from both studies, there were 88 patients each in the IPM and NPM groups. Both previous studies were approved by the Clinical Research Ethics Committee of the Joint Chinese University of Hong Kong – New Territories East Cluster, and both were registered at Clinicaltrials.gov (Registration number NCT00730743 and NCT01759901).
Patient selection
Patient selection was similar in both studies. Patients above the age 18 years who were scheduled for open hepatectomy were recruited. Patients were excluded if there was portal vein thrombosis, portal vein tumor thrombus or previous portal vein embolization. Patients with hepatic artery thrombosis or previous transarterial therapy were also excluded. Other exclusion criteria included cases of emergency hepatectomy, hepatectomy for ruptured HCC, anticipated portal vein resection or concomitant bowel or bile duct resection.
Randomization
Randomization was done in the operating room after laparotomy confirmed liver resection could proceed. Patients would be excluded if there were adhesions or any anatomical variations that might preclude safe application of IPM. Randomization was stratified according to the presence or absence of liver cirrhosis as judged by operating surgeons. Block randomization was used. Block size of 2 and 4 was randomly assigned to generate the randomization sequence. The computer-generated numbers were prepared and kept inside sealed envelopes by a research assistant who was not physically present in the operating room.
Operative technique
This was described in our previous two studies [9, 10]. IPM was done by encircling the hepaticoduodenal ligament and applying a vascular clamp in an intermittent manner: 15-min clamping followed by unclamping for 5 min till end of liver transection. The central venous pressure was kept below 5 mmHg during transection if possible. Liver resections were classified according to Brisbane 2000 terminology and was defined as a major when three or more liver segments were removed [15].
Outcome measurement
Operative outcomes including operative blood loss, need for transfusion, operative time and postoperative hospital stay were all recorded. Complications were graded according to the Clavien–Dindo classification [16]. Any death within 90 days after surgery was defined as operative mortality. After surgery, patients were followed up in outpatient clinics 3 monthly within first year and then half yearly afterward. Ultrasound or computed tomography was offered 3 monthly in first year and then half yearly afterward. Chest X-ray would be done half yearly. Alpha-feto protein (AFP) would be checked before each follow-up. Overall survival (OS) was calculated from time of hepatectomy to date of death or last available follow-up. Disease-free survival (DFS) was calculated from time of hepatectomy to date of diagnosis of first recurrence or last available follow-up.
Statistical analysis
All data were prospectively collected by a research assistant and kept in a computer database. Data were presented as median (range) unless specified otherwise. Statistical analysis was performed by Chi-square test or Fisher’s exact test to compare categorical variables and Mann–Whitney U test to compare continuous variables. The Kaplan–Meier method was used to estimate the survival rates, and the survival curves were compared by the log-rank test. Univariate and multivariable analyses were done by Cox regression. P < 0.05 was taken as the level of statistical significance. All statistical data analyses were performed using SPSS version 21.0 (IBM Corp 2012).
Results
There were 88 patients each in the IPM and the NPM groups. The two groups were comparable in age, sex, body mass index, number of comorbidities and American Society of Anesthesiologists (ASA) score (Table 1). All patients in this study were of Child’s grade A liver function. Most of the patients were hepatitis B carriers (75–80%). The two groups did not differ in preoperative complete blood counts, liver function, renal function, clotting profile and AFP value. Indocyanine green (ICG) test was performed for all patients, and there was no difference in retention rate at 15 min (R15) between the two groups (Table 1).
The proportion of major hepatectomy was similar between the two groups (39–42%) (Table 2). There was no difference in types of liver resection between the two groups. The operative time, blood loss and blood transfusion rate were similar. There was one operative mortality in each group. Complication rate and severity of complication according to Clavien–Dindo classification were similar except pleural effusion was significantly more in the IPM group (13.6% vs 3.4%, P = 0.015). Length of postoperative hospital stay was similar (Table 2).
Concerning the histopathological findings, there was no difference between the two groups in terms of American Joint Committee on Cancer (AJCC) staging, number of tumors, size of largest tumor, resection margin and incidence of vascular invasion (Table 3).
The median follow-up period for the IPM group and the NPM group was 48.7 months and 39.9 months, respectively. There was no difference in the rate of tumor recurrence, intrahepatic recurrence and extrahepatic recurrence (Table 4). There was no difference in non-disease-related deaths. Besides, types of treatment for recurrent disease did not differ between the two groups. The 1-, 3-, 5-year OS in the IPM and the NPM group was 92.0%, 82.0%, 72.1% and 93.2%, 68.8%, 58.1%, respectively. The 1-, 3-, 5-year DFS in the IPM and NPM groups were 73.6%,56.2%, 49.7% and 71.6%, 49.4%, 40.3%, respectively. Log-rank test revealed a significant better OS (P = 0.030) but not DFS (P = 0.366) in the IPM group (Fig. 1).
Subgroup analysis in cirrhotic patients revealed IPM had a significant better OS (P = 0.009) than NPM, but DFS was similar. In the non-cirrhotic patients, both the OS and DFS were similar between the two groups (Table 5). Subgroup analysis was also performed in major and minor hepatectomy patients, and in patients with or without vascular invasion, no difference in both the OS and DFS was found between IPM and NPM in these subgroups.
The total clamp time was categorized into four different groups (4 patients ≤ 15 min, 9 patients 16–30 min, 56 patients 31–45 min and 19 patients > 45 min) to compare with 88 patients in the NPM group. When the overall survival of the NPM group was compared with the overall survival of the four different clamp time groups individually by log-rank test, the P values were 0.296, 0.024, 0.066 and 0.422, respectively. Hence, only the group with clamp time 16–30 min had significantly better overall survival than the NPM group. For DFS, there was no difference in the NPM group compared with all the four different clamp time groups.
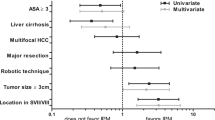
Univariate and multivariable analyses were done to determine the factors affecting OS and DFS. For OS, factors which were significant on univariate analysis were ASA score, ICG (R15), preoperative albumin and alkaline phosphatase, use of IPM, blood loss, multiplicity of tumor, tumor size and vascular invasion. Only IPM or NPM, blood loss ( > 320 ml or ≤ 320 ml) and solitary/multinodular tumor were significant factors on multivariable analysis (Table 6). For DFS, significant factors on univariate analysis were ASA score, preoperative albumin and alkaline phosphatase, type of hepatectomy, multiplicity of tumor, tumor size and vascular invasion. Only preoperative albumin, type of hepatectomy, tumor size and vascular invasion were significant factors on multivariable analysis (Table 6).
Discussion
IPM is still commonly used by many liver surgeons during hepatectomy in an attempt to reduce intra-operative blood loss. Though initial randomized controlled trials showed that it was effective in reducing blood loss [6, 7], subsequent trials did not confirm this effect [8,9,10]. The other concern for IPM is its ischemia/reperfusion injury [17]. Use of IPM may increase chance of postoperative liver failure and surgical complications. Our previous trial revealed no increase in complications or liver, failure but there was an increase in subclinical ascites and pleural effusion [10]. Even more worrying issue about the use of IPM is its long-term effect. In animal studies, ischemia/reperfusion injury might negatively impact the oncological outcomes by accelerating tumor growth and promoting metastases [18,19,20]. In a survey among European hepatobiliary surgeons, 71% of surgeons indicated that they applied PM when it was deemed appropriate and, when applied, 65% applied it intermittently [21]. According to a national survey from Japan, PM was performed in 93.5% of the hospitals (routinely in 25%) [22]. Thus an understanding of IPM on the impact of long-term outcomes after hepatectomy is crucial for the liver surgeons to adopt the technique or not.
Retrospective studies have yielded controversial results surrounding the impact of PM on long-term outcomes after hepatectomy for HCC. While some studies showed that there was no effect [13, 14, 23], other studies revealed a negative impact on long-term survival and disease recurrence [11, 12, 24,25,26]. Similarly, retrospective studies on the impact of IPM on the long-term outcomes of hepatectomy for colorectal liver metastasis (CRM) revealed controversial results [27,28,29,30]. One meta-analysis [31] and one randomized controlled trial [32] reached the same conclusion that IPM did not seem to affect the survival of patients with CRM.
To our knowledge, the present analysis is the first to study data from randomized controlled trials to evaluate the impact of IPM on the long-term outcomes after liver resection for HCC. Although the study was a combination of two separate studies to evaluate the short-term outcomes, the two groups were comparable and the follow-up treatment remained essentially the same throughout the period. Interestingly, patients with IPM had significantly longer overall survival than the NPM group, while the DFS was not different. This is something that cannot be easily explained. An even more interesting finding is that the benefit of improved OS is seen in cirrhotic patients but not in non-cirrhotic patients. This is contrary to the usual belief that pathological liver including cirrhosis tolerate ischemia/reperfusion poorer. The survival benefit was only seen with total clamp time of 16–30 min. Actually there was also a survival benefit seen in the clamp group 31–45 min group but not reaching statistically significance (P = 0.066). This can be due to a Type II error. It seems that a modest duration of clamp 16–45 min is most desirable to get the survival benefit. Theoretically, a clamp time less than 15 min is almost equivalent to the no-clamp group. Prolonged clamp time more than 45 min may reflect a more difficult and lengthy operation with more blood loss. The animal study by van der Bilt et al. showed that the outgrowth of micrometastases in occluded liver for 45 min was accelerated five- to sixfold of the nonoccluded lobe [33]. However, the authors also showed that such accelerated tumor growth was completely prevented by IPM for 3 cycles. A clinical study in human showed that prolonged IPM time more than 60 min had a significantly shorter OS compared with that < 60 min for HCC [11]. Another study on CRM showed that prolonged IPM time more than 45 min was associated with decreased time to hepatic tumor recurrence [27]. Since the median clamp time in the present study was only 45 min, any adverse effect due to prolonged clamping might not show up in the result.
Conversely, a study has reported that use of IPM might actually reduce extrahepatic recurrence in HCC [34]. It was postulated that clamping during liver resection might reduce the risk of tumor cell shedding and dissemination into the portal venous system. In our study, since there is no difference in tumor recurrence rate and DFS rate, it is unlikely that the application of IPM affects the incidence of tumor recurrence. An improved OS but not DSF survival may imply that patients can receive more radical re-treatment. However, on further analysis, there was no difference between the two groups in proportion of radical re-treatment like re-hepatectomy and local ablation (Table 4).
In this study, the use of IPM has been shown to be a positive predictor in multivariable analysis for OS. It was not a predictor for DFS. Improved survival associated with the use of IPM was rarely reported in the literature. One case-matched study involving CRM patients reported that the 5-year recurrence-free survival rate of IPM patients was significantly higher than that of the NPM group [35]. Another recent study showed that AJCC stage IIIB HCC patients (with macrovascular invasion) had better OS and recurrence-free survival with clamp time > 12 min than those with NPM or clamp time < 12 min [36]. The protective effect of IPM in this group of patient was believed to be due to the blockage of tumor cell dissemination in the portal tributaries and into remnant liver. We have performed subgroup analysis for tumor with or without vascular invasion, but no significant difference was seen on OS and DFS. The mechanism for the superior OS in the IPM group in this study is still poorly understood.
Since only retrospective data and pooled samples were used in this study, the results were considered to be preliminary and further studies are needed. However, this analysis gave an insight that IPM might have real positive impact on HCC patients after hepatectomy. Apart from designing a new randomized control trial, a systemic review on randomized control study of IPM and individual patient data (IPD) meta-analysis could help reduce bias between different randomized control trials.
In conclusion, this study suggested that IPM provided a better OS after hepatectomy for HCC. Such survival benefit was noted in cirrhotic patients, and the beneficial duration of clamp was 16–30 min.
References
Bruix J, Gores GJ, Mazzaferro V (2014) Hepatocellular carcinoma: clinical frontiers and perspectives. Gut 63:844–855
Nuzzo G, Giuliante F, Giovannini I et al (2001) Liver resections with or without pedicle clamping. Am J Surg 181:238–246
Jarnagin WR, Gonen M, Fong Y et al (2002) Improvement in perioperative outcome after hepatic resection: analysis of 1803 consecutive cases over the past decade. Ann Surg 236:397–406
Imamura H, Takayama T, Sugawara Y et al (2002) Pringle's manoeuvre in living donors. Lancet 360:2049–2050
Belghiti J, Noun R, Malafosse R et al (1999) Continuous versus intermittent portal triad clamping for liver resection: a controlled study. Ann Surg 229:369–375
Man K, Fan ST, Ng IO et al (1997) Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg 226:704–711
Man K, Lo CM, Liu CL et al (2003) Effects of the intermittent Pringle manoeuvre on hepatic gene expression and ultrastructure in a randomized clinical study. Br J Surg 90:183–189
Capussotti L, Muratore A, Ferrero A et al (2006) Randomized clinical trial of liver resection with and without hepatic pedicle clamping. Br J Surg 93:685–689
Lee KF, Cheung YS, Wong J et al (2012) Randomized clinical trial of open hepatectomy with or without intermittent Pringle manoeuvre. Br J Surg 99:1203–1209
Lee KF, Wong J, Cheung SYS et al (2018) Does intermittent Pringle maneuver Increase postoperative complications after hepatectomy for hepatocellular carcinoma? A randomized controlled trial. World J Surg 42:3302–3311. https://doi.org/10.1007/s00268-018-4637-3
Ishizuka M, Kubota K, Kita J et al (2011) Duration of hepatic vascular inflow clamping and survival after liver resection for hepatocellular carcinoma. Br J Surg 98:1284–1290
Hao S, Chen S, Yang X et al (2016) Impact of intermittent portal clamping on the early recurrence of hepatocellular carcinoma after surgery. Surg Today 46:1290–1295
Xia F, Lau WY, Xu Y et al (2013) Does hepatic ischemia-reperfusion injury induced by hepatic pedicle clamping affect survival after partial hepatectomy for hepatocellular carcinoma? World J Surg 37:192–201. https://doi.org/10.1007/s00268-012-1781-z
Huang J, Tang W, Hernandez-Alejandro R et al (2014) Intermittent hepatic inflow occlusion during partial hepatectomy for hepatocellular carcinoma does not shorten overall survival or increase the likelihood of tumor recurrence. Medicine (Baltimore) 93:e288
Strasberg SM (2005) Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 systems. J Hepatobiliary Pancreat Surg 12:351–355
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort 6336 patients and results of a survey. Ann Surg 240:205–213
Kim YI (2003) Ischemia-reperfusion injury of the human liver during hepatic resection. J Hepatobiliary Pancreat Surg 10:195–199
Doi K, Horiuchi T, Uchinami M et al (2002) Hepatic ischemia-reperfusion promotes liver metastasis of colon cancer. J Surg Res 105(243):247
van der Bilt JD, Soeters ME, Duyverman AM et al (2007) Perinecrotic hypoxia contributes to ischemia/reperfusion-accelerated outgrowth of colorectal micrometastases. Am J Pathol 170:1379–1388
Lim C, Broqueres-You D, Brouland JP et al (2013) Hepatic ischemia-reperfusion increases circulating bone marrow-derived progenitor cells and tumor growth in a mouse model of colorectal liver metastases. J Surg Res 184:888–897
van der Bilt JD, Livestro DP, Borren A et al (2007) European survey on the application of vascular clamping in liver surgery. Dig Surg 24:423–435
Nakajima Y, Shimamura T, Kamiyama T et al (2002) Control of intraoperative bleeding during liver resection: analysis of a questionnaire sent to 231 Japanese hospitals. Surg Today 32:48–52
Famularo S, Giani A, Di Sandro S et al (2018) Does the Pringle maneuver affect survival and recurrence following surgical resection for hepatocellular carcinoma? A western series of 441 patients. J Surg Oncol 117:198–206
Wang CC, Iyer SG, Low JK et al (2009) Perioperative factors affecting long-term outcomes of 473 consecutive patients undergoing hepatectomy for hepatocellular carcinoma. Ann Surg Oncol 16:1832–1842
Liu S, Li X, Li H et al (2016) Longer duration of the Pringle maneuver is associated with hepatocellular carcinoma recurrence following curative resection. J Surg Oncol 114:112–118
Hao S, Chen S, Yang X et al (2017) Adverse impact of intermittent portal clamping on long-term postoperative outcomes in hepatocellular carcinoma. Ann R Coll Surg Engl 99:22–27
Nijkamp MW, van der Bilt JD, Snoeren N et al (2010) Prolonged portal triad clamping during liver surgery for colorectal liver metastases is associated with decreased time to hepatic tumour recurrence. Eur J Surg Oncol 36:182–188
Wong KH, Hamady ZZ, Malik HZ et al (2008) Intermittent Pringle manoeuvre is not associated with adverse long-term prognosis after resection for colorectal liver metastases. Br J Surg 95:985–989
Weiss MJ, Ito H, Araujo RL et al (2013) Hepatic pedicle clamping during hepatic resection for colorectal liver metastases: no impact on survival or hepatic recurrence. Ann Surg Oncol 20:285–294
Giuliante F, Ardito F, Pulitanò C et al (2010) Does hepatic pedicle clamping affect disease-free survival following liver resection for colorectal metastases? Ann Surg 252:1020–1026
Matsuda A, Miyashita M, Matsumoto S et al (2013) Hepatic pedicle clamping does not worsen survival after hepatic resection for colorectal liver metastasis: results from a systematic review and meta-analysis. Ann Surg Oncol 20:3771–3778
Ferrero A, Russolillo N, Viganò L et al (2010) Does Pringle maneuver affect survival in patients with colorectal liver metastases? World J Surg 34:2418–2425. https://doi.org/10.1007/s00268-010-0682-2
van der Bilt JD, Kranenburg O, Nijkamp MW et al (2005) Ischemia/reperfusion accelerates the outgrowth of hepatic micrometastases in a highly standardized murine model. Hepatology 42:165–175
Tanaka K, Shimada H, Matsuo K et al (2008) Clinical features of hepatocellular carcinoma developing extrahepatic recurrences after curative resection. World J Surg 32:1738–1747. https://doi.org/10.1007/s00268-008-9613-x
De Carlis L, Di Sandro S, Giacomoni A et al (2013) Colorectal liver metastases: hepatic pedicle clamping during hepatectomy reduces the incidence of tumor recurrence in selected patients. Case-matched analysis Eur J Surg Oncol 39:726–733
Li X, Liu S, Li H et al (2016) Proper hepatic pedicle clamping during hepatectomy is associated with improved postoperative long-term prognosis in patients with AJCC stage IIIB hepatocellular carcinoma. Oncotarget 7:24623–24632
Acknowledgements
We would like to thank Mr. Philip IP for his assistance in patient randomization, data collection, data processing and statistical analysis.
Funding
Source of funding or grant support for the study: nil.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors have no conflicts of interest or financial ties to disclose.
Informed consent
Informed consent was obtained from all individual participants included in the study. Studies were approved by the Clinical Research Ethics Committee of the Joint Chinese University of Hong Kong—New Territories East Cluster.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, K.F., Chong, C.C.N., Cheung, S.Y.S. et al. Impact of Intermittent Pringle Maneuver on Long-Term Survival After Hepatectomy for Hepatocellular Carcinoma: Result from Two Combined Randomized Controlled Trials. World J Surg 43, 3101–3109 (2019). https://doi.org/10.1007/s00268-019-05130-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-019-05130-8