Abstract
Background
Dysphagia after Nissen fundoplication is challenging for patients. High-resolution manometry (HRM) has rarely been studied preoperatively to determine whether manometry values correlated with postoperative dysphagia after fundoplication. We aim to determine whether HRM criteria could predict dysphagia after Nissen fundoplication.
Methods
A retrospective review of single-institution laparoscopic Nissen fundoplications (LNF) from 2013 to 2015 was completed. Dysphagia was graded using a standard scale. Four groups were: those with new postoperative dysphagia (ND), never had dysphagia (NV), continued dysphagia (CD), and resolved dysphagia (RD). Manometry criteria included distal contractile integral (DCI), contraction front velocity (CFV), distal latency (DL), integrated relaxation pressure (IRP), percent peristalsis (PP), and distal esophageal contraction amplitude (DECA). Statistical bootstrapping was used to power sample sizes for ANOVA analysis.
Results
Ninety-four patients were included in the original cohort. Statistical bootstrapping sample size was 2992 patients. Among patients who did not have dysphagia prior to LNF, no HRM metric was associated with developing new dysphagia. Among those with dysphagia prior to LNF, a higher DCI, CFV, DL, PP, and DECA were associated with resolution of dysphagia. The RD group was 2.77 times more likely to have a DCI ≥ 1000 mmHg-s-cm compared with the CD group.
Conclusions
HRM criteria could not predict the development of postoperative dysphagia. However, in those with preoperative dysphagia and strong manometry criteria, dysphagia is more likely to resolve after Nissen fundoplication. Meanwhile, in those with preoperative dysphagia and weak manometry, dysphagia may persist and these patients may be better served with a partial fundoplication.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastroesophageal reflux disease (GERD) is a common disease with increasing incidence [1]. Therapy includes antisecretory medications and antireflux surgery (ARS). Preoperative workup includes pH testing, upper endoscopy, and esophageal manometry. Conventional manometry has been replaced by high-resolution manometry (HRM). Benefits over conventional manometry include more sensors along the catheter and color-rich contour plots allowing for better assessment of esophageal function.
Studies have shown excellent patient satisfaction after Nissen fundoplication [2,3,4]. Although early postoperative dysphagia is relatively common, in most patients it resolves by 8–12 weeks postoperatively. However, in some series up to 25% of patients required endoscopic dilation or reoperation [5]. Several papers have studied the association of preoperative conventional manometry metrics and postoperative dysphagia. However, no manometric variable has been found to be consistently associated with dysphagia [6,7,8,9,10]. Few studies have examined the association of dysphagia and postoperative HRM, and even less have analyzed preoperative HRM metrics and postoperative dysphagia [11,12,13].
The aim of this study was to determine whether preoperative HRM criteria could be used to predict whether patients will have new onset or persistent dysphagia after a laparoscopic Nissen fundoplication.
Materials and methods
Patient selection and workup
We performed a retrospective review of all laparoscopic Nissen fundoplications (LNF) at our center from 2013 to 2015. LNF were offered to patients with confirmed GERD (DeMeester Score > 14.7 on pH testing, abnormal impedance–pH testing, or endoscopic evidence of esophagitis), percent peristalsis (PP) > 50%, and distal esophageal contraction amplitude (DECA) greater than 25 mmHg on manometry [14, 15]. The high-resolution parameters, DCI, CFV, DL, IRP, were not used in the selection criteria for LNF, though the authors did use conventional manometry values of PP and DECA to select for LNF. Patients were included if they had preoperative HRM and clinical follow-up three or more months postoperatively. Exclusion criteria included incomplete manometry data, paraesophageal hernias (type 2–4), or functional dysmotility (e.g., achalasia). The Institutional Review Board approved this study.
Dysphagia was graded using a standard system (0 = never, 1 = 1–2 times a month, 2 = 1–2 times per week, 3 = daily). Patients graded their dysphagia on the questionnaire during their initial and postoperative clinic visits. A score of 2 or more defined clinically significant dysphagia for this study. Four groups were identified: those who never had dysphagia before or after surgery (NV), those with new postoperative dysphagia (ND), those with preoperative dysphagia that continued (CD), and those with preoperative dysphagia that resolved after LNF (RD).
HRM was performed in standard fashion as previously reported, evaluating distal contractile integral (DCI), contraction front velocity (CFV), distal latency (DL), and integrated relaxation pressure (IRP) [16]. Other manometry data collected were percent peristalsis (PP) and distal esophageal contraction amplitude (DECA). All patients had a standard laparoscopic 360° (Nissen) fundoplication over a large-diameter bougie (54–60 French) as previously described [9, 17].
Statistical analysis
Primary outcomes of this study were statistical differences in HRM metrics between dysphagia groups. Chi-square test was utilized to determine whether preoperative dysphagia status alone was associated with the resolution or continuation of dysphagia. Mann–Whitney test was used to detect differences between continuous HRM variables. Power analysis was performed based on sample statistics gathered from a literature review for manometry data DECA, DCI, IRP, and CFV to detect 10% difference from the mean using significance (alpha) = 0.05 and power = 0.80 (Online Resource 1). Power calculations were conducted for differences in a one-sample mean between groups. Differences between groups with and without preoperative dysphagia were evaluated with analysis of variance (ANOVA). To achieve statistical power, one naive bootstrapped sample was created for each manometry value using unrestricted random sampling with the assumption that each group was a simple unbiased random sample from a representative population of normal patients (Online Resource 2). The sample size for each bootstrapped sample by HRM variable was CFV = 720, IRP = 532, DCI = 360, and DECA = 424. Literature review revealed insufficient data on healthy patient populations for DL and PP; thus, the bootstrapped sample size selected was chosen to be the same as IRP and DECA, respectively, as the distributions in sample manometry data were similar.
Results
Ninety-four patients (median age 53 years) met study inclusion criteria. There were 56 females (59.8%). Preoperative dysphagia was present in 23 patients (24.5%). Median follow-up was 13 months (maximum 43 months). Dysphagia resolved (RD) in 15 of the 23 patients (65%) but continued (CD) in 8 patients (35%). Seventy-one patients (75.5%) did not have dysphagia prior to LNF, and new dysphagia (ND) (at least once per week, lasting more than 3 months) developed in 24 patients (33.8%). The remaining 47 (66.2%) patients never had dysphagia (NV) preoperatively or beyond 3 months postoperatively. The average time from HRM to surgery was 3.3 months. The average DeMeester Scores in each group were as follows: RD = 68.8, CD = 30.3, ND = 23.2, NV = 18.7.
There was no statistical association between preoperative and postoperative dysphagia (p = 0.96) using Chi-square analysis. Among patients in the original sample without preoperative dysphagia, there were no statistical differences in DECA, DCI, CFV, DL, IRP, or PP values between those who developed new dysphagia and those who never had dysphagia (p > 0.05). Among patients who had dysphagia prior to fundoplication, manometric values were not statistically different (p > 0.05) between the RD group and the CD group.
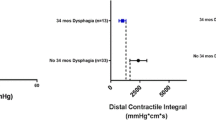
Using the bootstrapping statistical technique, there were 748 samples in each group and a total population sample size of 2992. In the sample without preoperative dysphagia prior to LNF (Table 1), there were no HRM metrics that significantly correlated with the development of dysphagia (p > 0.05). Among patients with dysphagia prior to LNF (Table 2), there were significant differences in HRM metrics between patients whose dysphagia resolved and whose dysphagia continued. A statistically higher HRM mean value for DCI, CFV, and DL was noted in patients whose dysphagia resolved compared with those with continued dysphagia. The mean PP and DECA were also statistically higher in the resolved dysphagia group compared with the continued dysphagia group. There were no statistical differences in IRP between the bootstrap groups.
Chi-square analysis of DCI was completed to determine whether a particular cutoff value was associated with the resolution of dysphagia after fundoplication. Though the Chicago Classification defines “Normal” DCI as greater than 450 mmHg-s-cm, the CD group has median DCI well above this value [18]. As such, a hypothesized cutoff of 1000 mmHg-s-cm was used to compare groups to see whether stronger contractile vigor was associated with the resolution of dysphagia. For those with preoperative dysphagia, the RD group was 2.77 (95% CI: 1.5, 5.1) times more likely to have a DCI ≥ 1000 mmHg-s-cm compared with the CD group (p = 0.001). There was no difference between ND and NV groups when using a DCI cutoff value of 1000 mmHg-s-cm (p = 0.233).
Discussion
Antireflux surgery has proven efficacy for the elimination of heartburn and regurgitation symptoms and for improving quality of life in patients with GERD [2,3,4]. Dysphagia is common in patients with GERD who present for antireflux surgery (24.5% in our series), though it resolves in most (65%) after a Nissen fundoplication. However, dysphagia persists in some and develops in a small percentage of patients after a Nissen fundoplication. Though uncommon, it is a frustrating complication for the patient and the surgeon. Some patients required dilation, and less commonly revision surgery is recommended. In some series, as many as 35% of patients may continue to experience occasional long-term dysphagia symptoms [19].
Early studies using conventional esophageal manometry aimed to identify the minimal esophageal strength required to overcome the newly created fundoplication. A minimum DECA of 25 mmHg in at least 70% of test swallows was sufficient to minimize postoperative dysphagia [14]. From this, fundoplication tailoring was born with the idea that partial fundoplication would induce less outflow resistance in patients with weak (DECA < 25 mmHg) esophageal strength. Since then, those thresholds have been tested with inconsistent results leading some surgeons to offer a full fundoplication (Nissen) for all. On the other hand, both laparoscopic Toupet and Nissen fundoplications provide normalization of esophageal acid exposure on postoperative pH testing resulting in other surgeons favoring a partial fundoplication on patients with impaired esophageal body function on manometry [4, 20].
Many studies have investigated preoperative factors attributable to prolonged dysphagia after a fundoplication. Herron showed that preoperative dysphagia was associated with postoperative dysphagia at least three months after LNF. In this study, no preoperative conventional manometry criteria were associated with postoperative dysphagia [9]. Cole and colleagues studied 401 laparoscopic Toupet fundoplications and found no association between preoperative lower esophageal sphincter (LES) pressure, distal esophageal pressure, or hypomotility on conventional manometry and postoperative dysphagia [8]. However, these studies all utilized conventional manometry and this issue has not been sufficiently tested using the current standard, high-resolution manometry.
A recent French study of 20 patients analyzed both preoperative and postoperative HRM after laparoscopic Nissen–Rossetti fundoplication [11]. No preoperative HRM metric was associated with the development of dysphagia. Postoperative elevated IRP was associated with prolonged dysphagia, but this likely correlates with the ‘tightness’ of the fundoplication and expectedly causes dysphagia. Montenovo and colleagues used multichannel intraluminal impedance (MII) and manometry prior to LNF in 74 patients and found no correlation with postoperative dysphagia [21]. Though the summary of the literature to date fails to show consistent preoperative factors reliably associated with dysphagia, many studies are not powered to show these differences.
To our knowledge, this is the first study using a statistically powered sample. Similar to prior studies, our initial analysis of the cohort failed to find significant associations between HRM metrics and postoperative dysphagia. We assumed the reason was twofold: lack of statistical power and absence of severely impaired esophageal function in our cohort due to our practice of tailoring. Though we acknowledge that postoperative dysphagia is a concern in patients with poor motility preoperatively, we specifically chose to study patients without esophageal hypomotility in order to establish preliminary baseline data. After using the bootstrapping statistical technique, the overall sample size was nearly 3000. Among patients who did not have dysphagia prior to LNF, there continued to be no HRM metric associated with new dysphagia. We believe this signifies that among patients without preoperative dysphagia and acceptable esophageal body function, HRM may not be able to help surgeons identify patients at increased risk of developing dysphagia after a Nissen fundoplication.
On the contrary, we were able to find statistical differences among patients whose preoperative dysphagia persisted compared to those in whom it resolved. A higher mean DCI, CFV, and DL on HRM were associated with the resolution of dysphagia after surgery (p < 0.05). Higher values of conventional manometry criteria (PP and DECA) were also associated with the resolution of dysphagia (p < 0.05). Though the mean CFV among the resolved dysphagia group (5.8 cm/s) was higher than the continued group (3.6 cm/s), the median value (3.0 cm/s) was marginally lower than the CFV in the continued group (3.6 cm/s). This is due to a single outlier in the original resolved group whose CFV was 30 cm/s. Despite these differences, all CFV values remain below the accepted normal value for CFV.
Given a higher mean DCI and DECA, we hypothesize that a stronger esophagus is better able to clear a bolus past the new fundoplication and thus is less likely to cause dysphagia. The original statistical analysis used HRM criteria as a continuous variable, thus not providing certain cutoff values to guide surgeons. The authors subsequently used Chi-square analysis of DCI values greater than 1000 mmHg-s-cm to determine whether this value of contractile vigor would better allow for bolus clearance past the fundoplication, thus less likely to cause dysphagia. The results show that the group whose dysphagia resolved was much more likely to have a DCI ≥ 1000 mmHg-s-cm, supporting this hypothesis.
Similarly, higher PP may indicate more coordinated esophageal clearance. The CFV and DL values were higher among those whose dysphagia resolved, though all values were within the normal established cutoff. Though the lower CFV and DL may indicate a steadier propagation of the esophageal pressure wave in the CD group, this may be outweighed by the lower overall pressure (DCI and DECA) and its inability to clear a bolus past the lower sphincter.
Not surprisingly, the lowest average DeMeester Score was noted in the group that never developed dysphagia; however, the worst reflux disease by pH testing was seen in the group whose dysphagia resolved. This may support the hypothesis that esophageal motility, rather than the extent of reflux disease, has more of a role in determining postoperative dysphagia. We selected a patient population without preoperative esophageal hypomotility and thus acknowledged that these conclusions may not generalize to patients with poor esophageal motility. These patients may be more at risk of dysphagia and will be the subject of future investigation. It is also important to note that while we found statistical differences in the absolute values of HRM metrics with regard to dysphagia, the difference may be small and still range within the normal accepted values according to the Chicago Classification. Additionally, we did not identify specific thresholds. We suspect that previously established threshold values may not be accurate to answer the specific clinical question of predicting postoperative dysphagia. Further work in this area may provide updated objective and clinically relevant manometric criteria to help guide fundoplication tailoring.
There are limitations to this study. Our practice is to tailor patients based on manometric findings. That is, patients with DECA < 25 mmHg and PP < 50% undergo partial fundoplication, while those with stronger values get full fundoplications. As such, this study is unable to assess whether tailoring is useful and led to less dysphagia in those with impaired esophageal body function. We did not measure esophageal manometry nor perform barium testing postoperatively to compare the results to preoperative data. We acknowledge that a tight fundoplication can lead to dysphagia. However, esophagography demonstrating tight or slow transit of contrast cannot determine solely that symptoms are due to a tight fundoplication. We use standardized bougie sizes in order to prevent this from confounding results.
Further, the statistical technique of bootstrapping estimates statistics for the original sample; thus, minor assumptions are made. The review did not reveal data on DL and PP. Thus, sample sizes were selected based on IRP and DECA, as the distributions were similar. There were differences in the standard deviation of PP between the original and bootstrapped sample for the RD group. This analysis did change from insignificant to significant between samples on ANOVA, which could have been affected by the change in the standard deviation. As such, bootstrapped samples do not serve as a “fix” for small sample sizes, but provide robust estimates of the distribution of the original sample to allow for achieving sufficient statistical power for the analysis.
In conclusion, in patients without dysphagia prior to laparoscopic fundoplication and acceptable esophageal body function, HRM criteria could not predict the development of postoperative dysphagia. Preoperative dysphagia is common and typically resolves after fundoplication, especially in those with stronger manometry pressure and percent peristalsis. In those with preoperative dysphagia and weaker manometry criteria, dysphagia is more likely to persist and these patients may be better served with a partial fundoplication, though further testing is required to support this inference.
References
El-Serag HB, Sweet S, Winchester CC et al (2014) Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 63:871–880
Cowgill SM, Arnaoutakis D, Villadolid D et al (2006) Results after laparoscopic fundoplication: does age matter? Am Surg 72:778–783; discussion 783–774
Dassinger MS, Torquati A, Houston HL et al (2004) Laparoscopic fundoplication: 5-year follow-up. Am Surg 70:691–694; discussion 694–695
Tian ZC, Wang B, Shan CX et al (2015) A meta-analysis of randomized controlled trials to compare long-term outcomes of Nissen and Toupet fundoplication for gastroesophageal reflux disease. PLoS ONE 10:e0127627
Stefanidis D, Hope WW, Kohn GP et al (2010) Guidelines for surgical treatment of gastroesophageal reflux disease. Surg Endosc 24:2647–2669
Anvari M, Allen C (1998) Esophageal and lower esophageal sphincter pressure profiles 6 and 24 months after laparoscopic fundoplication and their association with postoperative dysphagia. Surg Endosc 12:421–426
Blom D, Peters JH, DeMeester TR et al (2002) Physiologic mechanism and preoperative prediction of new-onset dysphagia after laparoscopic Nissen fundoplication. J Gastrointest Surg 6:22–27; discussion 27–28
Cole SJ, van den Bogaerde JB, van der Walt H (2005) Preoperative esophageal manometry does not predict postoperative dysphagia following anti-reflux surgery. Dis Esophagus 18:51–56
Herron DM, Swanstrom LL, Ramzi N et al (1999) Factors predictive of dysphagia after laparoscopic Nissen fundoplication. Surg Endosc 13:1180–1183
Tsuboi K, Lee TH, Legner A et al (2011) Identification of risk factors for postoperative dysphagia after primary anti-reflux surgery. Surg Endosc 25:923–929
Marjoux S, Roman S, Juget-Pietu F et al (2012) Impaired postoperative EGJ relaxation as a determinant of post laparoscopic fundoplication dysphagia: a study with high-resolution manometry before and after surgery. Surg Endosc 26:3642–3649
Mello MD, Shriver AR, Li Y et al (2016) Ineffective esophageal motility phenotypes following fundoplication in gastroesophageal reflux disease. Neurogastroenterol Motil 28:292–298
Myers JC, Nguyen NQ, Jamieson GG et al (2012) Susceptibility to dysphagia after fundoplication revealed by novel automated impedance manometry analysis. Neurogastroenterol Motil 24:812
Kahrilas PJ, Dodds WJ, Hogan WJ (1988) Effect of peristaltic dysfunction on esophageal volume clearance. Gastroenterology 94:73–80
Schwameis K, Lin B, Roman J et al (2018) Is pH testing necessary before antireflux surgery in patients with endoscopic erosive esophagitis? J Gastrointest Surg 22:8–12
Arnold BN, Dunst CM, Gill AB et al (2011) Postoperative impedance-pH testing is unreliable after Nissen fundoplication with or without giant hiatal hernia repair. J Gastrointest Surg 15:1506–1512
Patterson EJ, Herron DM, Hansen PD, et al (2000) Effect of an esophageal bougie on the incidence of dysphagia following nissen fundoplication: a prospective, blinded, randomized clinical trial. Arch Surg 135:1055–1061; discussion 1061–1052
Kahrilas PJ, Bredenoord AJ, Fox M et al (2015) The Chicago classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 27:160–174
Makris KI, Cassera MA, Kastenmeier AS et al (2012) Postoperative dysphagia is not predictive of long-term failure after laparoscopic antireflux surgery. Surg Endosc 26:451–457
Du X, Hu Z, Yan C et al (2016) A meta-analysis of long follow-up outcomes of laparoscopic Nissen (total) versus Toupet (270 degrees) fundoplication for gastro-esophageal reflux disease based on randomized controlled trials in adults. BMC Gastroenterol 16:88
Montenovo M, Tatum RP, Figueredo E et al (2009) Does combined multichannel intraluminal esophageal impedance and manometry predict postoperative dysphagia after laparoscopic Nissen fundoplication? Dis Esophagus 22:656–663
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors SS, CD, BR, ED, LS, SD have no disclosure or conflicts of interest that pertain to this research paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
268_2018_4870_MOESM2_ESM.pdf
Online Resource 2. Caption: Statistical Analysis Software (SAS) code used for bootstrapping and to generate samples (PDF 90 kb)
Rights and permissions
About this article
Cite this article
Siegal, S.R., Dunst, C.M., Robinson, B. et al. Preoperative High-Resolution Manometry Criteria are Associated with Dysphagia After Nissen Fundoplication. World J Surg 43, 1062–1067 (2019). https://doi.org/10.1007/s00268-018-4870-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-018-4870-9