Abstract
Background
Treatment of patients with liver metastasis of differentiated thyroid carcinoma (DTC) has not been sufficiently defined, because liver metastasis of DTC has been described mostly as case reports. Additionally, such patients are considered end-of-treatment responders. A relatively new approach using tyrosine kinase inhibitors (TKIs) may provide opportunities to manage systemic metastasis. This study aims to define the clinical features of DTC patients with liver metastasis and evaluate the benefits of TKIs.
Methods
We retrospectively analyzed clinical features of 29 patients (mean age 67.8 years) diagnosed with liver metastasis of DTC at our institution between January 1981 and May 2017.
Results
All patients had distant metastasis at other organ sites upon diagnosis of liver metastasis; 41% of them developed new metastasis afterward. Management after diagnosis of liver metastasis comprised palliative care (48%), radioactive iodine therapy (28%), and TKI therapy (24%). The median survival after diagnosis of liver metastasis was only 4.8 months. Survival rates were significantly better in patients with performance statuses between 0 and 2 on the Eastern Cooperative Oncology Group scale at diagnosis of liver metastasis (n = 22, 76%) treated with TKI compared to those who were not (P = 0.017; log-rank test; hazard ratio 0.19). One-year survival rates were 71.4 and 26.7% for patients treated with or without TKI, respectively.
Conclusions
Patients with liver metastasis had poor clinical prognosis. When other distant metastases existed at diagnosis of liver metastasis, TKI therapy was considered an effective therapeutic option for patients with liver metastasis of DTC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Differentiated thyroid carcinomas (DTCs), including papillary thyroid carcinoma (PTC) and follicular thyroid carcinoma (FTC), are slow-growing tumors with ten-year relative survival rates of 80–93% [1,2,3]. Distant metastasis develops in 4–15% [4,5,6,7,8,9] of patients with DTC, usually in the lungs and bones. Other sites of distant metastasis, such as the liver and brain, are rare [10, 11]. The appearance of liver metastasis in DTC is considered end-stage, and the prognosis is poor [11, 12].
A standard treatment for liver metastasis of DTC has not yet been established and the clinical status of these patients is not well defined, because liver metastasis in DTC has been described mostly in case reports. Therefore, diagnosis may be delayed and opportunities for patient management may be lost. However, a relatively new treatment approach using tyrosine kinase inhibitor (TKI) therapy may provide opportunities to treat patients with systemic metastasis refractory to radioactive iodine (RAI) therapy. Therefore, a comprehensive understanding of the clinical status of patients with liver metastasis is important for their management. The present paper aimed to define the clinical status of patients with liver metastasis from DTC and determine the benefits of treatment including TKI.
Materials and methods
The Ethics Committee of Ito Hospital approved this retrospective review of the medical records of 54 patients with thyroid cancer who were diagnosed with liver metastasis between January 1981 and May 2017 (Approval No. 169). The histological types of the primary thyroid cancer comprised PTC (n = 22, 41%), medullary thyroid carcinoma (n = 10, 19%), anaplastic thyroid carcinoma (n = 9, 17%), FTC (n = 9, 17%), poorly differentiated thyroid carcinoma (n = 3, 6%), and squamous cell thyroid carcinoma (n = 1, 2%). Distant metastases were diagnosed by biopsy, CT imaging, ultrasonography, or at autopsy. Among the 31 patients with primary DTC tumors, patients were excluded if liver metastases were diagnosed at autopsy (n = 2). The final study population comprised 29 patients. The follow-up rate was 96.6% (28/29). When we compared patients received TKI with patients without TKI, we excluded patients with poor performance status (PS) of 3 or 4 on the Eastern Cooperative Oncology Group (ECOG) scale because the sicker patients are not offered TKIs.
Data were statistically analyzed using STATA software 13.0 (Stata Corporation, College Station, TX, USA). Overall survival rates were estimated using the Kaplan–Meier method and compared using stratified log-rank tests.
Results
Patient characteristics
Table 1 shows the baseline characteristics of the patients at the time they were diagnosed with liver metastasis. Among the 29 patients (male n = 5; female n = 24; mean age 67.8 years; range 45–85 years), 20 and nine had PTC and FTC, respectively. The majority (n = 18, 62%) of patients had a PS of 0 or 1 on the ECOG scale, and four (14%) had a PS of 2. But seven patients (24%) had poor PS (3 or 4 on the ECOG scale). The median amount of time that elapsed from the initial therapy to being diagnosed with liver metastasis was 7.0 years. Almost all patients (n = 27, 93%) underwent thyroidectomy and 16 patients (55%) were treated with RAI. None of the patients had received TKI therapy before being diagnosed with liver metastasis.
Recurrence sites at diagnosis of liver metastasis
Table 2 shows the sites of distant metastases at the time liver metastasis was diagnosed. All patients had other distant metastases at the time they were diagnosed with liver metastasis. The most frequent sites were the lungs (86%) followed by bone (59%). Less frequent sites comprised the pleura (14%), skin (7%), and adrenal glands (3%). The brain was not a site of metastasis in any patient at the time of diagnosis with liver metastasis. Lymph node recurrence developed after primary surgery in 69% of patients, and local recurrence was identified in 17% of patients when liver metastasis was diagnosed.
Recurrence sites after diagnosis of liver metastasis
After being diagnosed with liver metastasis, only one patient had lymph node recurrence while none had local recurrence (Table 2). Distant metastasis occurred in 41% of patients at new organ sites, including bone (17%), pleura (7%), lung (7%), brain (7%), kidney (7%), skin (3%), muscle (3%), and soft tissue within the orbit (3%).
Additional therapy after diagnosis of liver metastasis
Slightly less than half of the patients (n = 14, 48%) did not undergo additional therapy and were given palliative care after liver metastasis was diagnosed because of poor PS and/or existing I131-refractory metastasis. Fourteen patients received additional therapy and the treatment modalities and outcomes for one patient remain unknown because they were lost to follow-up due to changing hospitals.
Among eight (28%) patients who were treated with RAI therapy for metastasis in the liver and other sites, one was subsequently switched to TKI therapy (Table 3). Five patients with iodine-avid liver metastases underwent treatment with I131. Disease progression occurred in three patients and stable disease was documented in two patients.
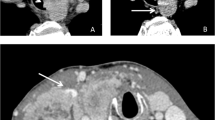
Seven (24%) patients were treated with TKIs due to iodine-refractory DTC (n = 6) or unresectable primary DTC (n = 1) after being diagnosed with liver metastasis. In our series, all of them received lenvatinib only. Lenvatinib was discontinued due to common terminology criteria for adverse events (CTCAE) Grade 3/4 anorexia, a well-known adverse event of lenvatinib, in two (29%). This has occurred within one month of treatment initiation in both of them. Therapy with lenvatinib was able to maintain in five patients (71%); four patients continued lenvatinib for more than 1 year, and one patient started lenvatinib within 1 year. The size of liver metastases was reduced in half of the patients treated for over 1 year with lenvatinib (Table 3; Fig. 1), whereas the size in the remainder of the patients remained stable with necrotic changes and a reduction in CT contrast enhancement.
a Liver metastasis is absent at the time of starting RIA therapy. b Several liver metastases have appeared during RIA therapy. c Liver metastases have decreased and are replaced with necrosis after induction of lenvatinib. d Liver metastases remain stable without signs of progression two years after starting lenvatinib. RAI radioactive iodine
Survival outcomes after diagnosis of liver metastasis
Figure 2a shows Kaplan–Meier survival estimates for all patients. The median survival after being diagnosed with liver metastasis of DTC was poor at 4.8 months, and the survival rate was 48.3 and 31.0% at six and 12 months, respectively. The cause of death was all related to thyroid cancer. None of patients died by comorbidities.
a Overall survival curves for all patients after diagnosis of liver metastasis. b Overall survival curves for patients with PS 0–2 on ECOG scale. The group that was not treated with TKI includes patients receiving palliative care or RAI, and one patient whose additional therapy remains unknown. The survival rate was significantly better for patients who received TKI therapy compared to those who did not (P = 0.017; log-rank test). ECOG Eastern Cooperative Oncology Group, PS performance status, RAI radioactive iodine, TKI tyrosine kinase inhibitor
Figure 2b shows Kaplan–Meier survival curves for the patients with PS 0–2 at the time of being diagnosed with liver metastasis (n = 22), who might have benefitted from TKI therapy had it been approved for thyroid carcinoma at the time. The survival rate was significantly better for patients who received lenvatinib (n = 7) compared to those who did not (n = 15) (P = 0.017; log-rank test; hazard ratio [HR] for death, 0.19; 95% confidence interval [CI], 0.042–0.86). The one-year survival rates were 71.4 and 26.7% among patients with and without lenvatinib, respectively.
Discussion
Most previous studies have found a low clinical incidence of liver metastasis of DTC and higher incidence at autopsy. The reported rates of liver metastasis and distant metastasis of DTC are 0.43–0.97% [13,14,15] and 2.4–14.7% [13,14,15,16], respectively, with the rate of distant liver metastasis at autopsy being 4.5–25.0% [17,18,19].
Surgical resection may offer the best chance for prolonged survival if liver metastasis is solitary [20,21,22,23]. In a previous report, one patient was treated with radiofrequency ablation (RFA) of liver metastasis from FTC without signs of other metastasis, and CT imaging suggested partial necrosis of the metastasis [24]. Although RFA might have achieved local control [12], the patient did not survive over the long term. However, DTC liver metastases are usually found concurrently with other distant sites [15, 23, 25] as in our series. Systemic therapies such as RAI and TKI are required to control metastases at multiple sites.
RAI is one approach to treating distant metastasis. Song et al. [11] suggested in their review that I131-positive liver metastasis is extremely rare. In fact, most I131-positive liver metastases have been described in case reports [23, 26,27,28]. Shah et al., who described one of the largest patient cohorts with liver metastasis of DTC (n = 11), found that three (37.5%) of eight patients treated with RAI had I131-negative liver metastasis [15]. To our knowledge, this is the only report on rates of patients with radioiodine uptake in liver metastasis. The I131-negative rate was identical in the present study. Both Song’s report and the present study showed that over 50% of patients with liver metastasis had the option of repeated RAI therapy, although RAI was non-cytoreductive against I131-positive liver metastasis in our series. As such, RAI should be an option for patients with liver metastasis after considering the balance between metastatic progression in the liver and other recurrent sites.
Treatment with TKIs such as lenvatinib and sorafenib represents another therapeutic choice for distant metastasis [29, 30], but few reports have described the therapeutic processes or effects of TKI on liver metastasis from DTC. Farina et al. [25] described one patient with liver, lung, bone, and lymph node metastases that were treated with sorafenib. The patient died six months after beginning sorafenib. Brient et al. [31] reported comparison of eight patients received TKIs and six patients without TKIs after diagnosis of liver metastasis from DTC. In their study, patients with TKIs had a significantly longer median survival than patients without TKIs (23.6 months and 3.9 months, respectively). However, their study had some limitations. TKIs which eight patients received, including vandetanib, sunitinib, pazopanib, and sorafenib, were not standardized. And control group (patients without TKIs) included the patient in an altered general condition who could not be offered TKIs. Even though these limitations were present, their study was valuable for presenting the possibility of TKI application in patients with liver metastasis from DTC. In the present study, patients with liver metastasis were treated with a single type of TKI, namely lenvatinib; we considered that lenvatinib may have induced shrinkage of active metastatic tumors. What is more, we excluded patients with poor PS from the control group (patients without TKI) because the sicker patients are not offered TKIs. Together, our results indicate more precisely that patients with liver metastasis from DTC lived longer when treated with lenvatinib.
Limitations of this study included that the period surveyed was over the last few decades to collect enough cases, because liver metastasis is rare in patients with DTC. Treatment strategies for systemic metastasis of DTC have not undergone major changes in the last few decades before the appearance of TKIs. The use of RAI in our hospital remained approximately unchanged during the target period (1981–2017) of this study. Differences in the timing of treatment and in sites of coexisting metastases also limited meaningful statistical comparisons in this study.
Conclusions
Liver metastases in patients with DTC are associated with widespread disease and historically poor prognosis. Although the sample was small and many patients were not able to tolerate long-term therapy, this study demonstrated high rates of response to lenvatinib with associated significant survival advantage in this group of patients. Lenvatinib allows opportunities for further treatment, even though patients with liver metastasis from DTC were previously considered end-of-treatment responders. The presence of liver metastasis may indicate that lenvatinib is an appropriate intervention. Because TKIs for DTC have a short history, future prospective trials are needed to validate our findings.
References
Mazzaferri EL, Kloos RT (2001) Clinical review 128: current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab 86:1447–1463
Hundahl SA, Fleming ID, Fremgen AM et al (1998) A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995. Cancer 83:2638–2648
Schlumberger MJ (1998) Papillary and follicular thyroid carcinoma. New Engl J Med 338:297–306
Casara D, Rubello D, Saladini G et al (1993) Different features of pulmonary metastases in differentiated thyroid cancer: natural history and multivariate statistical analysis of prognostic variables. J Nucl Med 34:1626–1631
Shaha AR, Shah JP, Loree TR (1997) Differentiated thyroid cancer presenting initially with distant metastasis. Am J Surg 174:474–476
Lin JD, Huang MJ, Juang JH et al (1999) Factors related to the survival of papillary and follicular thyroid carcinoma patients with distant metastases. Thyroid 9:1227–1235
Clark JR, Lai P, Hall F et al (2005) Variables predicting distant metastases in thyroid cancer. Laryngoscope 115:661–667
Benbassat CA, Mechlis-Frish S, Hirsch D (2006) Clinicopathological characteristics and long-term outcome in patients with distant metastases from differentiated thyroid cancer. World J Surg 30:1088–1095. https://doi.org/10.1007/s00268-005-0472-4
Lang BH, Wong KP, Cheung CY et al (2013) Evaluating the prognostic factors associated with cancer-specific survival of differentiated thyroid carcinoma presenting with distant metastasis. Ann Surg Oncol 20:1329–1335
Madani A, Jozaghi Y, Tabah R et al (2015) Rare metastases of well-differentiated thyroid cancers: a systematic review Ann. Surg Oncol 22:460–466
Song HJ, Xue YL, Xu YH et al (2011) Rare metastases of differentiated thyroid carcinoma: pictorial review. Endocr Relat Cancer 18:R165–R174
Haugen BR, Alexander EK, Bible KC et al (2015) American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016(26):1–133
Brown AP, Greening WP, McCready VR et al (1984) Radioiodine treatment of metastatic thyroid carcinoma: the Royal Marsden Hospital experience. Br J Radiol 57:323–327
Mizukami Y, Michigishi T, Nonomura A et al (1990) Distant metastases in differentiated thyroid carcinomas: a clinical and pathologic study. Hum Pathol 21:283–290
Shah DH, Samuel AM (1996) Metastasis to the liver in well-differentiated carcinoma of the thyroid. Thyroid 6:607–611
Wood WJ Jr, Singletary SE, Hickey RC (1989) Current results of treatment for distant metastatic well-differentiated thyroid carcinoma. Arch Surg 124:1374–1377
Silliphant WM, Klinck GH, Levitin MS (1964) Thyroid carcinoma and death. A clinicopathological study of 193 autopsies. Cancer 17:513–525
Silverberg SG, Hutter RV, Foote FW Jr (1970) Fatal carcinoma of the thyroid: histology, metastases, and causes of death. Cancer 25:792–802
Heitz P, Moser H, Staub JJ (1976) Thyroid cancer: a study of 573 thyroid tumors and 161 autopsy cases observed over a thirty-year period. Cancer 37:2329–2337
Kouso H, Ikegami T, Ezaki T et al (2005) Liver metastasis from thyroid carcinoma 32 years after resection of the primary tumor: report of a case. Surg Today 35:480–482
Ohwada S, Iino Y, Hosomura Y et al (1993) Solitary metastasis from papillary thyroid carcinoma in cirrhotic liver with hepatocellular carcinoma. Jpn J Clin Oncol 23:309–312
Kondo T, Katoh R, Omata K et al (2000) Incidentally detected liver metastasis of well-differentiated follicular carcinoma of the thyroid, mimicking ectopic thyroid. Pathol Int 50:509–513
Salvatori M, Perotti G, Rufini V et al (2004) Solitary liver metastasis from Hurthle cell thyroid cancer: a case report and review of the literature. J Endocrinol Invest 27:52–56
Wertenbroek MW, Links TP, Prins TR et al (2008) Radiofrequency ablation of hepatic metastases from thyroid carcinoma. Thyroid 18:1105–1110
Farina E, Monari F, Tallini G et al (2016) Unusual thyroid carcinoma metastases: a case series and literature review. Endocr Pathol 27:55–64
Guglielmi R, Pacella CM, Dottorini ME et al (1999) Severe thyrotoxicosis due to hyperfunctioning liver metastasis from follicular carcinoma: treatment with (131)I and interstitial laser ablation. Thyroid 9:173–177
Agriantonis DJ, Hall L, Wilson MA (2009) Utility of SPECT/CT as an adjunct to planar whole body I-131 imaging: liver metastasis from papillary thyroid cancer. Clin Nucl Med 34:247–248
Kraft O (2005) Hepatic metastasis of differentiated thyroid carcinoma. Nucl Med Rev Cent East Eur 8:44–46
Brose MS, Nutting CM, Jarzab B et al (2014) Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 384:319–328
Schlumberger M, Tahara M, Wirth LJ et al (2015) Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. New Engl J Med 372:621–630
Brient C, Mucci S, Taieb D et al (2015) Differentiated thyroid cancer with liver metastases: lessons learned from managing a series of 14 patients. Int Surg 100:490–496
Author information
Authors and Affiliations
Contributions
YS, KS, HT, TU, and KO contributed to study conception and design. YS, KS, and KM helped in analysis and interpretation of data. YS, KS, and HT contributed to drafting manuscript. WK, MN, HK, KI, and YK helped in revising the manuscript. YK contributed to final approval of the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Rights and permissions
About this article
Cite this article
Saito, Y., Sugino, K., Takami, H. et al. Clinical Status and Treatment of Liver Metastasis of Differentiated Thyroid Cancer Using Tyrosine Kinase Inhibitors. World J Surg 42, 3632–3637 (2018). https://doi.org/10.1007/s00268-018-4676-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-018-4676-9