Abstract
Background
Recently, the American Joint Committee on Cancer published the 8th edition of its Cancer Staging Manual with major changes regarding the staging of thyroid cancer, including the raising of the age cutoff from 45 to 55 years. Using the clinical and genetic data of 505 papillary thyroid cancer (PTC) cases, we aimed to compare overall survival (OS) and recurrence-free survival (RFS) with different age cutoff values, and also investigate the efficacy of the new staging system on a genomic level.
Methods
We downloaded gene expression data, somatic mutation profile, copy number alteration data and clinical data of 505 PTC patients from The Cancer Genome Atlas data portal. We used multiple statistical analysis and multiplatform genomic analysis to evaluate the efficacy of the 8th edition.
Results
When using 55 years as the cutoff value for analyzing RFS, the Kaplan–Meier plot showed a significant p value but not when using 45 years (p = 0.006 vs. p = 0.493), but both cutoff values were significant when analyzing OS (p = 1.1 × 10−9 with age 55 vs. p = 4.4 × 10−5 with age 45). When looking at stage-dependent survival, both the 7th and 8th edition had significant p values (p = 0.048 vs. p = 3.1 × 10−9 in RFS and p = 5.9 × 10−10 vs. p = 2.2 × 10−10 in OS). Multiplatform genomic analysis showed patients ≥55 years had 103 differently expressed genes when compared with other age groups. Signaling pathway analysis revealed that patients ≥55 years had altered pathways associated with aggressiveness of thyroid cancer.
Conclusion
In conclusion, this is the first study to show clinical and genetic evidence supporting the altered age cutoff point of 55 years in the AJCC 8th edition for PTC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Papillary thyroid cancer (PTC) staging is different from most cancers as age is a crucial prognostic factor, independent from the other factors considered in the staging of most cancers such as primary tumor size, nodal status, and distant metastasis [1, 2]. Young patients tend to have a better prognosis than elderly patients, with the cutoff value being regarded as an age of 45 years. Previously, in the 7th edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, classification for patients under 45 was limited to either stage I or II depending on the absence or presence of metastatic disease, while patients age 45 and above were divided into stages I through IV [3]. This cutoff of 45 years has been implemented ever since the second edition. In October 2016, the AJCC published the 8th edition of the Cancer Staging Manual, and two of the major changes seen in the staging of thyroid cancer are (1) the increase in the age limit from 45 years of age at diagnosis to 55 years, and (2) the removal of regional lymph node metastases and microscopic extrathyroidal extension from the definition of T3 disease [4]. These changes will most likely result in the down-staging of a considerable number of patients and thus will hopefully more accurately be indicative of their better prognosis. However, as of yet, there have been few reports about the effect of down-staging when using the 8th edition for anticipating overall survival (OS) or recurrent-free survival (RFS) in PTCs in a clinical setting.
The aim of the present study was to compare OS and RFS with different cutoff values (age 45 vs. age 55) using the clinical data of 505 PTC cases in The Cancer Genome Atlas (TCGA) data. We also compared gene expression data, altered canonical pathways, copy number alteration and somatic mutation profiles according to age distribution using the genomic data in order to investigate the efficacy of the new staging system on a genomic level.
Materials and methods
Genomic and clinical data sets
All genomic data of papillary thyroid carcinomas from TCGA project were obtained from The Cancer Genome Atlas (TCGA) Data Portal (https://tcga-data.nci.nih.gov). Gene-level gene expression data from mRNA-seq (N = 505), copy number variation data (N = 505), somatic mutation data (N = 505) and clinical information (N = 505) were included in data analyses. Clinical data included age (range 15–89 years), gender, tumor size, uni-/multifocality, extrathyroidal extension, TNM stage, and BRAF mutation state (Supplementary Data 1).
Analysis of gene expression data and supervised clustering with age distribution
The patients were divided into three groups according to age—age < 45 (group 1), age 45–54 (group 2), and age ≥ 55 (group 3). BRB-ArrayTools software program (http://linus.nci.nih.gov/BRB-ArrayTools.html) was used to analyze gene expression data [5]. First, gene expression data were gene-median-centered. Gene variability was then computed using the median absolution deviation. A total of 3120 most variable genes were selected. A heatmap was generated using Cluster and TreeView software programs [6] to visualize the gene expression pattern, with red representing highly expressed genes and green representing lowly expressed genes.
Selection of specific gene signature in each age group
To select genes that were differentially expressed among the three different age groups (<45, 45–54, ≥55 years), we performed multiple two-sample t tests for each gene using a combination of the three age groups. A stringent cutoff criterion of p < 0.001 and having at least a 1.5-fold difference were used. Any gene that was observed in at least two age groups was removed. We drew a Venn diagram to show the number of specific gene signatures in each age group.
Significant canonical signaling pathways enriched in each subgroup
In computational and systems biology, canonical pathways are defined as a collection of reference pathways that reflect the biologic alteration of specific molecules. Canonical pathway analysis was performed using Ingenuity Pathways Analysis (IPA, Ingenuity, Redwood City, CA, USA) to identify whether there were significant genes belonging to pre-defined pathways upregulated in each group. Genes associated with canonical pathways in the Ingenuity Pathways Knowledge Base were considered for analysis. A p value was calculated using Fisher’s exact test, and p < 0.001 was considered significant.
Analysis of copy number alteration and somatic mutation
Copy number alteration and somatic mutation data analysis were performed using OncoPrint at cBioportal website (https://www.cbioportal.org). OncoPrint is a means of visually identifying distinct genomic alterations, such as copy number alterations, somatic mutations, and mRNA expression. We rearranged the order of the 505 cases according to the three age groups (<45, 45–54, ≥55 years) to compare the molecular profiles of each group.
Statistical analysis
The RFS and OS distribution was analyzed using the Kaplan–Meier method and compared by the log rank test. The association of each group with each clinical variable was evaluated using the Chi-square test and Fisher’s exact test. Significant difference was considered at p value <0.05. All statistical analyses were conducted in R language environment (http://www.r-project.org).
Results
Distribution of clinical characteristics according to different age cutoff values
We divided the patients into two groups for each age cutoff value (<45 vs. ≥45, and <55 vs. ≥55) and compared BRAF mutation, extrathyroidal extension (ETE), histology, multifocality, site, gender, and mass size (Table 1). There was no significant difference in BRAF mutation, multifocality, or site between the younger group and older group, regardless of the age cutoff value. There was a statistical significance in ETE between the younger and older groups when using either cutoff value. As for gender and mass size, there was a significant difference only when using the cutoff value of 55 years per the 8th edition. There were significantly more female patients in the under-55-year group, while those at least 55 years old had a statistically significant larger mass size than those under 55 years old.
Comparison of RFS and OS with two different cutoff values (age 45 vs. age 55)
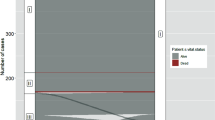
When using 45 years as the cutoff value as per the AJCC 7th edition, there was no statistically significant difference in RFS between those younger than 45 and those 45 and older (p = 0.493). However, when setting the cutoff value at 55 years as per the 8th edition, the Kaplan–Meier plot showed a statistically significant difference between the two age groups (p = 0.006, Fig. 1). As for OS, both age 45 and age 55 as a cutoff value showed a statistically significant difference (p = 1.1 × 10−9 with age 55 vs. p = 4.4 × 10−5 with age 45) (Fig. 2).
Kaplan–Meier plot for recurrence-free survival (RFS) according to different age cutoff values. a We used age 45 years to divide papillary thyroid cancer (PTC) patients into two groups. b We used age 55 years instead of age 45. When we used age 45, KM plot for RFS did not show a statistically significant difference between under 45 and over 45. However, when we used age 55 years, KM plot for RFS showed a statistically significant p value (p = 0.006)
Kaplan–Meier plot for overall survival (OS) according to different age cutoff values. a We used age 45 years to divide papillary thyroid cancer (PTC) patients into two groups. b We used age 55 years instead of age 45. Both KM plots for OS showed a statistically significant difference (p = 4.4 × 10−5 for age cutoff value 45 and p = 1.1 × 10−9 for age cutoff value 55)
Change of TNM staging from AJCC 7th edition to AJCC 8th edition and frequency of event in OS and RFS
Of the 505 cases, we were able to obtain sufficient information to precisely stage 493 patients. Of those cases, 203 patients (41%) were down-staged when we applied the AJCC 8th staging system instead of 7th (Table 2). Forty-two cases were down-staged from stage II to stage I, 53 cases from stage III to stage II, 53 cases from stage III to stage I, 16 cases from stage IV to stage III, 22 cases from stage IV to stage II, and 17 cases from stage IV to stage I. When we checked for events of RFS (recurrence) and OS (death), nine cases had recurred of the 53 cases where stage III was altered to stage II. There were also four deaths in the group of 16 cases where stage IV was down-staged to stage III and three deaths in the group of 22 cases where stage IV was down-staged to stage II (Table 2).
Comparison of stage-dependent RFS and OS using the two different staging systems (AJCC 7th vs. AJCC 8th)
Stage-dependent recurrence-free survival analysis results revealed that both the 7th edition (p = 0.048) and 8th edition (p = 3.1 × 10−9) staging systems showed a significant difference in the probability of RFS across the different stages (Fig. 3). The Kaplan–Meier plot for stage-dependent OS also showed a statistically significant p value when using either staging system (p = 5.9 × 10−10 vs. p = 2.2 × 10−10) (Fig. 4).
Stage-dependent Kaplan–Meier plot for recurrence-free survival (RFS), using two different staging systems. a We followed the staging system using AJCC 7th edition, and b we used the AJCC 8th edition. When we used AJCC 8th staging system, KM plot for RFS showed a much more significant p value than when using the 7th edition (p = 0.048 in AJCC 7th and p = 3.1 × 10−9 in AJCC 8th)
Selection of specific gene signature in each age group
The 505 patients were divided into three groups—age < 45 (group 1), age 45–54 (group 2), and age ≥ 55 (group 3). A heatmap generated from the gene expression data showed that there were no specific molecular subtypes between the three groups (Fig. 5). Next, we sought to find genes whose expression was specific to each age group by applying multiple two-class t tests among these three age groups for each gene. Those genes that had a statistically significant difference (with a p value of <0.001 and at least 1.5-fold difference) between any two age groups were designated as a differently expressed gene (DEG). Interestingly, the number of genes specific to just that particular age group was 14 in <45 years and zero in 45–54 years (Fig. 6). In contrast, the total number of age-specific genes was 103 for those in the ≥55 years group (Supplement Data 2).
Gene expression pattern according to age distribution. Supervised clustering of papillary thyroid carcinomas (PTCs) did not show distinct molecular subtypes. Gene with expression levels that were at least twofold different in at least 50 cases relative to the median value across cases was selected for hierarchical clustering analysis. Data are given in matrix format, in which rows represent individual genes and columns represent each patient. Each cell in the matrix represents the expression level of a gene feature in an individual pattern. Color red or green in cell reflects a relatively high or low expression, respectively, as indicated in the scale bar
Schematic diagram of the number of each-age-specific genes. When we applied stringent cutoff of p < 0.001 (Student’s t test) and 1.5-fold difference, we identified 103 genes which were differently expressed in patients ≥55 years. As for age under 45 and age between 45 and 55, there were only 14 and zero differently expressed genes (DEG), respectively
Significant canonical signaling pathways enriched in ≥55 years group
Ingenuity Pathways Analysis revealed that patients at least 55 years and older had alterations in Sirtuin signaling pathway, MSP-RON signaling pathway, ATM signaling, FXR/RXR activation pathway, and TGF-beta pathway.
Analysis of copy number alteration somatic mutation data according to age distribution
Oncoprint analysis failed to show a difference in copy number alteration or somatic mutation patterns between the three age groups (<45, 45–54, ≥55 years) (Fig. 7). We compared seven of the most frequently mutated genes—BRAF, HRAS, NRAS, OTUD4, EIF1AX, NUP93 and NLRP6, according to each age group, but there was no statistically significant frequency difference of somatic mutation or copy number alteration (Fig. 7).
Copy number (CPN) alteration and somatic mutation profiles according to each age group. We compared seven of the most frequently mutated genes (BRAF, HRAS, NRAS, OTUD4, EIF1AX, NUP93, and NLRP6) according to each age group, but there was no statistically significant frequency difference of somatic mutation or copy number alteration
Discussion
The AJCC Cancer Staging Manual had been using an age cutoff value of 45 years for PTCs since the 2nd edition published in 1983. The new staging system with a cutoff point of 55 years in the 8th edition was published in 2016 and is effective starting from January 2018. The object of this study was to determine how accurately the 8th edition reflects the prognosis and recurrence of PTC compared to the 7th edition by analyzing the overall survival and recurrent-free survival of 505 cases of PTC when using the cutoff value of 45 years of age and 55 years. Furthermore, we aimed to find out how the stage of each of those 505 PTC cases changed when using the 8th edition staging system and to investigate if the down-staging correctly reflected the disease prognosis by evaluating the recurrence and overall survival in those cases where the stage had changed.
This study showed that when setting the age cutoff value at 45 years, there was no significant difference in recurrent-free survival between those younger than 45 and those 45 and older. In contrast, there was a statistically significant difference in recurrent-free survival when raising the cutoff value to 55 years (Fig. 1). There was a significant difference in overall survival whether the age cutoff value was 45 or 55 years, but the difference was much more striking when the cutoff value was 55 years (Fig. 2). These data indicate that raising the cutoff age value from 45 to 55 years more effectively predicts the disease prognosis of PTCs and support the use of the 8th edition of the AJCC Cancer Staging Manual.
We also analyzed the clinical features of the 505 PTC cases using the two different age cutoff values and interestingly discovered that the size of the tumor was significantly increased in those 55 years or older when compared with patients younger than 55 years, while there was no statistically significant difference when the cutoff value was 45 years (Table 1). In the 7th edition, stage I and stage II were divided according to mass size, as 2–4-cm tumors were included in stage II and less than 2-cm-sized tumors were included in stage I. However, in the 8th edition, all patients younger than 55 years without distant metastases are stage I regardless of tumor size, and those 55 years or older with tumors ≤4 cm are also stage I when negative for lymph node metastases, despite there being many reports regarding the correlation between mass size and prognosis of PTCs [7,8,9]. As we were able to show that tumor size was not independent of age when the age cutoff was raised to 55 years, this result may be supporting evidence for the exclusion of the size criterion from the 8th edition.
Overall, 203 cases were down-staged when using the 8th edition staging system, including 53 cases changing from stage III to stage II and 53 cases changing from stage III to stage I (Table 2). Most of the down-staging occurred in PTC cases with minimal ETE or central neck lymph node metastasis because they were allocated to stage III according to the 7th edition, but to either stage I or II according to the 8th edition. Of the 53 cases that were down-staged from stage III to stage II, there were nine RFS events (recurrence) and two OS events (death), suggesting that prudent follow-up care may be warranted for such down-staged cases. There were 3 deaths of the 22 cases that were down-staged from stage IV to stage II, and 4 deaths of the 16 cases that were down-staged from stage IV to stage III, further supporting the need for careful monitoring of down-staged cases, as gross extension into strap muscle (stage II) or into subcutaneous tissue, larynx, trachea, esophagus, and recurrent laryngeal nerve (stage III) definitely is presenting finding of poor prognosis of PTCs in many reports [10,11,12].
When we performed multiplatform genomic analysis, remarkably, gene expression pattern analysis showed that of the three age groups, group-specific genes were only observed in the group of 55 years and older, as there were 103 differentially expressed genes (DEG) found for group 3 but only 14 genes for group 1 and zero for 2 (Fig. 6). This means that that there were only 14 genes having a significant difference in mRNA expression between groups 1 and 2, implying that there is no significant genetic difference in the biological pathways of PTC patients less than 45 years and those at least 45 years and less than 55 years old. This result demonstrates that raising the age cutoff value to 55 years is not only clinically appropriate but also genetically sound. Although there are gene differences, they are not clearly seen on the heatmap because we performed supervised clustering by fixing the X-axis (505 cases) and clustering only the Y-axis (genes). This may cause distribution of the clustering of the highly expressed and lowly expressed genes when looking at the heatmap. In contrast to gene expression pattern, copy number alteration and somatic mutation profile analysis did not reveal any significant difference in mutations between the three groups (Fig. 7).
In this study, we carried out gene network analysis by using analysis tool in IPA to uncover potential signaling pathways activated in each subtype (Supplement Data 3). We used p value and expression ratio of differently expressed genes (Supplement Data 4). Analysis predicted that patients at least 55 years old (group 3) had alterations in Sirtuin signaling pathway, ATM signaling, FXR/RXR activation pathway, and TGF-beta pathway. Sirtuins are class III histone deacetylase enzymes that use NAD+ as a co-substrate for their enzymatic activities [13]. SIRT1, SIRT6, and SIRT7 are primarily nuclear enzymes which regulate transcription factors, and SIRT6 and SIRT7 play important roles in rRNA transcription and cell cycle regulation [14]. The ATM signaling pathway is associated with cell cycle regulation and is involved in cellular stress response. There are many reports supporting the association of the ATM pathway with PTC prognosis [15, 16]. The FXR/RXR pathway has been reported to be associated with tumor aggressiveness that affects patients’ survival in thyroid cancer [17].
Recently, there have been several studies investigating how effective the new staging system is in predicting the prognosis of PTC [18,19,20]. However, those previous studies had limitations in that they either used a heterogenous cohort or had a small study population. The strengths of our study are that we looked at both the clinical and genomic data of 505 PTC patients enrolled in the TCGA database and used a homogenous cohort. The limitation of this study is that it is a retrospective analysis using TCGA data. Prospective clinical trials are needed to provide more reliable results.
In conclusion, this study is the first study to show clinical and genetic evidence supporting the altered age cutoff point of 55 years in the 8th edition of the AJCC Cancer Staging Manual for PTC patients. Analysis of not only clinical data but also gene expression data, copy number alteration data, altered canonical pathway, and somatic mutation profile revealed that the 8th edition’s staging system can more accurately predict recurrence and also survival rate compared to the 7th edition.
References
Siegel R, Ward E, Brawley O et al (2011) Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 61:212–236
Davies L, Welch HG (2006) Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 295:2164–2167
Edge SB, Compton CC (2010) The American joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17:1471–1474
Perrier ND, Brierley JD, Tuttle RM (2018) Differentiated and anaplastic thyroid carcinoma: major changes in the American joint committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 68:55–63
LA Simor R, Li MC, Ngan M, Menenzes S, Zhao Y (2007) Analysis of gene expression data using BRB-array Tools. Cancer Inform 4(7): 11–21
Eisen MB, Spellman PT, Brown PO et al (1998) Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 95(8): 14863–14866
Cady B, Rossi R (1988) An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery 104(3): 947–995
Hay ID, Bergstralh EJ, Goellner JR et al (1993) Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery 114:1050–1057 (discussion 1057–1058)
Shaha AR, Loree TR, Shah JP (1994) Intermediate-risk group for differentiated carcinoma of thyroid. Surgery 116:1036–1040 (discussion 1040–1031)
Kim H, Jung HJ, Lee SY et al (2016) Prognostic factors of locally invasive well-differentiated thyroid carcinoma involving the trachea. Eur Arch Oto-Rhino-Laryngol 273(6): 1919–1920
Moritani S (2015) Surgical management of cricotracheal invasion by papillary thyroid carcinoma. Ann Surg Oncol 22:4002–4007
Hotomi M, Sugitani I, Toda K et al (2012) A novel definition of extrathyroidal invasion for patients with papillary thyroid carcinoma for predicting prognosis. World J Surg 36:1231–1240. https://doi.org/10.1007/s00268-012-1518-z
Kweon KH, Lee CR, Jung SJ et al (2014) Sirt1 induction confers resistance to etoposide-induced genotoxic apoptosis in thyroid cancers. Int J Oncol 45:2065–2075
Qu N, Hu JQ, Liu L et al (2017) SIRT6 is upregulated and associated with cancer aggressiveness in papillary thyroid cancer via BRAF/ERK/Mcl1 pathway. Int J Oncol 50:1683–1692
Gu Y, Liu X, Yu Y et al (2014) Association of ATM Gene Polymorphism with PTC Metastasis in Female Patients. Int J Endocrinol 2014:370825
Kang J, Deng XZ, Fan YB et al (2014) Relationships of FOXE1 and ATM genetic polymorphisms with papillary thyroid carcinoma risk: a meta-analysis. Tumour Biol 35:7085–7096
Giaginis C, Tsoukalas N, Alexandrou P et al (2017) Clinical significance of farnesoid X receptor expression in thyroid neoplasia. Future Oncol 13(2): 1785–1790
Steinschneider M, Muallem Kalmovich L, Koren S, et al (2017) Reassessment of differentiated thyroid cancer patients using the 8th TNM classification system: a comparative study Thyroid: Official Journal of the American Thyroid Association 2017
Lamartina L, Grani G, Arvat E, et al (2017) 8th edition of AJCC/TNM staging system of thyroid cancer: what to expect Endocrine-related cancer 2017
Suh S, Kim YH, Goh TS et al (2017) Outcome prediction with the revised American joint committee on cancer staging system and American thyroid association guidelines for thyroid cancer. Endocrine 58(2): 495–450
Acknowledgements
We appreciate the patients and their families who generously donated their tissues to TCGA, as well as the members of TCGA who collected and disclosed valuable data.
Funding
This research was supported by Hallym University Research Fund 2018 (H20180239).
Author information
Authors and Affiliations
Ethics declarations
Conflict of interest
All authors have no conflict of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, K., Kim, J.H., Park, I.S. et al. The Updated AJCC/TNM Staging System for Papillary Thyroid Cancer (8th Edition): From the Perspective of Genomic Analysis. World J Surg 42, 3624–3631 (2018). https://doi.org/10.1007/s00268-018-4662-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-018-4662-2