Abstract
Background
Total pharyngolaryngoesophagectomy (PLE) is used as a curative treatment for synchronous laryngopharyngeal and thoracic esophageal cancer or for multiple cancers in the cervical and thoracic esophagus. Gastric pull-up is commonly used after PLE, but postoperative complications are common. The present study evaluated these procedures in patients with esophageal cancer.
Methods
Fourteen patients (7 with synchronous pharyngeal and thoracic esophageal cancer, 4 with synchronous cervical and thoracic esophageal cancer, and 3 with cervicothoracic esophageal cancer) underwent reconstructive surgery after PLE involving gastric pull-up combined with free jejunal graft between 2004 and 2015.
Results
Esophagectomy via right thoracotomy was performed in 9 patients, and transhiatal esophagectomy was used in 5. The posterior mediastinal route was used in 13 patients, excluding one patient with early gastric cancer. Interposition of a free jejunal graft included microvascular anastomosis using two arteries and two veins in all patients. Anastomotic leakage and graft necrosis did not occur in any of the 14 patients who underwent the above surgical procedures. Tracheal ischemia close to the tracheostomy orifice occurred in 4 patients (28.6%), but none of these patients developed pneumonia. No hospital deaths were recorded.
Conclusions
The results indicate that gastric pull-up combined with free jejunal graft is a feasible reconstructive surgery after PLE. This procedure is a promising treatment strategy for synchronous pharyngeal and thoracic esophageal cancer or multiple cancers in the cervical and thoracic esophagus. Larger series are needed to show the distinct advantages of this procedure in comparison with conventional methods of reconstruction after PLE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal cancer is one of the most virulent gastrointestinal cancers. It often spreads to regional and distant lymph nodes at a relatively early stage [1], and it sometimes invades adjacent structures, such as the trachea and left main bronchus, especially in advanced stages of the disease [2, 3]. Esophageal cancer, especially esophageal squamous cell carcinoma, is sometimes associated with head and neck cancers, such as laryngopharyngeal cancer, and it may exhibit multiple lesions in the area extending from the cervical to thoracic esophagus [4]. This characteristic primarily occurs because cancers of the upper aerodigestive tract share common risk factors, such as smoking and alcohol, which induces field cancerization [5]. Total PLE is often indicated as a curative treatment for patients with double cancer of the laryngopharynx and thoracic esophagus or multiple lesions of cervical and the thoracic esophagus.
PLE is one of the most invasive and challenging surgeries for gastrointestinal cancers based on reported relatively high morbidity and mortality rates [6,7,8,9]. In particular, reconstruction after PLE is associated with various problems, e.g., the need for a long reconstructed conduit to cover the defect after PLE, and insufficient blood flow to the oral end of conduits [10]. Gastric pull-up is commonly used for reconstruction after PLE. Elongated stomach roll [11, 12] and colon interposition [13, 14] is sometimes used when the conduit must cover a wide defect. Microvascular anastomosis is sometimes additionally performed when the blood supply of the oral end in the reconstruction conduit is insufficient [11, 12]. The first choice surgical technique in our hospital involves reconstructive surgery using gastric pull-up combined with free jejunal graft after PLE. This procedure resolves two problems that are associated with post-PLE reconstruction: the need to cover a wide defect and insufficient blood flow to the oral end of conduit. This study retrospectively assessed the clinical outcome of reconstructive surgery involving gastric pull-up combined with free jejunal graft after PLE.
Materials and methods
Patients
A total of 739 patients with esophageal cancers underwent surgery at the Department of Digestive Surgery, Osaka Medical Center for Cancer and Cardiovascular Diseases, between January 2004 and December 2015. Sixteen of these patients underwent PLE. Two of these 16 patients underwent reconstruction using colonic interposition after PLE because they had previously undergone total gastrectomy for gastric cancer. The remaining 14 patients underwent reconstructive surgery involving gastric pull-up combined with free jejunal graft after PLE. These 14 patients are the subject of this study. All 14 patients were diagnosed with squamous cell carcinoma of the hypopharynx and/or cervical and thoracic esophagus based on histopathological examination of biopsy material obtained before surgery.
Table 1 summarizes the clinical characteristics of the 14 patients. The mean age was 62 years (range 44–73 years). They included 13 men and 1 female. Seven patients had synchronous pharyngeal cancer and thoracic esophageal cancer, 4 patients had synchronous cervical esophageal cancer and thoracic esophageal cancer, and 3 patients had cervicothoracic esophageal cancer extending from the cervical esophagus to the upper thoracic esophagus. Four of the 14 patients with PLE received preoperative chemoradiotherapy to the esophagus including the cervical esophagus to thoracic esophagus, and one patient received preoperative chemotherapy. The remaining 9 patients underwent surgery alone. The Human Ethics Review Committees of Osaka Medical Center for Cancer and Cardiovascular Diseases, Osaka, Japan, approved the study protocol.
Surgical procedure
Esophagectomy was performed via right thoracotomy or using a transhiatal approach.
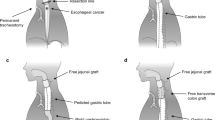
Surgery in PLE with esophagectomy via right thoracotomy began with a thoracic approach in the left lateral decubitus position. The thoracic esophagus and mediastinal lymphadenectomy were resected, and the patient was placed in a supine position to perform pharyngolaryngectomy with cervical esophagectomy and cervical lymph node dissection. Median laparotomy was performed in parallel with the cervical procedure to construct the gastric tube, together with upper abdominal lymphadenectomy and harvesting of a jejunal segment. The gastric tube which was 3.5–5 cm in width was made using linear stapler, and was pulled up to the cervical position, and the free jejunal graft was implanted immediately to cover the surgical defect in the neck and implanted in isoperistaltic position, with minimum ischemic time to ensure jejunal graft survival. Gastrojejunostomy was performed via a side-to-end anastomosis between the edge of the gastric tube and distal jejunum using 25-mm circular stapler, and the top of gastric conduit was resected using linear stapler. Microvascular anastomosis was performed after completion of gastrojejunostomy to shorten the ischemic time of the free jejunal graft. Basically, two sets of microvascular anastomoses using two arteries and two veins were performed in our hospital for free jejunal graft. The harvested jejunal graft was usually needed to be 60–70 cm in length for this technique, but the actually used jejunal graft for intestinal anastomosis was 15–20 cm in length while the remaining jejunal graft was sacrificed. The first set of microvascular anastomosis was completed under a surgical microscope, and an end-to-end anastomosis was performed via hand-sewn anastomosis between the hypopharynx and proximal jejunum. This was followed by a second set of microvascular anastomosis (Fig. 1a–c). The reason of this procedure is that we adjust the location of the second set of microvascular anastomosis in accordance with the position of free jejunal graft after completing intestinal anastomosis. Harvesting jejunal graft and two sets of microvascular anastomoses was performed by the reconstruction team consisting mainly of plastic surgeons.
a Reconstructive surgery involving gastric pull-up combined with free jejunal graft after total pharyngolaryngoesophagectomy. b Total pharyngolaryngoesophagectomy was completed, and the gastric tube was pulled up to the neck. The free jejunal graft was placed in the neck, and two sets of microvascular anastomose were performed (Case 10). c Arterial anastomosis was performed between the right superior thyroidal artery and the jejunal artery. Venous anastomosis was performed between the right superior thyroid vein and the jejunal vein (Case 10)
Surgery for PLE with transhiatal esophagectomy began with a cervical approach in the supine position and pharyngolaryngectomy with cervical esophagectomy and cervical lymph node dissection were performed. Partial sternotomy was added when necessary to complete the dissection of the upper mediastinal LN and/or to secure the distal margin of the trachea from the cervical approach. A median laparotomy was performed to conduct the transhiatal esophagectomy and construct the gastric tube, together with upper abdominal lymphadenectomy and harvesting of a jejunal segment. Intestinal reconstruction and vascular anastomosis were performed in a manner similar to PLE with esophagectomy via right thoracotomy.
Results
Operative factors
Esophagectomy via right thoracotomy was performed in 9 of the patients who underwent PLE, and transhiatal esophagectomy was performed in 5 patients (Table 2). Partial sternotomy was added to the cervical approach in 4 of the latter 5 patients. The posterior mediastinal route was used as the route of reconstruction in 13 patients, excluding one patient who underwent partial gastrectomy for synchronous early gastric cancer. Cervical tracheostomy was performed in 11 patients, and mediastinal tracheostomy was performed in 3 patients because they harbored tumors that infiltrated the mediastinal trachea.
Two sets of microvascular anastomoses using two arteries and two veins were performed in all 14 patients who underwent reconstruction involving gastric pull-up combined with free jejunal graft after PLE to interposition the free jejunal graft. The most commonly harvested recipient arteries were the superior thyroid artery and transverse cervical artery which arises from thyro-cervical trunk. The former was used in 12 patients, including two patients in whom bilateral superior thyroid arteries were used. The transverse artery was also used in 12 patients, including one patient in whom bilateral transverse arteries were used. The most commonly used recipient vein was the facial vein, which was used in 7 patients, including one patient in whom bilateral facial veins were used. The other most commonly used recipient veins were the transverse cervical vein and the external jugular vein, which were used in 6 patients. The superior thyroid vein and internal jugular vein were also used in some cases.
Operative complications
None of the 14 patients who underwent reconstruction involving gastric pull-up combined with free jejunal graft after PLE developed anastomotic leakage or graft necrosis (Table 3). Bleeding from the cervical wound occurred in one patient who underwent re-operation for hemostasis. None of the 14 patients developed pneumonia. Ischemia of the tracheal stump occurred in 4 patients (28.6%). Three of these 4 patients underwent only debridement of the necrotic tissue near the tracheostomy orifice and re-sewing the tracheal stump to the skin. The remaining one patient required resection of the ischemic tracheal ring and newly developing mediastinal tracheostomy because the extent of tracheal ischemia was relatively long. Ischemia of the tracheal stump orifice did not occur in 5 patients who underwent PLE with transhiatal esophagectomy, but it occurred in 4 of the 9 patients who underwent PLE with esophagectomy via right thoracotomy. The right bronchial artery was not preserved when PLE was performed in these 4 patients. Preoperative therapy did not affect the incidence of postoperative complications in this study.
No hospital deaths were recorded in this series.
Survival
The median survival time was 19.6 months. Recurrent disease occurred in 7 patients during the follow-up period: cervical lymph nodes in 2 patients, mediastinal lymph node in 1 patient, lung and brain in 1 patient, lung and liver in 1 patient, lung in 1 patient, and liver in 1 patient. Two cervical lymph node recurrences were within surgical field, and one mediastinal lymph node recurrence was without surgical field because the latter was occurred in right main bronchus lymph node in 1 patient who underwent transhiatal esophagectomy. Eight patients died during the follow-up period. Five patients died from cancer recurrence (esophageal cancer in 4 patients and hypopharyngeal cancer in 1 patient). One patient died from metachronous gum cancer despite treatment with radiation therapy, and one patient died from metachronous ethmoid cancer despite treatment with chemoradiotherapy. The remaining one patient death was due to choking on sputum at 8 months postoperatively. Therefore, 4 patients were alive without cancer recurrence, and 2 patients were alive with cancer recurrence.
Discussion
The present study described reconstruction involving gastric pull-up combined with free jejunal graft after PLE in 14 patients, including 7 patients with double cancer of the laryngopharynx and thoracic esophagus, 4 patients with multiple lesions of the cervical and thoracic esophagus, and 3 patients with cervicothoracic esophageal cancer. The results suggest that this procedure is a feasible reconstruction technique after PLE because neither anastomotic leakage nor graft necrosis occurred in this series.
Gastric pull-up via the posterior mediastinal route is the most commonly used procedure in previous studies [6, 15,16,17,18]. Gastric pull-up reconstruction has several advantages. This technique is the simplest procedure because it requires only one anastomosis within the alimentary tract. This technique also covers a long defect after PLE using a narrow gastric tube [19]. However, blood flow in the oral end of the gastric conduit tends to be poor, which leads to anastomotic leakage and graft necrosis. The recent reported incidence of anastomotic leakage and graft necrosis in patients who underwent reconstructive surgery involving gastric pull-up reconstruction after PLE is 3.5–17.2 and 0–5.3%, respectively [6,7,8,9, 20, 21]. Elongated gastric pull-up with microvascular anastomosis using short gastric vessels or left gastric vessels is sometimes applied after PLE to keep blood flow in the oral end of the gastric conduit [11, 12]. However, perigastric lymph node dissection can be inadequate in this procedure due to preservation of perigastric vessels. Therefore, elongated gastric pull-up with microvascular anastomosis seems to be unsuitable for patients with synchronous hypopharyngeal cancer and thoracic esophageal cancer because perigastric lymph node metastasis is common in thoracic esophageal cancer. The present study used gastric pull-up combined with free jejunal graft for reconstruction after PLE, and neither anastomotic leakage nor graft necrosis was encountered in this series. This result is likely due to the adequate blood supply in the oral end of the reconstruction conduit, which was used for anastomosis to the pharynx, and the lack of tension on the reconstruction conduit via interpositioning of the free jejunal graft.
Another commonly used reconstruction after PLE is colonic interposition. This procedure offers distinct advantages: it provides a long segment with minimum risk of sutures under tension; shorter time to restoration of good deglutition despite the absence of effective peristalsis; and fewer symptoms of gastric reflux than gastric pull-up [13, 15]. However, this procedure also has certain disadvantages, including the need for three intestinal anastomoses (pharyngocoloplasty, colon–colic and colon–gastric anastomosis) and tenuous blood supply to the oral end [13, 15]. The reported morbidity and mortality rates of colonic interposition are relatively high compared to reconstruction using the gastric pull-up [6, 13, 18]. The gastric pull-up combined with free jejunal graft used in our series has clear advantages over colonic interposition in terms of morbidity and mortality despite the need for microvascular anastomosis to the free jejunal graft. Colonic interposition may be suitable for patients in whom gastric tube cannot be used due to synchronous gastric cancer or history of gastric surgery.
This series performed microvascular anastomosis using two arteries and two veins for free jejunal graft in all cases. Advances in microvascular surgery have increased the patency rate of microvascular surgery up to 95% [22]. However, the reported incidence of graft ischemia in free jejunal graft remains 11.1% [18]. Graft ischemia is the most serious complication in free jejunal graft because re-operation is needed in most cases, and it sometimes leads to septic shock once graft ischemia occurs. Therefore, we usually used two sets of microvascular anastomoses using two arteries and two veins for free jejunal graft after PLE or cervical esophagectomy with or without laryngopharyngectomy in our hospital to minimize the risk of graft ischemia. The incidence of anastomotic leakage and graft necrosis in free jejunal grafting using two microvascular anastomosis techniques at our hospital is 4.3 and 0% in recent 231 cases. The transverse cervical artery and superior thyroidal artery were used in almost all cases in this series, but the recipient veins used for microvascular anastomosis in free jejunal graft varied considerably. In addition to the transverse cervical vein and superior thyroidal vein, the facial vein was also commonly used because pharyngojejunal anastomosis in reconstructive surgery involving gastric pull-up and free jejunal grafting after PLE tends to be located cranially. The internal and external jugular veins are also suitable for recipient veins in microvascular anastomosis in free jejunal graft, but the internal jugular vein was less frequently used in this series. One reason of this result is that microvascular anastomosis device is usually used for venous anastomosis in our hospital.
Ischemia of tracheal stump is one of the possible complications after PLE, especially in cases with mediastinal lymphadenectomy via right thoracotomy [11, 23]. In addition to the decrease in blood supply to the tracheal wall due to laryngectomy, complete resection of the cervical and thoracic paratracheal lymph nodes reduces blood supply to the trachea, with potential tracheal ischemia during PLE. In our series, all four patients who developed necrosis of tracheal stump underwent mediastinal lymph node dissection for thoracic esophageal cancer, including subcarinal lymph nodes, via right thoracotomy. The right bronchial artery could not be reserved in these four patients although we routinely try to preserve the bronchial arteries in mediastinal lymphadenectomy. However, none of the 5 cases of PLE with transhiatal esophagectomy developed ischemia of tracheal stump. Therefore, surgeons must be careful in PLE with mediastinal lymphadenectomy for thoracic esophageal cancer via right thoracotomy to avoid damage to bronchial arteries and may have to apply less aggressive mediastinal lymphadenectomy such as mediastinal lymphadenectomy without subcarinal lymph node dissection in consideration of the blood supply to the tracheal wall.
This retrospective study has several limitations. First, the functional and nutritional results such as regurgitation, dysphagia, and weight loss were not examined in this study. Further studies are needed to show the functional advantages of gastric pull-up combined with free jejunal graft over gastric pull-up only. The second limitation is relatively small sample size. In this series, blood loss in surgical procedure of PLE and reconstruction using gastric pull-up combined with free jejunal graft was substantial, compared with standard transthoracic esophagectomy with reconstruction using gastric tube. This may result from longer operative time in this procedure. However, efforts should be made to limit blood loss during this procedure because blood loss can have a negative influence on postoperative short-term and long-term outcomes.
In conclusion, we described reconstruction involving gastric pull-up combined with free jejunal graft in 14 patients who underwent PLE. The results indicate that this procedure is feasible and is not associated with anastomotic leakage or graft necrosis. Larger series are needed to show the distinct advantages of this procedure in comparison with conventional methods of reconstruction after PLE.
References
Peyre CG, Hagen JA, DeMeester SR et al (2008) Predicting systemic disease in patients with esophageal cancer after esophagectomy: a multinational study on the significance of the number of involved lymph nodes. Ann Surg 248:979–985
Ancona E, Ruol A, Castoro C et al (1997) First-line chemotherapy improves the resection rate and long-term survival of locally advanced (T4, any N, M0) squamous cell carcinoma of the thoracic esophagus: final report on 163 consecutive patients with 5-year follow-up. Ann Surg 226:714–723
Miyata H, Yamasaki M, Kurokawa Y et al (2012) Clinical relevance of induction triplet chemotherapy for esophageal cancer invading adjacent organs. J Surg Oncol 106:441–447
Morita M, Kuwano H, Ohno S et al (1994) Multiple occurrence of carcinoma in the upper aerodigestive tract associated with esophageal cancer: reference to smoking, drinking and family history. Int J Cancer 58:207–210
Slaughter DP, Southwick HW, Smejkel W (1953) Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer 6:963–968
Triboulet JP, Mariette C, Chevalier D et al (2001) Surgical management of carcinoma of the hypopharynx and cervical esophagus. Arch Surg 136:1164–1170
Pesko P, Sabljak P, Bjelovic M et al (2006) Surgical treatment and clinical course of patients with hypopharyngeal carcinoma. Dis Esophagus 19:248–253
Ferahkose Z, Bedirli A, Kerem M, Azili C, Sozuer EM, Akin M (2008) Comparison of free jejunal graft with gastric pull-up reconstruction after resection of hypopharyngeal and cervical esophageal carcinoma. Dis Esophagus 21:340–345
Denewer A, Khater A, Hafez MT, Hussein O, Roshdy S, Shahatto F, Elnahas W, Kotb S, Mowafy K (2014) Pharyngoesophageal reconstruction after resection of hypopharyngeal carcinoma: a new algorithm after analysis of 142 cases. World J Surg Oncol 12:182
Morita M, Saeki H, Ito S et al (2014) Technical improvement of total pharyngo-laryngo-esophagectomy for esophageal cancer and head and neck cancer. Ann Surg Oncol 21:1671–1677
Sagawa N, Okushiba S, Ono K et al (2000) Reconstruction after total pharyngolaryngoesophagectomy. Comparison of elongated stomach roll with microvascular anastomosis with gastric pull up reconstruction or something like that. Langenbecks Arch Surg 385:34–38
Watanabe M, Baba Y, Yoshida N, Ishimoto T, Sakaguchi H, Kawasuji M, Baba H (2014) Modified gastric pull-up reconstructions following pharyngolaryngectomy with total esophagectomy. Dis Esophagus 27:255–261
Bussi M, Ferrero V, Riontino E et al (2000) Problems in reconstructive surgery in the treatment of carcinoma of the hypopharyngoesophageal junction. J Surg Oncol 74:130–133
Carlson GW, Schusterman MA, Guillamondegui OM (1992) Total reconstruction of the hypopharynx and cervical esophagus: a 20-year experience. Ann Plast Surg 29:408–412
Surkin MI, Lawson W, Biller HF (1984) Analysis of the methods of pharyngoesophageal reconstruction. Head Neck Surg 6:953–970
Harrison DF, Thompson AE (1986) Pharyngolaryngoesophagectomy with pharyngogastric anastomosis for cancer of the hypopharynx: review of 101 operations. Head Neck Surg 8:418–428
Wei WI, Lam LK, Yuen PW et al (1998) Current status of pharyngolaryngo-esophagectomy and pharyngogastric anastomosis. Head Neck 20:240–244
Panhofer P, Springer C, Izay B et al (2013) Influence of resection extent on morbidity in surgery for squamous cell cancer at the pharyngoesophageal junction. Langenbecks Arch Surg 398:221–230
Sasaki CT, Salzer SJ, Cahow E et al (1995) Laryngopharyngoesophagectomy for advanced hypopharyngeal and esophageal squamous cell carcinoma: the Yale experience. Laryngoscope 105:160–163
Shuangba H, Jingwu S, Yinfeng W et al (2011) Complication following gastric pull-up reconstruction for advanced hypopharyngeal or cervical esophageal carcinoma: a 20-year review in a Chinese institute. Am J Otolaryngol 32:275–278
Cense HA, Law S, Wei W et al (2007) Pharyngolaryngoesophagectomy using the thoracoscopic approach. Surg Endosc 21:879–884
Harii K (1990) The free flap in head and neck cancer. BC Decker, Philadelphia, pp 33–35
Sekido M, Yamamoto Y, Minakawa H et al (2003) Use of the “supercharge” technique in esophageal and pharyngeal reconstruction to augment microvascular blood flow. Surgery 134:420–424
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Miyata, H., Sugimura, K., Motoori, M. et al. Clinical Assessment of Reconstruction Involving Gastric Pull-Up Combined with Free Jejunal Graft After Total Pharyngolaryngoesophagectomy. World J Surg 41, 2329–2336 (2017). https://doi.org/10.1007/s00268-017-3948-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-017-3948-0