Abstract
Background
There are many reports about limited surgery for intraductal papillary mucinous neoplasms (IPMNs) of the pancreas. However, there is no consensus on limited surgery for IPMNs. The primary objective of this study was to define the clinical indications for limited surgery for IPMNs.
Methods
The data of 98 patients who underwent curative resections for IPMN were retrospectively analyzed. IPMNs were classified into four different pathological grades: low-grade dysplasia (LGD), intermediate-grade dysplasia (IGD), high-grade dysplasia (HGD), and invasive carcinoma (Inv-IPMN). Inv-IPMNs were divided into T1a, T1b, and T1c or over T1c (≥T1c). Based on preoperative radiological findings, IPMNs were stratified into the three groups using the 2012 International Consensus Guidelines: worrisome features, high-risk stigmata (HRS), and others.
Results
There were no positive lymph node cases and no recurrent cases of LGDs, IGDs, and HGDs. On the other hand, positive lymph node cases in T1a, T1b, and ≥T1c were seen in 37.5, 20, and 22.2% of cases, respectively. The recurrence rates of T1a, T1b, and ≥T1c were 50, 40, and 55.6%, respectively. Of the HRS cases, 30 (73.2%) were malignant and 25 (61%) were Inv-IPMN. HRS showed sensitivity of 92.6%, specificity of 77.5%, and accuracy of 81.6% to identify Inv-IPMN by preoperative imaging.
Conclusions
Limited surgery such as parenchyma-sparing pancreatectomy should be avoided for all cases of Inv-IPMNs, because every Inv-IPMN including T1a has the potential for lymph node metastasis and recurrence. HRS had high preoperative diagnostic ability for predicting Inv-IPMN. For cases that meet HRS criteria, pancreatectomy with lymphadenectomy is needed, and limited surgery should be withheld.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Background
Intraductal papillary mucinous neoplasms (IPMNs) of the pancreas arise in the main pancreatic duct or its major branches. The precancerous nature of IPMNs is now widely accepted to imply a sequence of progression to malignancy, as with colonic polyps [1, 2]. In fact, IPMNs show various pathological grades, and the World Health Organization (WHO) 2010 classification system classifies them into low-grade dysplasia (LGD), intermediate-grade dysplasia (IGD), high-grade dysplasia (HGD), and invasive carcinoma (Inv-IPMN) [3].
IPMNs are said to have a better prognosis than ordinary pancreatic cancer, but their prognosis is poor if they progress to invasive cancer [4, 5]. However, there is also a concept of minimally invasive IPMN, and some reports found the prognosis to be good if the invasiveness remained modest [6, 7]. A comparative study that matched the pathological stage found that the prognosis of node-positive Inv-IPMN was the same as that of node-positive sporadic pancreatic carcinoma [8].
On the other hand, the operative indications for IPMNs have become widely adopted subsequent to the release of the 2012 International Consensus Guidelines (ICG 2012) [9]. The guidelines state that, in the case of IPMNs with no clear findings of invasive cancer, it is acceptable to perform limited surgery. However, there is no clear guidance in regard to findings suggestive of Inv-IPMN. Accordingly, at present, there is no clear consensus regarding the indications for pancreatic limited surgery, such as parenchyma-sparing pancreatectomy.
Research on IPMNs has made dramatic progress in the last decade, but several points remain at issue. How much invasion of IPMN increases the risk of lymph node metastasis and postoperative recurrence? Is preoperative image diagnosis possible for Inv-IPMN? It is problematic that, even though these and other questions remain unanswered, many IPMNs are being treated by limited surgical procedures such as laparoscopic surgery and parenchyma-sparing pancreatectomy [10–13].
The objective of this study was to define the clinical indications for limited surgery for IPMNs. To that end, IPMNs were first classified into subtypes based on their distance of invasion, and they were compared in regard to lymph node metastasis and prognosis. Thus, the indications for limited surgery were considered from the viewpoint of the distance of invasion. Moreover, in order to preoperatively predict the invasion distance, ICG 2012 was used for diagnosis of malignancy, and the indications for limited surgery were considered from the perspective of the preoperative diagnosis.
Materials and methods
Patients
A total of 98 patients with IPMN who underwent surgical resection at Osaka City University Hospital between 1994 and 2015 were included in this study. The study was approved by the Ethics Committee of Osaka City University and was in compliance with the Declaration of Helsinki. Informed consent was obtained from all patients to use specimens for this study in accordance with the institutional rules of the hospital. All patients had a confirmed histopathological diagnosis of IPMN of the pancreas based on WHO classifications [3]. Clinical records, radiological data, pathological results, and surgical reports were reviewed retrospectively. The median duration of follow-up for all 98 patients was 55 months (range 10–210 months).
Definition of radiological type of intraductal papillary mucinous neoplasms
IPMNs were classified into three macroscopic types based on preoperative radiological findings. Main duct-type IPMN (MD-IPMN) was defined as showing dilatation of the main pancreatic duct to over 5 mm. Branch duct-type IPMN (BD-IPMN) was defined as showing cystic dilatation of a branch pancreatic duct that communicated with a non-dilated main pancreatic duct. Mixed-type IPMN (MX-IPMN) displayed characteristics of MD-IPMN and BD-IPMN. Computed tomography and magnetic resonance cholangiopancreatography were used to determine the radiological type of IPMN, with endoscopic ultrasonography (EUS) added when needed.
Parameters of malignant predictors in the 2012 ICG
Based on ICG 2012, nine preoperative clinical and radiological parameters were assessed, and cases were then categorized as high-risk stigmata (HRS), worrisome features (WF), and the others, which were called no criteria (NC). HRS was defined as IPMN with at least one of the following factors: obstructive jaundice due to a cystic lesion at the head of the pancreas; a contrast-enhanced solid component within a cyst; or the main pancreatic duct ≥10 mm. WF was also defined as IPMN with at least one of the following factors: history of acute pancreatitis; cyst diameter ≥3 cm; thickened/enhancing cyst walls; main pancreatic duct size 5–9 mm; non-enhancing mural nodule; or an abrupt change in caliber of pancreatic duct with distal pancreatic atrophy and lymphadenopathy. Contrary to ICG 2006, which described predictors of malignancy for BD-IPMN alone, the ICG 2012 algorithm analyzes all IPMNs together, considering main duct dilatation as indicative of WF or HRS [14]. The present study population thus included both BD-IPMNs and MD-/MX-IPMNs.
Pathology
On the basis of the fourth edition of the WHO classification system, the degree of dysplasia was graded and categorized as LGD, IGD, HGD, or Inv-IPMN [ 3 ]. Moreover, Inv-IPMNs were divided into three subtypes: T1a (stromal invasion ≤5 mm), T1b (stromal invasion >5 mm and ≤10 mm), and T1c or over T1c (≥T1c) (stromal invasion >10 mm). In this study, LGD and IGD were considered benign, and both HGD and Inv-IPMN were considered malignant.
Statistical analysis
All statistical analyses were performed using JMP statistical software (version 11; SAS Institute, Cary, NC). Fisher’s exact probability test or the χ 2 test was used to evaluate differences in clinical factors between the two groups, and the Mann-Whitney U test was used for continuous data. The Kaplan–Meier method was used for univariate survival analysis, and log-rank testing was applied. Values of p < 0.05 were considered significant.
Results
Clinical characteristics of the study population
The demographic and clinical characteristics of the study patients are presented in Table 1. The study subjects were 54 men and 44 women, with a mean age of 68.3 years at the time of operation. Radiological type was identified as MD-IPMN in 27 cases (27.6%), BD-IPMN in 53 cases (54.1%), and MX-IPMN in 18 cases (18.4%).
In our institute, indications for surgical resection were decided according to ICG 2012 and, before ICG 2012 was published, according to ICG 2006. Before ICG 2006, surgical indications were decided as follows: main pancreatic duct >10 mm; a cyst greater than 50 mm; solid component within the cyst; or the presence of symptoms. All IPMNs were resected; limited surgery such as laparoscopic surgery and parenchyma-sparing pancreatectomy was not performed in any cases.
Lymph node metastasis, postoperative recurrence rate, and prognosis according to the pathological grade
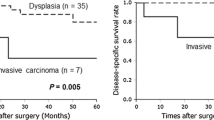
Table 1 shows the clinical characteristics of each pathological grade, including 33 LGD, 25 IGD, 13 HGD, 8 T1a, 10 T1b, and 9 ≥ T1c cases. Figure 1 presents characteristic HE-stained micrographs of HGD and Inv-IPMN. Table 1 also shows the lymph node metastasis rates and recurrence rates for each pathological grade. There were no cases of recurrence among LGD/IGD cases. Whereas there were no cases of lymph node metastasis or recurrence in HGD, including carcinoma in situ (CIS), the lymph node metastasis rates in T1a, T1b, and ≥T1c were 37.5, 20, and 22.2%, respectively, while their recurrence rates were 50, 40, and 55.6%, respectively. Figure 2 shows the prognostic curves for disease-specific survival for each pathological grade, showing recurrence patterns. The median survival times (MSTs) of T1a and T1b were comparatively good, at 51.4 and 50.5 months, respectively. On the other hand, the MST of ≥T1c was 16 months, and the prognosis of ≥T1c was about the same as for pancreatic ductal adenocarcinoma (PDA) cases resected at our institution (p = 0.36). T1b and PDA showed a significant difference in prognosis (p = 0.02). The prognosis for PDA and T1a differed, but not significantly (p = 0.0596). Of the total of eight T1a cases, 4 had recurrence, which included one case of lymph node recurrence. Of the total of 10 T1b cases, 4 had recurrence, which included two cases of lymph node recurrence.
Kaplan–Meier survival curves for disease-specific survival of malignant IPMNs and conventional pancreatic ductal adenocarcinoma (PDAC). Five-year survival rates of HGD, T1a, T1b, ≥T1c, and PDAC are 100, 45.0, 42.7, 25.4, and 19.6%, respectively. Median survival times of T1a, T1b, ≥T1c, and PDAC are 51.4, 50.5, 16.0, and 17.7 months, respectively. Of the total of eight T1a cases, 4 had recurrence, including one case of lymph node recurrence. Of the total of 10 T1b cases, 4 had recurrence, including two cases of lymph node recurrence
Progression of the risk of malignancy across ICG 2012 categories
Among patients with NC (n = 18), two cases (11.1%) were malignant, and none was Inv-IPMN. Among WF cases (n = 39), eight (20.5%) were malignant, and two (5.1%) were Inv-IPMN. On the other hand, among HRS cases, 30 (73.2%) were malignant, and 25 (61.0%) were Inv-IPMN. HRS showed higher rates of invasive lesions than NC (p < 0.001) and WF (p < 0.001). HRS showed high diagnostic accuracy for invasive IPMN, with sensitivity of 92.3%, specificity of 77.5%, positive predictive value of 61.0%, negative predictive value of 96.5%, and accuracy of 81.6% (Fig. 3).
Of the high-risk stigmata (HRS) cases, 30 (73.2%) are malignant (HGD + Inv-IPMN) and 25 (61.0%) are Inv-IPMN. HRS shows higher rates of invasive lesions than no criteria (p < 0.001) and worrisome features (p < 0.001). HRS shows high diagnostic accuracy for invasive IPMN, with sensitivity of 92.3%, specificity of 77.5%, positive predictive value of 61.0%, negative predictive value of 96.5%, and accuracy of 81.6%
Table 2 shows diagnostic ability by the criteria of HRS and WF in IPMN patients according to the pathological grade. All cases of T1b (n = 10) and all cases of ≥T1c (n = 9) satisfied the HRS criteria. That is, HRS showed sensitivity of 100% for the diagnosis of T1b or more severe Inv-IPMN. On the other hand, only 6 of 8 (75%) T1a cases satisfied the HRS criteria, while the remaining 2 did not. These 2 cases had the following characteristics. Case 1 was MX-IPMN of the pancreatic head, and although the main pancreatic duct (MPD) showed a tendency to be dilated, it did not reach 10 mm. Case 2 was also MX-IPMN of the pancreatic head, with dilation (35 mm) of a pancreatic duct branch and a thickened cyst wall of 5 mm. Both of these cases thus satisfied the WF criteria.
Discussion
As a result of broad use of ICG 2012, a consensus is being reached over which type of IPMNs should be operative indication. IPMNs are generally described as being low-malignant potential tumors, and limited surgery, such as laparoscopic surgery and parenchyma-sparing pancreatectomy, is indicated for many. However, not much study has been done regarding whether limited surgery should be indicated for all IPMN cases.
This study examined the prognosis of IPMNs in detail, and based on the distance of invasion, new findings not previously reported by studies of the indications for limited surgery were identified. Lymph node metastasis was seen in 37.5% of cases, even when there was minimal invasion of <5 mm, and there were also cases of lymph node recurrence. This can be thought to indicate that, even if an IPMN shows minimal invasion of <5 mm, limited surgery should not be performed, and laparoscopic surgery should be permitted only for cases up to HGD. Moreover, the International Consensus Guidelines note that 61% of HRS cases are Inv-IPMN and strongly suggest that limited surgery should not be indicated for HRS.
Some reports have defined Inv-IPMN with ≤5 mm invasion as minimally invasive IPMN. Nara et al. [6] examined 26 cases of minimally invasive IPMNs and reported that none had lymph node metastasis, while the 5-year survival rate was 100%. Conversely, a Korean group reported recurrence in 8.3% of cases with minimally invasive IPMNs [15]. In a larger-scale study, Jordan et al. investigated 70 cases of Inv-IPMN and reported that the rate of lymph node metastasis increased in proportion to the invasion distance: 8.6% for ≤5 mm, 18.8% for 5–9 mm, 28.6% for 10–14 mm, and 41.7% for 15–19 mm [7]. In the present study, the rates of lymph node metastasis were 37.5% even for T1a and 20% for ≥T1b. The rate of recurrence was around 50% for ≥T1a, and even when the invasion distance was within 5 mm, it was considered to have acquired a high degree of biological malignancy, unlike HGD. Accordingly, we propose that Inv-IPMN must be treated by pancreatectomy with lymph node dissection, regardless of the invasion distance.
Moreover, the prognostic curve for ≥T1c cases, with an invasion distance exceeding 10 mm, nearly overlapped the prognostic curve for ordinary pancreatic cancer, and it was confirmed that ≥T1c IPMN is a disease that has about the same degree of biological malignancy as conventional pancreatic ductal carcinoma and has a poor prognosis.
Limited surgery for pancreatic neoplasms has important advantages in that it preserves the organ and is minimally invasive. Remarkable technical advances have been made in laparoscopic surgery, and in recent years, it has been reported that it is possible to achieve equivalent lymph node dissection and nerve plexus dissection as with open surgery for pancreatic cancer [16–18]. However, no randomized controlled trial (RCT) has yet been carried out to compare the safety and prognosis of open surgery and laparoscopic surgery for pancreatectomy, and it cannot yet be declared that laparoscopic surgery permits lymph node dissection and peripancreatic nerve plexus dissection equivalent to that with open surgery. Nevertheless, there are many reports regarding limited surgery, such as laparoscopic surgery and parenchyma-sparing pancreatectomy, for curative resection of IPMNs [10–13]. Based on the findings of the present study, since there was not a single case of lymph node metastasis or recurrence for IPMN with HGD or less dysplasia, limited surgery may be indicated for such IPMN cases.
However, performing grade classification preoperatively is generally difficult, and preoperative diagnosis of IPMN with HGD or less dysplasia is in fact impossible. Several predictive factors for Inv-IPMN have been reported, including dilated branch (>30 mm) [19], MPD dilatation [20], the presence of mural nodule [21], elevated CEA [22], and elevated CA19-9 [22, 23]. Ogawa et al. [24] reported that diffusion-weighted MRI improved the ability to detect invasive malignancy in IPMN. The group from the University of Padua reported the efficacy of detection of malignancy by PET [25], while Camilo et al. reported that weight loss and a solid component were useful [26].
In the present study, high levels of sensitivity, specificity, and positive diagnosis rate were achieved for Inv-IPMN cases satisfying any of the three guideline criteria for HRS, i.e., jaundice, MPD ≥10 mm, and a contrast-enhanced solid component. The HRS criteria were originally defined for an algorithm for deciding the treatment policy for BD-IPMN. However, the criteria are also applicable to MD-IPMN, and their high diagnostic ability for Inv-IPMN was confirmed. However, in the present study, 2 cases of Inv-IPMN did not meet the HRS criteria and satisfied only the WF criteria. That finding indicates that, although few in number, some Inv-IPMN cases are included in the WF population. Thus, limited surgery should not be indicated for all cases of WF, and the surgical procedure must be decided for each individual case based on strict preoperative imaging diagnosis. Based on the present results, it can be concluded that limited surgery should not be performed for cases of HRS, since they include a high proportion of Inv-IPMN, whereas WF cannot be said to be an indication for limited surgery.
The present study has several limitations. First, this was a retrospective study conducted at a single institution, and the sample size was small. A large-scale, multicenter, cohort study is needed to evaluate the pathological features and prognosis of Inv-IPMN. Another significant limitation was that the modality for diagnosing each IPMN varied. In particular, EUS was performed in 75% of cases. EUS is today considered an essential examination for the diagnosis of IPMN, but it is necessary to note that the present patient series included some cases that predated the use of EUS and were diagnosed and underwent surgery without use of this modality.
In conclusion, limited surgery such as parenchyma-sparing pancreatectomy should be avoided for all cases of Inv-IPMNs, because every Inv-IPMN including T1a has the potential for lymph node metastasis and recurrence. HRS had high preoperative diagnostic ability for predicting Inv-IPMN. For cases that meet HRS criteria, pancreatectomy with lymphadenectomy is needed, and limited surgery should be withheld.
References
Maitra A, Fukushima N, Takaori K et al (2005) Precursors to invasive pancreatic cancer. Adv Anat Pathol 12:81–91
Hruban RH, Maitra A, Kern SE et al (2007) Precursors to pancreatic cancer. Gastroenterol Clin North Am 36:831–849
Bosman FT, Carneiro F, Hruban RH, Theise ND (eds) (2010) WHO classification of tumors of Digestive System. International Agency for Research on Cancer, Lyon
Jang JY, Hwang DW, Kim MA et al (2011) Analysis of prognostic factors and a proposed new classification for invasive papillary mucinous neoplasms. Ann Surg Oncol 18:644–650
Yopp AC, Katabi N, Janakos M et al (2011) Invasive carcinoma arising in intraductal papillary mucinous neoplasms of the pancreas: a matched control study with conventional pancreatic ductal adenocarcinoma. Ann Surg 253:968–974
Nara S, Shimada K, Kosuge T et al (2008) Minimally invasive intraductal papillary-mucinous carcinoma of the pancreas: clinicopathologic study of 104 intraductal papillary-mucinous neoplasms. Am J Surg Pathol 32:243–255
Winter JM, Jiang W, Basturk O et al (2016) Recurrence and survival after resection of small intraductal papillary mucinous neoplasm-associated carcinomas (≤20 mm invasive component): a multi-institutional analysis. Ann Surg 263:793–801
Wasif N, Bentrem DJ, Farrell JJ et al (2010) Invasive intraductal papillary mucinous neoplasm versus sporadic pancreatic adenocarcinoma: a stage-matched comparison of outcomes. Cancer 116:3369–3377
Tanaka M, Fernandez-del Castillo C, Adsay V et al (2012) International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 12:183–197
Thomas E, Matsuoka L, Alexopoulos S et al (2015) Laparoscopic hand-assisted parenchymal-sparing resections for presumed side-branch intraductal papillary mucinous neoplasms. J Laparoendosc Adv Surg Tech A 25:668–671
Fernandez-Cruz L, Cosa R, Blanco L et al (2007) Curative laparoscopic resection for pancreatic neoplasms: a critical analysis from a single institution. J Gastrointest Surg 11:1607–1621 (discussion 1621–1602)
Sauvanet A, Gaujoux S, Blanc B et al (2014) Parenchyma-sparing pancreatectomy for presumed noninvasive intraductal papillary mucinous neoplasms of the pancreas. Ann Surg 260:364–371
Schwarz L, Fleming J, Katz M et al (2016) Total laparoscopic central pancreatectomy with pancreaticogastrostomy for high-risk cystic neoplasm. Ann Surg Oncol 23:1035
Tanaka M, Chari S, Adsay V et al (2006) International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology 6:17–32
Kim J, Jang KT, Mo Park S et al (2011) Prognostic relevance of pathologic subtypes and minimal invasion in intraductal papillary mucinous neoplasms of the pancreas. Tumour Biol 32:535–542
Abu Hilal M, Richardson JR, de Rooij T et al (2016) Laparoscopic radical ‘no-touch’ left pancreatosplenectomy for pancreatic ductal adenocarcinoma: technique and results. Surg Endosc 30:3830–3838
Sharpe SM, Talamonti MS, Wang E et al (2015) The laparoscopic approach to distal pancreatectomy for ductal adenocarcinoma results in shorter lengths of stay without compromising oncologic outcomes. Am J Surg 209:557–563
Sulpice L, Farges O, Goutte N et al (2015) Laparoscopic distal pancreatectomy for pancreatic ductal adenocarcinoma: Time for a randomized controlled trial? Results of an all-inclusive national observational study. Ann Surg 262:868–873 (discussion 873–864)
Anand N, Sampath K, Wu BU (2013) Cyst features and risk of malignancy in intraductal papillary mucinous neoplasms of the pancreas: a meta-analysis. Clin Gastroenterol Hepatol 11:913–921 (quiz e959–960)
Murakami Y, Uemura K, Hayashidani Y et al (2007) Predictive factors of malignant or invasive intraductal papillary-mucinous neoplasms of the pancreas. J Gastrointest Surg 11:338–344
Shimizu Y, Yamaue H, Maguchi H et al (2013) Predictors of malignancy in intraductal papillary mucinous neoplasm of the pancreas: analysis of 310 pancreatic resection patients at multiple high-volume centers. Pancreas 42:883–888
Kanno A, Satoh K, Hirota M et al (2010) Prediction of invasive carcinoma in branch type intraductal papillary mucinous neoplasms of the pancreas. J Gastroenterol 45:952–959
Roch AM, Ceppa EP, Al-Haddad MA et al (2014) The natural history of main duct-involved, mixed-type intraductal papillary mucinous neoplasm: parameters predictive of progression. Ann Surg 260:680–688 (discussion 688–690)
Ogawa T, Horaguchi J, Fujita N et al (2014) Diffusion-weighted magnetic resonance imaging for evaluating the histological degree of malignancy in patients with intraductal papillary mucinous neoplasm. J Hepatobiliary Pancreat Sci 21:801–808
Pedrazzoli S, Sperti C, Pasquali C et al (2011) Comparison of International Consensus Guidelines versus 18-FDG PET in detecting malignancy of intraductal papillary mucinous neoplasms of the pancreas. Ann Surg 254:971–976
Correa-Gallego C, Do R, Lafemina J et al (2013) Predicting dysplasia and invasive carcinoma in intraductal papillary mucinous neoplasms of the pancreas: development of a preoperative nomogram. Ann Surg Oncol 20:4348–4355
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Kimura, K., Amano, R., Ymazoe, S. et al. The Clinical Indications for Limited Surgery of Intraductal Papillary Mucinous Neoplasms of the Pancreas. World J Surg 41, 1358–1365 (2017). https://doi.org/10.1007/s00268-016-3824-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-016-3824-3