Abstract
Background
The prognosis of intrahepatic cholangiocarcinoma (ICC) remains poor despite improvements in treatment and post-operative clinical management. We review our experiences and evaluate our current surgical approaches by comparing patients from two consecutive treatment periods.
Methods
One hundred forty-four patients who underwent hepatectomy for ICC between 1993 and 2014 were divided into groups that received treatment before (n = 65, first period) and after 2006 (n = 79, second period), when new treatment options such as adjuvant chemotherapy and multimodal therapy for recurrence were introduced. Clinicopathological characteristics and survival outcomes were compared between the groups.
Results
First-period patients exhibited more advanced tumor characteristics, including larger tumors, higher serum carbohydrate antigen 19–9 levels, and vascular invasion. Median overall survival (OS) durations of the first- and second-period groups were 21.4 and 57.7 months, respectively (p < 0.001); corresponding median disease-free survival (DFS) durations were 12.2 and 16.6 months, respectively (p = 0.027). Multivariate analysis found an independent association of the treatment time period with OS and DFS. Notably, second-period patients with N1 disease achieved a longer OS and DFS (median OS time: 12.4 and 26.0 months, p = 0.0018, and median DFS: 4.7 and 10.7 months p = 0.019, respectively). Among recurrent patients (first, n = 50 and second, n = 44), second-period patients had a significantly longer survival after recurrence (8.0 vs. 22.3 months, p < 0.001).
Conclusion
ICC patients, particularly those with N1 disease, achieved significant survival improvements that were partly attributable to patient selection, adjuvant chemotherapy, and multimodal treatment after recurrence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intrahepatic cholangiocarcinoma (ICC), which arises from the second or further peripheral biliary tree branches, is the second-most common type of primary liver cancer after hepatocellular carcinoma (HCC) [1, 2]. Surgical resection is considered the only curative treatment for ICC. However, the respective 5-year overall survival (OS) and postsurgical recurrence rates of 15–40 % [3–7] and 50–60 % [3, 8, 9] are unsatisfactory.
Recently, the clinical management of ICC has improved following advances in surgical techniques and preoperative imaging modalities and the introduction of effective chemotherapy regimens. We previously reported the significance of fluorine-18 fluorodeoxyglucose positron emission tomography (18FDG-PET) [10] and adjuvant chemotherapy [11] for ICC management. 18FDG-PET is predictive of lymph node metastasis and ICC recurrence after surgical resection and is useful for identifying patients at high risk for recurrence [10]. Additionally, after several studies, adjuvant gemcitabine chemotherapy effectively treated patients undergoing curative resection for advanced ICC [11], and gemcitabine-based systemic chemotherapy for unresectable biliary tract cancer is widely considered effective [12–14].
ICC is a rare entity, and therefore randomized clinical trials have been difficult to conduct. Although previous reports suggested improvements in the outcomes of patients with ICC [15, 16], it remains unclear whether these recent medical developments have influenced the survival outcomes of such patients. The aim of the present study was to review our experiences and evaluate the potential beneficial effects of our current surgical approaches by comparing cohorts from 2 consecutive time periods during a 22-year overall period at a single-center specializing in hepatobiliary surgery.
Methods
Patients
One hundred fifty-six ICC patients (excluding combined ICC–HCC patients) initially underwent hepatectomy with curative intent at Kyoto University Hospital, Kyoto, Japan between 1993 and 2014. Twelve patients with histological para-aortic lymph node metastases (a type of distant metastasis) were excluded. Finally, 144 patients were enrolled in this retrospective study and categorized according to whether they underwent hepatectomy during the first (February 1993–December 2005; n = 65) or second period (January 2006–January 2014; n = 79), as several advances in clinical ICC management occurred at our institute around 2006. Image diagnosis, including multi-detector computed tomography, was introduced in 2006, and 18FDG-PET data accumulation was established in 2007 [10]. Additionally, therapeutic systemic chemotherapy combined with surgical treatment (i.e., adjuvant chemotherapy and gemcitabine-based chemotherapy after recurrence) was introduced in 2006. Regarding adjuvant chemotherapy, gemcitabine hydrochloride and tegafur–gimeracil–oteracil–potassium (S-1) were authorized for use in Japanese patients with biliary tract cancer in 2006 and 2007, respectively, and are currently used for selected patients. Post-operative adjuvant chemotherapy was principally indicated for stage II–IV tumors, according to the American Joint Committee on Cancer/International Union Against Cancer (AJCC/UICC) classification, seventh edition [17]. Neoadjuvant chemotherapy was not considered in this study.
Data collection
Clinicopathologic data, including sex, age, hepatitis virus markers, liver function (e.g., Child–Pugh classification), and primary tumor characteristics were collected. Treatment-related variables (e.g., surgical procedures including lymphadenectomy, chemotherapy) were also determined. Primary tumor characteristics and resection margins were ascertained from final pathologic assessments. Operative mortality was defined as death within 30 days of surgery or during the same admission period. Morbidity was evaluated using the Clavien–Dindo classification [18]. Patients underwent regular postsurgical clinical follow-ups to evaluate blood chemistry and tumor markers (e.g., carbohydrate antigen 19–9 [CA19-9] and carcinoembryonic antigen), routine computed tomography examinations at 3–6-month intervals, and magnetic resonance imaging or 18FDG-PET as needed. Recurrence diagnoses were entirely based on imaging studies and tumor markers. Follow-up data were updated in January 2015. The study protocol was approved by the Ethical Committee of the Graduate School of Medicine, Kyoto University. Written informed consent was obtained from all study participants.
Surgery
Patients who were able to undergo macroscopically curative resection, with a Child–Pugh grade of A or B and future liver remnant volume >30 % of the whole liver, were indicated for surgical treatment. Preoperative portal vein embolization was considered when the future liver remnant volume was <30 % of the whole liver in patients with an undamaged liver, or <40 % in those with a damaged liver. Procedure type was defined according to the hepatic anatomy and resection terminology, as proposed by the International Hepato-Pancreato-Biliary Association in 2000 [19]. Major and minor hepatectomy were defined as bisectionectomy or more and sectionectomy or less, respectively. Extended hepatectomy was defined as the removal of ≥5 segments.
We routinely sampled para-aortic lymph nodes to determine surgical indications. Regional lymphadenectomy around the hepatoduodenal ligament was routinely performed except for patients with poor conditions and those preoperatively diagnosed with HCC or other diseases. Intra-operative findings indicated the necessity of extended lymphadenectomy around the celiac trunk or hepatogastric ligament. The pathological lymph node status was defined as follows: N0, negative lymph node metastasis; N1: positive lymph node metastasis; and Nx: uncertain lymph node status. Residual tumor status was defined as follows: R0 resection, no macroscopic or microscopic tumor remaining; R1 resection, microscopically positive surgical margins; and R2 resection, not all gross tumors removed.
Treatment strategy for recurrent patients
Treatments were determined according to the initial recurrence pattern. Surgery was initially considered as a loco-regional therapy for selective patients with intrahepatic or extrahepatic recurrences. Patients with synchronous intrahepatic and extrahepatic recurrences and those with recurrences in multiple extrahepatic organs were treated with systemic chemotherapy, but not surgery. Radiofrequency ablation therapy was only performed in patients who refused surgery.
Statistical analysis
Using the Kaplan–Meier method, overall survival (OS) was calculated from the date of surgery to the date of death or end of follow-up; disease-free survival (DFS) was calculated with the date of death or recurrence as the terminal event; and survival after recurrence (SAR) was calculated from the date of recurrence to the date of death or end of follow-up. Survival was compared according to time period using the generalized Wilcoxon test. Prognostic factors for survival were identified through multivariate Cox proportional hazards models with stepwise selection; variables that were identified as significant (p < 0.1) in a univariate analysis were placed in a multivariate model. Continuous variables were expressed as medians (ranges) and compared using the Mann–Whitney U test. Categorical variables were compared using χ 2 tests. All analyses were 2-sided, and differences were considered significant at a p value <0.05. Statistical analyses were performed using JMP ver. 12.1 software (SAS Institute, Inc., Cary, NC, USA).
Results
Patient characteristics
Table 1 presents the characteristics of 144 patients who underwent curative intent hepatectomy. Although second-period patients were older than first-period patients, there were no significant intergroup differences in terms of sex ratios and the number of patients with hepatitis-B surface antigen or hepatitis-C antibody positivity. Liver function, defined according to the presence of liver cirrhosis or Child–Pugh classification, was comparable between the groups. Regarding tumor characteristics, patients in the first period had significantly larger tumors, higher serum CA19-9 levels, and less frequent vascular invasion. Second-period patients more frequently had an Nx nodal status; however, a proportion of patients with N0 or N1 disease were comparable. Moreover, a proportion of patients with major vascular or biliary invasion were also comparable. Forty-six patients, all in the second-period group, underwent adjuvant chemotherapy.
Short-term outcomes after hepatectomy
Table 2 summarizes short-term outcomes after hepatectomy. Major hepatectomy [first period, n = 26 (40 %) and second period, n = 27 (34 %)] and extended hepatectomy [n = 33 (51 %) and n = 34 (34 %), respectively] were most common, and no significant intergroup differences in surgical procedures were observed. Lymphadenectomy was less frequent in the second period [n = 64 (98 %) and n = 70 (89 %), p = 0.023]. Lymphadenectomy was not performed in 3 patients with preoperatively diagnosed HCC, 6 patients with poor liver function, and 1 elderly patient. Biliary [n = 27 (42 %) and n = 22 (28 %)] and vascular reconstruction [n = 14 (22 %) and n = 11 (14 %), respectively] was performed aggressively in both groups. Regarding pathology, the residual tumor statuses were R0 in 118 patients [n = 52 (80 %) and n = 66 (84 %)] and R1 in 26 patients [n = 13 (20 %) and n = 13 (16 %)]; R2 was not observed. Mortality was observed in eight patients [n = 4 (6 %) and n = 4 (5 %)], with no significant intergroup difference. Seventy patients experienced post-operative complications [n = 34 (52 %) and n = 36 (46 %)]; the groups did not differ significantly in terms of the frequency of Clavien–Dindo grade III/VI disease [n = 15 (23 %) and n = 13 (16 %), respectively, p = 0.57].
Survival analysis
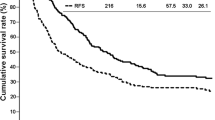
The overall median observation time was 26.1 (range: 0–264) months, with values of 21.4 and 29.2 months in the first and second periods, respectively. Of the 144 ICC patients, the median survival time (MST) and 1/3/5 year survival rates were 30.9 months and 78/48/38 %, respectively, whereas the median DFS time and 1/3/5 year survival rates were 13.8 months and 53/28/24 %, respectively. Both OS and DFS were significantly better in the second-period group than in the first-period group (OS: MST, 21.4 and 57.7 months, p < 0.001, DFS: MST, 11.5 vs. 16.6 months, p = 0.022, respectively; Fig. 1).
Although a total cohort analysis revealed the achievement of significantly better OS and DFS in the second-period group, these results were influenced by patient selection bias. Accordingly, we conducted a multivariate analysis and observed that the second-time period was independently associated with better OS and DFS (OS: hazard ratio [HR] = 0.49, 95 % confidence interval [CI]: 0.31–0.78, p = 0.0018; DFS: HR = 0.66, 95 % CI: 0.43–0.98, p = 0.042; Table 3). These results suggest that ICC patients who underwent surgery during the second period achieved better survival outcomes, regardless of disease progression.
To assess whether these treatment improvements provided survival benefits within specific subgroups, patients were further stratified according to significant prognostic factors identified through a multivariate analysis (Table 4). Notably, patients with N1 disease, a known significant prognostic factor, achieved significantly better OS and DFS in second period (OS: MST: 12.4 and 26.0 months, p = 0.0012; DFS: MST: 4.7 and 10.7 months, p = 0.017, respectively; Fig. 2). In contrast, patients with multiple tumors did not obtain a survival benefit in second period (OS: MST: 12.8 and 16.6 months, p = 0.43, DFS: MST: 4.7 and 4.7 months, p = 0.88, respectively). Patients with an R1 resection status achieved a longer OS and DFS, although these differences were only marginally significant (OS: MST: 8.1 and 25.4 months, p = 0.075; DFS: MST: 3.3 and 10.7 months, p = 0.062).
Treatment and survival of recurrent patients
During follow-up, 94 (72 %) of the 130 patients remaining alive postoperatively (excluding 6 who died from other diseases without recurrence) developed recurrences, including 50 patients before 2006 (82 %) and 44 patients after 2006 (59 %, p = 0.0034). First- and second-period patients with N1 disease, multiple tumors, and R1 resection had comparable recurrence rates [N1: n = 20 (95 %) and n = 14 (82 %), p = 0.20; multiple tumor: n = 15 (100 %) and n = 13 (93 %), p = 0.29; R1 resection: n = 10 (100 %) vs. n = 11 (92 %), p = 0.35, respectively]. Table 5 presents initial recurrence patterns and treatments. Twenty-seven (29 %) patients developed intrahepatic recurrences [first, n = 13 (26 %) and second, n = 14 (32 %)]; 36 (38 %) had extrahepatic recurrences [n = 19 (36 %) and n = 17 (39 %), respectively]; and 31 (33 %) had both types [n = 18 (32 %) and n = 13 (30 %), respectively]. The groups did not differ significantly regarding the initial recurrence site (p = 0.75).
Multimodal treatment, including surgery for selected patients, was performed continuously, regardless of time period. More second-period patients underwent gemcitabine-based chemotherapy [n = 37 (84 %)], and only 3 received best supportive care. The median time to recurrence was 9.6 (range: 0.4–173.3) months [first, 8.8 (0.4–173.3) months and second, 9.1 (0.8–69.3) months; p = 0.71]. SAR was significantly better in the second period than in the first period (MST, 8.1 vs. 22.3 months, p = 0.0036; Fig. 3).
Discussion
The present study demonstrates improvements in long-term prognosis for patients with ICC who underwent hepatectomy during the last decade. Significantly better OS and DFS were achieved during the second period versus the first period. Several potential factors might have influenced this long-term outcome, including (1) patient selection, (2) adjuvant chemotherapy, and (3) combined multimodal treatment and developed systemic chemotherapy for recurrent patients.
Both OS and DFS improved significantly over time, possibly because of intergroup differences in background characteristics (e.g., tumor characteristics). Although the total patient analysis was influenced by patient selection bias, we demonstrated the significance of treatment improvements through a multivariate analysis; specifically, adjuvant chemotherapy and multimodal treatment combined with developed systemic chemotherapy might have conferred survival benefits upon recurrent patients. A recent large nationwide study demonstrated the significance of adjuvant chemotherapy for patients with ICC [20, 21] and suggested that surgery with adjuvant chemotherapy is a promising treatment strategy, which is consistent with our data. Furthermore, a significantly better SAR might have influenced OS, given the high recurrence rate of this disease. The introduction of gemcitabine-based chemotherapy improved the prognosis of unresectable biliary tract cancer [12, 13], and a recent study reported the significance of aggressive multimodal treatment for recurrent patients [16]. Together with improved systemic chemotherapy, more effective multimodal treatments might contribute to improvements in SAR and OS.
The poor post-operative prognosis of patients with ICC is likely multifactorial and might associate with the fact that many patients present with advanced disease. We have demonstrated significant improvements in OS and DFS for patients with N1 disease, which is considered the poorest [3–6] prognostic factor. Our treatment strategy, which includes routine lymphadenectomy, adjuvant chemotherapy, and multimodal treatment for recurrent patients, significantly affected the outcomes of patients with N1 disease. Preoperative predictions of the nodal status could promote the effectiveness of further therapies such as neoadjuvant therapy. In contrast, patients with multiple tumors and poor differentiation exhibited no improvements. In particular, multiple tumors were independently associated with early death and recurrence. Multiple tumors might therefore be a more accurate morphological indicator of biological tumor behavior and independent survival factor, as reported previously [7, 22, 23]. Regarding presence, the prognostic weight of multiple tumors was not large in the AJCC/UICC, seventh edition [17]. Our results suggest that new strategies or treatment options should be considered for patients with multiple tumors.
The present study has several limitations, including the retrospective design and consequent analytical limitations. Additionally, patient selection bias existed between the consecutive time frames; notably, multivariate and subgroup analyses of patients revealed better survival among those in the second group, especially those with N1 disease or vascular invasion. We cannot definitively conclude the specific reasons for this improvement. Undoubtedly, patient selection and improved treatment options were the largest contributors to positive outcomes not only among overall patients, but also among patients with N1 disease.
In conclusion, this present study demonstrates significant improvements in the prognosis of ICC. Particularly, the prognosis of patients with N1 disease improved significantly. However, the presence of multiple tumors remains a poor prognostic factor after surgery, and efforts to overcome this factor will be addressed in future.
References
Aljiffry M, Abdulelah A, Walsh M et al (2009) Evidence-based approach to cholangiocarcinoma: a systematic review of the current literature. J Am Coll Surg 208:134–147
Ikai I, Arii S, Okazaki M et al (2007) Report of the 17th nationwide follow-up survey of primary liver cancer in Japan. Hepatol Res 37:676–691
Bridgewater John, Peter R, Shahid A et al (2014) Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 60:1268–1289
Ribero D, Pinna AD, Guglielmi A et al (2012) Italian Intrahepatic Cholangiocarcinoma Study Group. Surgical approach for long-term survival of patients with intrahepatic cholangiocarcinoma: a multi-institutional analysis of 434 patients. Arch Surg 147:1107–1113
Farges O, Fuks D, Boleslawski E et al (2011) Influence of surgical margins on outcome in patients with intrahepatic cholangiocarcinoma: a multicenter study by the AFC-IHCC-2009 study group. Ann Surg 254:824–830
de Jong MC, Nathan H, Sotiropoulos GC et al (2011) Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol 29:3140–3145
JiangW Zeng ZC, Tang ZY et al (2011) A prognostic scoring system based on clinical features of intrahepatic cholangiocarcinoma: the Fudan score. Ann Oncol 22:1644–1652
Hyder O, Hatzaras I, Sotiropoulos GC et al (2013) Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery 153:811–818
Sulpice L, Rayar M, Boucher E et al (2012) Treatment of recurrent intrahepatic cholangiocarcinoma. Br J Surg 99:1711–1717
Seo S, Hatano E, Higashi T et al (2008) Fluorine-18 fluorodeoxyglucose positron emission tomography predicts lymph node metastasis, P-glycoprotein expression, and recurrence after resection in mass-forming intrahepatic cholangiocarcinoma. Surgery 143:769–777
Yamanaka K, Hatano E, Kanai M et al (2014) A single-center analysis of the survival benefits of adjuvant gemcitabine chemotherapy for biliary tract cancer. Int J Clin Oncol 19:485–489
Eckel F, Schmid RM (2007) Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer 96:896–902
Valle J, Wasan H, Palmer DH et al (2010) Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362:1273–1281
Valle JW, Furuse J, Jitlal M et al (2014) Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann Oncol 25:391–398
Endo I, Gonen M, Yopp AC et al (2008) Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg 248:84–96
Ercolani G, Vetrone G, Grazi GL et al (2010) Intrahepatic cholangiocarcinoma: primary liver resection and aggressive multimodal treatment of recurrence significantly prolong survival. Ann Surg 252:107–114
Edge SB, Compton CC (2010) The American Joint Committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17:1471–1474
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 40:205–213
Strasberg SM, Phillips C (2013) Use and dissemination of the Brisbane 2000 nomenclature of liver anatomy and resections. Ann Surg 257:377–382
Miura JT, Johnston FM, Tsai S et al (2015) Chemotherapy for surgically resected intrahepatic cholangiocarcinoma. Ann Surg Oncol 22:3716–3723
Sur MD, In H, Sharpe SM et al (2015) Defining the benefit of adjuvant therapy following resection for intrahepatic cholangiocarcinoma. Ann Surg Oncol 22:2209–2217
Mavros MN, Economopoulos KP, Alexiou VG (2014) Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg 149:565–574
Nathan H, Aloia TA, Vauthey JN et al (2009) A proposed staging system for intrahepatic cholangiocarcinoma. Ann Surg Oncol 16:14–22
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Yoh, T., Hatano, E., Nishio, T. et al. Significant Improvement in Outcomes of Patients with Intrahepatic Cholangiocarcinoma after Surgery. World J Surg 40, 2229–2236 (2016). https://doi.org/10.1007/s00268-016-3583-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-016-3583-1