Abstract
Introduction
Systemic chemotherapy is able to convert colorectal liver metastases (CRLM) that are initially unsuitable for local treatment into locally treatable disease. Surgical resection further improves survival in these patients. Our aim was to evaluate disease-free survival (DFS), overall survival, and morbidity for patients with CRLM treated with RFA following effective downstaging by chemotherapy, and to identify factors associated with recurrence and survival.
Materials and methods
Included patients had liver-dominant CRLM initially unsuitable for local treatment but eligible for RFA or RFA with resection after downstaging by systemic chemotherapy. Chemotherapeutic regimens consisted predominantly of CapOx, with or without bevacizumab. Follow-up was conducted with PET-CT or thoraco-pelvic CT.
Results
Fifty-one patients had a total of 325 CRLM (median = 7). Following chemotherapy, 183 lesions were still visible on CT (median = 3). Twenty-six patients were treated with RFA combined with resection. During surgery, 309 CRLM were retrieved on intraoperative ultrasound (median = 5). Median survival was 49 months and was associated with extrahepatic disease at time of presentation and recurrences after treatment. Estimated cumulative survival at 1, 3 and 4 years was 90, 63 and 45 %, respectively. Median DFS was 6 months. Twelve patients remained free of recurrence after a mean follow-up of 32.6 months.
Conclusion
RFA of CRLM after conversion chemotherapy provides potential local control and a good overall survival. To prevent undertreatment, the involvement of a multidisciplinary team in follow-up imaging and assessment of local treatment possibilities after palliative chemotherapy for liver-dominant CRLM should always be considered.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The prognosis of patients with colorectal liver metastases (CRLM) has improved significantly in recent years. As recently as 1976, 5-year survival for multiple metastases was zero [1], whereas studies now report survival rates of 30–50 % [2, 3]. Although curative surgical resection is the gold standard, 70–80 % of patients are excluded as candidates due to site, size or location of their metastases.
The local ablative therapies that have emerged over recent decades, the most important of which is currently radiofrequency ablation (RFA), have led to a considerable broadening of curative and palliative treatment options. RFA was introduced for CRLM unsuitable for hepatic resection in the mid-1990s and was regarded as a treatment option with palliative intent for patients that failed to respond to systemic therapy. With greater experience and more refined techniques, results have improved significantly and RFA now offers improved survival with the possibility of cure in selected patients [4–7].
Patients who are not candidates for local CRLM therapy commonly rely on systemic chemotherapeutics to slow disease progression. Modern chemotherapeutics are effective and can prolong survival, achieving a median survival of 20–30 months without additional local treatment [8]. Chemotherapy is still considered a palliative treatment since reports of complete tumour clearance are scarce [9]. However, chemotherapy can downstage primarily irresectable CRLM in selected patients, allowing curative hepatic resection to be performed. This so-called ‘conversion chemotherapy’ enables hepatic resection in 10–20 % of the patients with previously irresectable disease, and reported survival rates are similar to the patients with primarily resectable tumours [10–12].
Patients with CRLM who are initially unsuitable for any local treatment, and thus receive palliative chemotherapy, can also become candidates for RFA after downstaging of the tumour load. Although literature on this subject is scarce [13, 14], the rationale behind this use of RFA is complete tumour eradication and improved survival compared to chemotherapy alone. The aim of the present study was to evaluate disease-free survival (DFS), overall survival, and morbidity in patients treated with RFA after conversion chemotherapy, and to identify factors associated with improved survival.
Materials and methods
Patients
All patients with CRLM treated with RFA, or RFA combined with resection between January 2006 and July 2013 at a university hospital and a large teaching hospital, were recorded in a database. This retrospective study included all patients with liver-dominant CRLM who were initially ineligible for local treatment but became candidates for RFA alone or in combination with resection after their metastases were significantly downstaged by chemotherapy. Limited extrahepatic metastatic disease was not considered a contra-indication if all metastases could be locally controlled, e.g. resected, ablated or treated with stereotactic radiotherapy. The decision for initial treatment was made by our multidisciplinary liver board based on integrated FDG PET-CT and diagnostic contrast-enhanced (ce)CT or enhanced magnetic resonance imaging (MRI) including DWI-MRI. All images (pre- and post-chemotherapy) were revised to confirm that inclusion criteria were met. Lesions were considered irresectable when, after resection with a 1-cm margin, the future remnant liver should not exceed 30 %, the absence of adequate vascular in- and outflow and biliary drainage and less than two contiguous liver segments [15]. Patients were deemed unfit for initial local treatment because number, size or site would not permit complete eradication of their CRLM or a safe 1-cm ablation margin. These patients were referred for systemic chemotherapy. Chemotherapy only ceased when local treatment could be performed, on disease progression or due to unacceptable toxicity. Treatment response was evaluated every 3–6 cycles using FDG PET-CT and a ceCT of the liver. Effective downstaging was defined as the decrease of tumour load in the liver, in number and/or in size of the lesions that allowed complete tumour clearance by RFA, or RFA combined with resection [13, 14]. Patients with persistent liver-dominant disease were re-evaluated for local treatment by our multidisciplinary liver board. The decision to perform local treatment after downstaging of the metastases was only made when the overall strategy could potentially achieve complete treatment of the tumour load. Maximum time between the last cycle of chemotherapy and imaging was 4 weeks and surgery followed within 6 weeks after. Patients were not included when chemotherapy was only indicated to treat additional extrahepatic disease.
The definite decision to perform RFA rather than resection was made during surgery and was aided by intraoperative ultrasound (IOUS), palpation and inspection.
Chemotherapy
Patients followed a variety of chemotherapeutic regimes initiated at their local hospital. Capecitabine and oxaliplatin (CapOx) were given in 3-week cycles, usually 6–8 cycles in total; intravenous oxaliplatin 130 mg/m2 (day 1) followed by oral capecitabine 1000 mg/m2 twice daily (day 1, evening, to day 15, morning), followed by a week’s rest before starting the next cycle.
When bevacizumab was added to this regime, 7.5 mg/kg was administered on the first day of every cycle.
FOLFOX was administered as follows: oxaliplatin, administered as a 85 mg/m2 intravenous infusion over 2 h on day 1, concomitantly with leucovorin (LV) as a 400 mg/m2 infusion over 2 h, followed by 5-FU, given as a 400 mg/m2 bolus injection, and then as a 2400 mg/m2 continuous infusion over 46 h. Cycle length was 2 weeks and consisted of approximately 49 h of infusion and 12 days of rest.
RFA
An open approach was chosen as an initial treatment [16–18]. Based upon size and site of the lesion, 2.0–5.0 cm expandable-needle electrodes (LeVeen, Boston Scientific, USA) were placed using ultrasound. Ablation was performed using a commercially available generator (RF3000, Boston Scientific, USA) and tract ablation was performed routinely. A successful ablation was defined as an ablation zone including a 1-cm tumour-free margin as seen on IOUS. Tumours exceeding 5 cm were treated with multiple repositions of the single RFA needle for one or more overlapping ablations.
Follow-up
Perioperative morbidity and mortality were defined as complications and death occurring within 90 days after treatment. Complications were graded according to the Clavien Dindo classification of surgical complications. Patients were subsequently followed-up at regular intervals of 3–6 months using full-body FDG PET-CT or thoraco-pelvine ceCT. A local site recurrence was defined as an area on PET-CT of focally increased FDG uptake in or within 1 cm of the treated lesion, or growth of a lesion on two consecutive ceCT scans. A new lesion was defined as recurrence anywhere in the liver 1 cm outside of the ablation zone. In case of intra- or extrahepatic recurrence, renewed (local) treatment options were always considered.
Statistical analyses
Quantitative and qualitative variables are described as the mean, median and frequencies. Categorical variables were compared using the Chi-square or Fisher’s exact test and continuous variables were compared using the Student’s t test and a p value < 0.05 was considered significant. Overall survival and DFS were estimated using the Kaplan–Meier method with the log-rank test and a Cox regression analysis to determine differences between groups. A confidence interval of 95 % was used for each analysis, and all calculations were performed using SPSS ver. 20 (SPSS Inc., Chicago, IL, USA).
Results
A total of 56 patients received induction chemotherapy and subsequent laparotomy with the intention for local treatment using RFA. However, IOUS revealed additional lesions in 5 patients (8.9 %) who were too extensive for local treatment. These patients were excluded from further analyses.
The studied population therefore consisted of 51 patients, of whom 30 were male (59 %) with a mean age of 60 years (range 35–75 years). Baseline characteristics are presented in Table 1.
Chemotherapy
More than half of patients (30 patients; 59 %) received CapOx combined with bevacizumab. Twelve patients were treated with CapOx, but switched to capecitabine mono-therapy in two patients due to toxicity. The remaining patients received FOLFOX (n = 6) or capecitabine mono-therapy (n = 3). The number of therapeutic cycles ranged from 3 to 18 (mean 7.12, SD 3.2). Nineteen patients received chemotherapy only before RFA; two patients continued chemotherapy after RFA, and in 30 patients chemotherapy was resumed following diagnosis of recurrent disease. None of the patients who remained free of disease received chemotherapy following RFA.
Imaging before and after chemotherapy
All patients underwent ceCT and PET-CT analyses before starting chemotherapy (Table 2). The total number of lesions on CT at time of diagnosis was 325. All showed FDG uptake on PET-CT. After chemotherapy, the total number of lesions visible on ceCT was reduced to 183.
Following chemotherapy, PET-CT was completely negative in 19 patients (43.2 %) and only 68 of the initial 325 lesions still showed FDG uptake (in 25 patients; 56.8 %). Six of the 19 (31.6 %) FDG-negative lesions proved to be >3 cm on IOUS, compared to 12 of the 25 FDG-positive lesions (42 %) (p = 0.22). Seven patients (13.7 %) were only evaluated using ceCT without PET-CT after chemotherapy.
Extrahepatic disease in 4 patients showed a complete response after chemotherapy. All other patients showed partial extrahepatic disease responses after chemotherapy and were locally treated using stereotactic body radiotherapy or resection.
Intraoperative ultrasound
The total number of lesions made visible during surgery was 309, compared to 183 on ceCT (Table 2). The mean size was 31 mm (range 9–70 mm), with 21 lesions >3 cm. In 24 patients (46 %), the number of lesions found during surgery was compatible to the number of lesions on pre-chemotherapy imaging. More lesions than expected on pre-chemotherapy CT were found in 10 patients, and in the remaining 17 patients not all lesions were retrieved on IOUS.
Follow-up
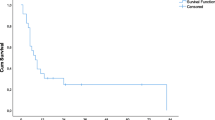
Six grade II and 3 grade III complications occurred in 9 patients after RFA and complications were more frequent in patients who underwent RFA in combination with resection (p = 0.02) (Table 3). Mean hospital stay was 8.34 days (SD 3.47). This did not differ significantly between RFA (7.7, SD 2.8) or RFA and resection (8.9 days, SD 4,0) (p = 0.24). Mean follow-up was 32.6 months (range 5–80, SD 19.3), including 12 patients who remained free of disease (23.5 %). Median DFS was 6 months (95 %CI 4–8 months) (Fig. 1). The number of patients with recurrent disease after treatment was comparable in patients treated with RFA or RFA combined with resection (p = 0.938). First recurrences had solely intrahepatic locations in 26 patients and were both intra- and extrahepatic in 13 patients. A better DFS was associated with a lower number of lesions found on IOUS compared to the number originally diagnosed on pre-chemotherapy ceCT (p = 0.03). A worse DFS was associated with patients with extrahepatic disease at time of presentation (p = 0.01). The median overall survival was 49 months. Estimated cumulative survival at 1, 3 and 4 years was 90 % (CI 82.8–97.2 %), 63 % (CI 47.7–78.3 %) and 45 % (95 % CI 26–64), respectively. Impaired overall survival was associated with extrahepatic disease at time of presentation (p < 0.01) and the presence of recurrence after initial treatment (p < 0.01). A trend towards worse survival was noted in tumours that measured >5 cm on IOUS (p = 0.06). Both extrahepatic disease at time of presentation and the presence of recurrence after initial treatment remained independent predictors of overall survival in a multivariate analysis (Table 4).
Discussion
Overall survival in this selected study population was comparable to the top-end survival rates reported after RFA or resection, despite the relatively short DFS. Factors that may have contributed to good overall survival include a good response to repeated chemotherapy and local treatment of recurrences. The number of complications was relatively high but only when RFA was combined with surgical resection, which accords with the relatively high complication rate suggested in literature reports on surgical resection after conversion chemotherapy [19]. Despite treated, extrahepatic disease was associated with decreased overall survival and disease-free survival. Despite the impressive survival improvement in patients treated with modern systemic chemotherapy, cure is still extremely rare. Complete radiologic response to chemotherapy does not predict complete remission. Other studies that investigated recurrence rates of lesions that had disappeared after chemotherapy, and were therefore not resected during surgery, found that 83 % of these lesions recurred in the first year after treatment [20]. This was confirmed by a study that showed that complete radiologic response is not correlated to complete pathologic response [9]. That a complete pathologic response is very rare and cannot be predicted by pre-operative imaging which has been shown by studies in which only 4–8 % of the CRLM resected after systemic treatment lack tumour cells in the surgical specimen [9, 21]. This inability to distinguish between complete and incomplete responses after conversion chemotherapy makes complete eradication of remnant lesions essential to achieving cure in patients. Paradoxically, our study showed that failure to retrieve all lesions on IOUS, which were therefore left in situ, led to improvements in DFS. However, this did not translate to a better overall survival. This outcome supports the theory that not all tumour cells are eradicated by chemotherapy, but that some metastases show a better response and therefore take longer to recur.
RFA is now a valuable local treatment option for patients with tumours ineligible for surgical resection. Although unsupported by a RCT, numerous studies have shown that RFA can result in complete tumour clearance and an increased life-expectancy, with 5-year survival rates of 30–43 %. RFA also appears to be superior to chemotherapy alone [4, 6, 22]. RFA combined with resection in bilateral disease is associated with improved overall survival compared to resection alone [23]. Ruers et al. compared RFA combined with chemotherapy and chemotherapy alone in an EORTC study (CLOCC trial). They found that the combination with RFA resulted in a significantly prolonged DFS [24] and, recently, they were the first to even show an significant improved OS (median 45.6 vs 40.5 months, p = 0.01) [7]. This is the first study that prospectively compares the role of RFA in patients with CRLM. Although our results are comparable, our patient population differ. Patients in the CLOCC trial were primarily amenable for RFA without the need for downstaging the tumour load using conversion chemotherapy. Evrard et al. described a population of patients that received RFA combined with surgical resection for their irresectable CRLM [25]. Although all patients were treated with chemotherapy prior to local hepatic treatment, a positive response to chemotherapy was not required and even patients with progression during chemotherapy were treated. This suggests that at least some lesions were amenable to local treatment prior to the start of systemic treatment. Studies that describe the effect of RFA after conversion chemotherapy remain scarce; similarly to resection after chemotherapy, they report improvements in overall survival [13, 14].
However, as shown by the relatively limited median DFS, a risk of incomplete ablation remains. In contrast, the median overall survival in this study is comparable to the top end of studies reporting on patients primarily candidates for RFA [26]. This discrepancy can be explained by two mechanisms. Firstly, the opportunity to ablate local recurrences in the liver is known to be able to achieve complete tumour eradication and therefore prolonged survival [4]. Secondly, modern chemotherapeutic regimens have changed considerably over recent years and the addition of bevacizumab in particular is believed to be responsible for an improved pathological response of CRLM compared to oxaliplatin-based chemotherapy [27]. A recent study was not able to show improved DFS or OS after resection of bevacizumab-treated CRLM [28].
A good radiological response of the lesion to chemotherapy may be a preferred outcome for the oncologist and the patient, but it can present a problem during surgery [29]. Complete radiological resolution of the target lesions makes it hard to plan liver surgery. As over 80 % of these lesions will recur during follow-up, local treatment remains obligatory when pursuing cure [20]. Imaging half-way through the chemotherapeutic regime may indicate response to systemic therapy and local treatment can be initiated in time before complete disappearance on imaging. IOUS is essential due to its ability to identify 20–40 % of additional lesions after chemotherapy compared to any other pre-operative imaging modality [16, 17]. In this study, very few patients were evaluated with DW-MRI, although the authors acknowledge the increasing role of DW-MRI after chemotherapy.
Several limitations, some inherent to local ablative treatment, also played a role in this study. At first, the median follow-up of 32.6 months prevents us from drawing any definitive long-term conclusions. All patients included in the study were eventually deemed eligible for local treatment using RFA. Patients who never became candidates were excluded. Since tumour shrinkage was a condition for later local treatment, the tumour biology in these patients is likely to differ and thus introduce a selection bias. An additional bias may have resulted from selection of patients for this study with intrinsically better median survival prospects compared to patients in other studies evaluating the effect of chemotherapy. To improve comparability, all images were reevaluated after inclusion to confirm patient suitability for local treatment. The lack of a uniform definition of unsuitability for local ablative treatment is a limitation in the evaluation of our results. In the past, contra-indications for resection of CRLM were clear and included > 3 metastases, presence of extrahepatic disease or when a tumour-free margin of 1 cm could not be achieved [30]. It has become clear that patients who do not meet the original inclusion criteria derive benefit from surgical resection [31]. New inclusion criteria to define resectability state that the future remnant liver should exceed 25 % of a healthy liver, and 30 % after systemic chemotherapy with adequate vascular in- and outflow and biliary drainage and two contiguous liver segments [32]. The influence of time to progression, age and localisation is still a subject of considerable debate. To the best of our knowledge, no such proposal exists for ablative therapies. As a consequence, inclusion and exclusion criteria for treatment rely on personal experience and interpretation of the literature by a multidisciplinary liver team. Without clear definitions, the exact role of chemotherapy in the treatment effect will remain hard to determine.
This study reinforces the need to evaluate all patient responses to chemotherapy in case of liver-dominant disease, even when the initial stage precluded any prospect of cure. The field of CRLM treatment is rapidly evolving and new surgical techniques such as two-staged hepatectomy and ablative techniques like RFA, MWA and recently irreversible electroporation have extended the therapeutic possibilities. The need for specialization and centralization is therefore only increasing and non-specialist decision making may lead to undertreatment [33].
References
Wilson SM, Adson MA (1976) Surgical treatment of hepatic metastases from colorectal cancers. Arch Surg 111(4):330–334
Pawlik TM, Scoggins CR, Zorzi D et al (2005) Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg 241(5):715–722 discussion
Abdalla EK, Vauthey JN, Ellis LM et al (2004) Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 239(6):818–825
Nielsen K, van Tilborg A, Meijerink M et al (2013) Incidence and treatment of local site recurrences following RFA of colorectal liver metastases. World J Surg 37(6):1340–1347. doi:10.1007/s00268-013-1997-6
Van Tilborg AAJM, Meijerink MR, Sietses C et al (1002) Long-term results of radiofrequency ablation for unresectable colorectal liver metastases: a potentially curative intervention. Br J Radiol 2011(84):556–565
Wong SL, Mangu PB, Choti MA et al (2010) American Society of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol 28(3):493–508
Ruers T, Punt C, Coevorden van F et al (2015) Radiofrequency ablation (RFA) combined with chemotherapy for unresectable colorectal liver metastases (CRC LM): Longterm survival results of a randomized phase II study of the EORTC-NCRI CCSG-ALM Intergroup 40004 (CLOCC). J Clin Oncol 33(suppl; abstr 3501)
Loupakis F, Cremolini C, Masi G et al (2014) Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med 371(17):1609–1618
Adam R, Wicherts DA, de Haas RJ et al (2008) Complete pathologic response after preoperative chemotherapy for colorectal liver metastases: myth or reality? J Clin Oncol 26(10):1635–1641
Adam R, Delvart V, Pascal G et al (2004) Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg 240(4):644–657
Masi G, Cupini S, Marcucci L et al (2006) Treatment with 5-fluorouracil/folinic acid, oxaliplatin, and irinotecan enables surgical resection of metastases in patients with initially unresectable metastatic colorectal cancer. Ann Surg Oncol 13(1):58–65
Adam R, Wicherts DA, de Haas RJ et al (2009) Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J Clin Oncol 27(11):1829–1835
Mima K, Beppu T, Chikamoto A et al (2012) Hepatic resection combined with radiofrequency ablation for initially unresectable colorectal liver metastases after effective chemotherapy is a safe procedure with a low incidence of local recurrence. Int J Clin Oncol 18(5):847–855
Knudsen AR, Kannerup AS, Mortensen FV et al (2009) Radiofrequency ablation of colorectal liver metastases downstaged by chemotherapy. Acta Radiol 50(7):716–721
Clavien PA, Petrowsky H, DeOliveira ML et al (2007) Strategies for safer liver surgery and partial liver transplantation. N Engl J Med 356(15):1545–1559
Spatz J, Holl G, Sciuk J et al (2011) Neoadjuvant chemotherapy affects staging of colorectal liver metastasis—a comparison of PET, CT and intraoperative ultrasound. Int J Colorectal Dis 26(2):165–171
Sietses C, Meijerink MR, Meijer S et al (2010) The impact of intraoperative ultrasonography on the surgical treatment of patients with colorectal liver metastases. Surg Endosc 24(8):1917–1922
Eisele RM, Neumann U, Neuhaus P et al (2009) Open surgical is superior to percutaneous access for radiofrequency ablation of hepatic metastases. World J Surg 33(4):804–811
Lam VW, Spiro C, Laurence JM et al (2012) A systematic review of clinical response and survival outcomes of downsizing systemic chemotherapy and rescue liver surgery in patients with initially unresectable colorectal liver metastases. Ann Surg Oncol 19(4):1292–1301
Benoist S, Brouquet A, Penna C et al (2006) Complete response of colorectal liver metastases after chemotherapy: does it mean cure? J Clin Oncol 24(24):3939–3945
Rubbia-Brandt L, Giostra E, Brezault C et al (2007) Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol 18(2):299–304
Govaert K, van Kessel C, Lolkema M et al (2012) Does radiofrequency ablation add to chemotherapy for unresectable liver metastases? Curr Colorectal Cancer Rep 8(2):130–137
Karanicolas P, Jarnagin W, Gonen M et al (2013) Long-term outcomes following tumor ablation for treatment of bilateral colorectal liver metastases. JAMA Surg 148(7):597–601
Ruers T, Punt C, Van Coevorden F et al (2012) Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup phase II study (EORTC 40004). Ann Oncol 23(10):2619–2626
Evrard S, Rivoire M, Arnaud J et al (2012) Unresectable colorectal cancer liver metastases treated by intraoperative radiofrequency ablation with or without resection. Br J Surg 99(4):558–565
Solbiati L, Ahmed M, Cova L et al (2012) Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology 265(3):958–968
Ribero D, Wang H, Donadon M et al (2007) Bevacizumab improves pathologic response and protects against hepatic injury in patients treated with oxaliplatin-based chemotherapy for colorectal liver metastases. Cancer 110(12):2761–2767
Rong Z, Martel G, Vandenbroucke-Menu F et al (2014) Impact of peri-operative bevacizumab on survival in patients with resected colorectal liver metastases: an analysis of the LiverMetSurvey. HPB (Oxford) 16(4):342–349
De Bruyne S, Van Damme N, Smeets P et al (2012) Value of DCE-MRI and FDG-PET/CT in the prediction of response to preoperative chemotherapy with bevacizumab for colorectal liver metastases. Br J Cancer 106(12):1926–1933
Pawlik TM, Schulick RD, Choti MA (2008) Expanding criteria for resectability of colorectal liver metastases. Oncologist 13(1):51–64
Smith MD, McCall JL (2009) Systematic review of tumour number and outcome after radical treatment of colorectal liver metastases. Br J Surg 96(10):1101–1113
Adams RB, Aloia TA, Loyer E et al (2013) Selection for hepatic resection of colorectal liver metastases: expert consensus statement. HPB (Oxford) 15(2):91–103
Jones RP, Vauthey JN, Adam R et al (2012) Effect of specialist decision-making on treatment strategies for colorectal liver metastases. Br J Surg 99(9):1263–1269
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Nielsen, K., Scheffer, H.J., Volders, J.H. et al. Radiofrequency Ablation to Improve Survival After Conversion Chemotherapy for Colorectal Liver Metastases. World J Surg 40, 1951–1958 (2016). https://doi.org/10.1007/s00268-016-3554-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-016-3554-6