Abstract
Background and objectives
Hepatic resection is established as the treatment for HCC. However, patients sometimes experience early recurrence of HCC (ER HCC) after curative resection.
Methods
A retrospective analysis was conducted for 193 patients with single HCC who underwent curative liver resection in our medical center between April 2000 and March 2013. We divided the cohort into two groups; early recurrence group (ER G) which experienced recurrence within 6 months after resection, and non-early recurrence group (NER G). Risk factors for ER HCC were analyzed.
Results
Thirty-nine out of 193 (20.2 %) patients had ER HCC. Univariate analysis showed Glasgow prognostic score (GPS, p = 0.036), neutrophil to lymphocyte ratio (NLR, p = 0.001), level of PIVKA-II (p = 0.0001), level of AFP (p = 0.0001), amounts of blood loss (p = 0.001), operating time (p = 0.002), tumor size (p = 0.0001), stage III and IV (p = 0.0001), and microvascular invasions (portal vein: p = 0.0001 and hepatic vein: p = 0.001) to be associated with ER HCC. By multivariate analysis, there were significant differences in high NLR (p = 0.029) and high AFP (p = 0.0001) in patients with ER HCC.
Conclusions
Preoperative high AFP (more than 250 ng/ml) and high NLR (more than 1.829) were independent risk factors for ER HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the second leading cause of cancer-related deaths worldwide, and is the fourth leading cancer deaths in Japan [1]. During the past 20 years, HCC-related cancer death was experiencing steady decrease, and survival rate improved about 4 times (9.5–39.5 %), due to improvement of survival rate of resection cases (54.2 %). The best management of HCC is to make an early diagnosis, and perform radical therapy [2]. Surgical resection is the first-line therapeutic option for HCC [1, 2]. Overall survival after resection of HCC has improved in recent years; however, the recurrence rate still remains high, and early recurrence (ER) of HCC is still a controversial issue regarding HCC patients who underwent resection [3]. Risk factors for postoperative recurrence of HCC are classified into 3 categories: (1) tumor factors such as tumor size and numbers (2) host factors such as the presence of cirrhosis and hepatitis viral load, and (3) surgical and pathological factors such as surgical margin and microvascular invasions [4, 5]. Prominent risk factors for ER HCC are HBV DNA and HBs Ag levels, gender and level of tumor markers in previous reports [6–8].
In general, early recurrence was defined as recurrence within one year, and previous reports had analyzed some risk factors. However, sometimes we encountered earlier recurrent cases, such as within 6 month, and thus perhaps need to rethink the timing of curative resection. The risk factors about ER HCC within 6 months were also never described in past studies. In this retrospective study, we evaluated and revealed risk factors for ER HCC within 6 months, using preoperative factors.
Recent years, some reports revealed that various inflammation-based prognostic scores, such as Glasgow prognostic score (GPS) and neutrophil to lymphocyte ratio (NLR), have been associated with overall and disease-free survival in patients with several types of cancer including HCC [9–15]. However, it is still too early to evaluate the association of such factors to ER HCC patients’ survival.
In this retrospective study, we evaluated and revealed risk factors for ER HCC, within 6 months, using preoperative and pathological factors.
Patients and methods
ER study population
Data of patients with single lesion HCC, who underwent initial curative resection of the liver at the Second Department of Surgery Dokkyo Medical University between April 2000 and February 2013, were retrieved from a collected database and analyzed retrospectively. Curative resection (R0 resection) was defined as that leaving behind no gross or microscopic tumor on the cut surface and in the remnant liver. Clinical and pathologic staging was performed according to the general rules for the clinical and pathological study of primary liver cancer (3rd edition).
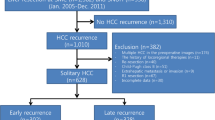
During the study period, 518 cases received initial liver resection at our department, and 353 of 518 cases had single lesion HCC. One hundred ninety-three out of 353 patients had recurrence of HCC. For this retrospective analysis, we selected and evaluated the 193 patients with recurrence of single lesion HCC. Those patients never received any adjuvant therapies after liver resection (Fig. 1). We divided them into two groups based on whether the patients experienced early recurrence (less than 6 months after surgery, ER G: n = 39) or no early recurrence (NER G: n = 154).
HCC was diagnosed before operation based on at least two kinds of radiological examinations such as, multi-detector row computed tomography (MDCT), magnetic resonance imaging (MRI), and abdominal ultrasound (US). If HCC was more than 5 cm in diameter, we implemented bone scintigraphy or fluorodeoxy glucose-positron emission tomography (FDG-PET), and evaluated extrahepatic metastasis. The indication for liver resection was determined based on each patients liver functional reserve, mainly assessed by Makuuchi criteria, which comprise preoperative measurements of ascites, serum bilirubin level, and indocyanine green retention rate at 15 min (ICG R15) after administration.
All HCC were confirmed histolopathologically after resection, and combined CCC and other kinds of malignancies were excluded in this series. The histologic grade of tumor differentiation was assigned by the Edmondson grading system. Clinicopathologic factors that potentially were related to survival and recurrence were selected in this study, including age, gender, hepatitis, tumor markers (serum alpha-fetoprotein: AFP and protein induced by vitamin K absence or antagonist-II: PIVKA-II), liver cirrhosis, ICG R15, hyaluronic acid (HA), type IV collagen (T4 C), type of liver resection (anatomical resection: AR or limited resection: LR), neutrophil to lymphocyte ratio (NLR), Glasgow prognostic score (GPS), operative time, amount of blood loss, pringle time, tumor size, tumor differentiation, pathological stage and pathological microvascular invasion.
Operative procedure
We confirmed the absence or presence of intrahepatic metastasis by US, and the location of HCC at laparotomy.
Accurate resection area in accordance to Couinaud classification was determined with a staining techniques an intraoperative ultrasound [16]. In sub or segmentectomy, under ultrasonographic guidance, about 5-ml indigo carmine dye (indigocarmine injection 20 mg/5 ml; Daiichi Sankyo, Tokyo, Japan) was injected into a branch of feeding portal vein. Additional branches were punctured, if necessary, depending on the location of the HCC. In a LR, we dissected liver along a line so as to secure surgical margin of at least 2 cm, when possible. Final decision to perform LR in operating room was based on factors such as tumor location by ultrasound, liver condition (with or without cirrhosis), and technical difficulties for AR. When AR was impossible, liver parenchymal transection was performed so as not to expose the tumor surface with at least 5 mm margin. We divided the liver parenchyma by the clamp-crushing method or an ultrasonic dissector. For control of bleeding during transection of parenchyma, we used the intermitted Prinlge’s maneuver (15 or 20 min clamp and 5 min release) in this series. After resection, abdominal drains were inserted in the liver cut surface, and a chest drain was inserted in the right pleural space. Drainage tubes were removed when there was no visible bile leakage, the fluid bilirubin level was less than 2.0 mg/dl, amount of fluid collection was less than 100 ml/day, and bacteriological culture was negative from day 7 after operation.
Follow-up
After discharge, all patients were followed from initial liver resection until either death or recurrence. We evaluated recurrence as follows: monthly monitoring using measurements of AFP and PIVKA-II, and every 3 months by dynamic computed tomography or ultrasonography. Follow-up period ranged from to one to 12 years after surgery in this study. For those patients who had recurrence of 1–3 lesions HCC, we performed repeat LR, while we selected transcatheter arterial chemoembolization for cases with more than four lesions.
The GPS was estimated as described previously. Briefly, patients with both an increased CRP level (>1.0 mg/dL) and hypoalbuminemia (<3.5 g/dL) were allocated a score of 2, patients with only one of these biochemical abnormalities were allocated a score of 1, and patients with neither of these abnormalities were allocated a score of 0 [9]. The NLR was defined as the absolute neutrophil count divided by the absolute lymphocyte count [10].
These two groups were mainly compared with each clinico-pathological characteristic in both groups.
Statistics
Statistical analyses were performed with the SPSS statistical software package (version 13.0; SPSS Inc, Chicago, IL). Receiver operating characteristic (ROC) curve analysis was used to define the ideal cut-off values of laboratory parameters (such as, NLR, AFP, HA, T4 C, amount of bleeding operating time and tumor size). The recommended cut-off value for each parameter was determined as the most prominent points on the ROC curve for sensitivity and specificity, respectively. Survival analysis was performed using Kaplan–Meier analysis and the log rank test to compare patient survival. Univariate and multivariate analyses were performed to clarify the laboratory parameter most significantly associated with ER HCC, and also were used to assess risk factors to predict the ER HCC after resection. Odds ratios with 95 % CI were calculated using logistic regression model analyses. P values of less than 0.05 were considered to be statistically significant.
Results
Characteristic of ER HCC
During the study period, all of 193 patients received pathological margin negative resection. Thirty-nine of 193 patients (20.2 %) were ER G, 23 patients had intrahepatic recurrence; 18 of 23 patients had multiple and five had simple lesion recurrence. Sixteen of 39 patients had extrahepatic recurrence; 9 had lung, four had bone, and remaining three patients had brain dissemination, and lymph node recurrence, respectively.
Cancer-specific patients survival (CSS) comparison
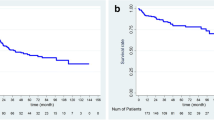
The 1-, 3-, and 5-year CSS rates were 74.3, 13.2, and 0 percent, respectively, for ER G, and 99.3, 88.9, and 82.3 per cent for NER G. There was difference in CSS between the two groups (p = 0.0001) (Fig. 2).
Univariate analysis
Clinical data
There were no significant differences in the median age, gender, and positive hepatitis C or B between the two groups. GPS score 2 (p = 0.036, OR = 3.13, 95 % CI 1.07–10.2), high of NLR (more than 1.829, p = 0.001, OR = 3.58, 95 % CI 1.66–7.71), AFP (more than 250 ng/ml, p = 0.0001, OR = 9.21, 95 % CI 4.21–20.14) and PIVKA-II (more than 1240 mA U/ml, p = 0.0001, OR = 7.38, 95 % CI 3.38.13–16.1) were significant risk factors for ER of HCC (Table 1).
Surgical factors
No patients died within 30 days after surgery. There were no significant differences in surgical procedures (AR or LR) between the two groups. There were significant differences in amount of bleeding (more than 783 ml, p = 0.001, OR = 3.561, 95 % CI 1.71–7.37) and longer operating time (more than 288 min, p = 0.002, OR = 3.39, 95 % CI 1.57–7.30) between the two groups (Table 2).
Histopathological factors
Four factors were significant prognostic factors for ER HCC, such as tumor size (more than 5.45 cm, p = 0.0001, OR = 5.86, 95 % CI 2.76–12.43), advanced stage (II or IV, p = 0.0001, OR = 9.42, 95 % CI 4.19–21.18), portal vein invasion (p = 0.0001, OR = 9.84, 95 % CI 4.40–22.0) and hepatic vein invasion (p = 0.001, OR = 9.16, 95 % CI 2.59–32.3) (Table 2).
Multivariate analysis
Multivariate analysis using the six clinical and surgical characteristics (GPS, NLR, AFP, PIVKA-II, amount of bleeding, and operating time) selected above (excluding pathological factors: tumor size, portal vein and hepatic vein invasion) revealed that preoperative NLR (p = 0.029, odd ratio = 2.78, 95 % CI 1.11–6.99) and high level of AFP (more than 250 ng/ml, p = 0.0001, odd ratio = 6.93, 95 % CI 2.67–16.69) were significant prognostic factors for ER HCC (Table 3).
Discussion
In this study, 39 out of 193 patients had ER HCC after curative liver resection including intra- and extrahepatic recurrences. The risk factors of ER HCC were high level of AFP and high NLR.
Up to now, ER HCC has been described in several reports. Zhu et al showed that level of AFP (>800 ng/ml) multiple tumors and microvascular invasion were risk factors for HBV-related early ER HCC (less than 1 year) [7]. Another Chinese group, Zhou et al revealed that AFP level (> 100 ng/ml) and microscopic vascular invasion were independent factors for ER HCC (less than 1 year) in patients with single lesion (less than 3 cm) [17]. Du ZG et al also revealed that independent risk factors for ER HCC (less than 2 years) were multiplicity of tumors and venous infiltration [18]. Our study revealed that the AFP level (more than 250 ng/ml) and microvascular invasion were key factors for ER HCC. AFP is routinely measured in the diagnosis of cancer, monitoring therapeutic effectiveness, detecting recurrence, and in predicting prognosis. More than 70 % of HCC patients have a high serum concentration of AFP because of its secretion from dedifferentiated tumor. High level of AFP was associated with greater tumor size, bilobar disease, portal vein thrombosis, and high patient mortality [19]. However, no studies, as far as we know, had discussed and evaluated the cut-off value of AFP by ROC curve analysis; previous studies were using median or average, in association with the ER HCC. In our study, we used AFP cut-off level (more than 250 ng/ml), and according to the results of the multivariate analysis, discovered that it was in fact a risk factor for ER HCC.
Moreover, tumor venous invasion is an important factor for recurrence of HCC. Postoperative HCC recurrence is thought to take place in two ways, intrahepatic metastasis in the residual liver, and metachronous, multicentric hepatocarcinogenesis based on hepatitis [20]. Similar to the findings of other investigators [4, 7, 16, 17], microvascular invasion was also an independent risk factor in our analysis. This finding strongly supports the hypothesis that ER HCC may be mainly attributable to intrahepatic metastasis [20]. Furthermore, our study revealed that ER HCC is attributed to EHR. In our patients, the incidence of EHR in the lung bone and brain was high. Their intra- and extrahepatic recurrences were explained by the high incidence of portal venous invasion. Significantly high level of AFP may reflect such advanced stage of ERHCC.
In the last decade, numerous retrospective studies have revealed that the clinical utility of systemic inflammation-based prognostic systems, such as GPS, NLR, and modified GPS, was effective prognostic factors for several types of cancers [9, 12, 14, 21, 22]. It also has been confirmed that such systems are useful for predicting the postoperative survival of cancer patients, and are able to classify such patients before surgery. NLR is a useful predictor of postoperative mortality in patients with different types of cancer, easy to use, and costs are lower than that of tumor makers and biomarkers [10, 12]. From 2008, several reports revealed that the preoperative NLR level was a prognostic factor for recurrence free and overall survival after curative resection of HCC [11, 15, 23–25]. To the best of our knowledge, there is no previous study that reported on the association between prognostic factor of NLR and the patients with ER HCC after curative liver resection, and we also demonstrated that high preoperative NLR could predict the ER HCC. This result suspected that systemic inflammatory response plays critical roles and exist along the path of HCC progression, and NLR could be useful for one of the prognostications but also perioperative surveillance of the patients with ER HCC. Comparison between NLR and AFP suggested that cost of NLR is much lower, furthermore sequential follow-up using NLR would be a useful adjunct to CT, MRI, and PET scan. On the other hand, NLR value was still controversial. Previous reports evaluated using different cut-off values of NLR, which were not able to provide a consistent standard for comparison among different patients' populations [11, 15, 26]. We used the NLR as cut-off level for this study, and demonstrated that NLR (≥1.829) was a significant independent prognostic factor for ER HCC. We suggest to unify the NLR level by each cancer, especially HCC.
Finally, on the basis of our present findings, patients with ER HCC were experienced significantly lower CSS, and were comparatively severe in preoperative condition than patients without ER HCC.
Conclusion
With regard to ER HCC, because the outcome of patients with a NLR and high AFP level was much worse than that of patients without those factors. Pre- or postoperative treatments including chemotherapy or transcatheter chemoembolization should add for improving prognosis.
References
Kudo M, Izumi N, Kokudo N et al (2011) Management of hepatocellular carcinoma in Japan: consensus-based clinical practice guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis 29(3):339–364
Bruix J, Sherman M (2005) Practice Guidelines Committee AAftSoLD. Management of hepatocellular carcinoma. Hepatology 42(5):1208–1236
Lim KC, Chow PK, Allen JC et al (2012) Systematic review of outcomes of liver resection for early hepatocellular carcinoma within the Milan criteria. Br J Surg 99(12):1622–1629
Shirabe K, Kanematsu T, Matsumata T et al (1991) Factors linked to early recurrence of small hepatocellular carcinoma after hepatectomy: univariate and multivariate analyses. Hepatology 14(5):802–805
Poon RT, Fan ST, Lo CM et al (2002) Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg 235(3):373–382
Sohn W, Paik YH, Kim JM et al (2014) HBV DNA and HBsAg levels as risk predictors of early and late recurrence after curative resection of HBV-related hepatocellular carcinoma. Ann Surg Oncol 21(7):2429–2435
Zhu WJ, Huang CY, Li C et al (2013) Risk factors for early recurrence of HBV-related hepatocellular carcinoma meeting milan criteria after curative resection. Asian Pac J Cancer Prev 14(12):7101–7106
Li T, Qin LX, Gong X et al (2014) Clinical characteristics, outcome, and risk factors for early and late intrahepatic recurrence of female patients after curative resection of hepatocellular carcinoma. Surgery 156(3):651–660
Ishizuka M, Kubota K, Kita J et al (2012) Impact of an inflammation-based prognostic system on patients undergoing surgery for hepatocellular carcinoma: a retrospective study of 398 Japanese patients. Am J Surg 203(1):101–106
Walsh SR, Cook EJ, Goulder F et al (2005) Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol 91(3):181–184
Gomez D, Farid S, Malik HZ et al (2008) Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg 32(8):1757–1762
Ishizuka M, Oyama Y, Abe A, Kubota K (2014) Combination of platelet count and neutrophil to lymphocyte ratio is a useful predictor of postoperative survival in patients undergoing surgery for gastric cancer. J Surg Oncol 110(8):935–941
Feng JF, Huang Y, Chen QX (2014) The combination of platelet count and neutrophil lymphocyte ratio is a predictive factor in patients with esophageal squamous cell carcinoma. Transl Oncol 7(5):632–637
McMillan DC (2013) The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev 39(5):534–540
Liao R, Tang ZW, Li DW et al (2015) Preoperative neutrophil-to-lymphocyte ratio predicts recurrence of patients with single-nodule small hepatocellular carcinoma following curative resection: a retrospective report. World J Surg Oncol 13:265
Makuuchi M, Hasegawa H, Yamazaki S (1985) Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet 161(4):346–350
Zhou YM, Yang JM, Li B et al (2010) Risk factors for early recurrence of small hepatocellular carcinoma after curative resection. Hepatobiliary Pancreat Dis Int 9(1):33–37
Du ZG, Wei YG, Chen KF, Li B (2014) Risk factors associated with early and late recurrence after curative resection of hepatocellular carcinoma: a single institution’s experience with 398 consecutive patients. Hepatobiliary Pancreat Dis Int 13(2):153–161
Tangkijvanich P, Anukulkarnkusol N, Suwangool P et al (2000) Clinical characteristics and prognosis of hepatocellular carcinoma: analysis based on serum alpha-fetoprotein levels. J Clin Gastroenterol 31(4):302–308
Poon RT, Fan ST, Ng IO et al (2000) Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer 89(3):500–507
Shimoda M, Katoh M, Kita J et al (2010) The Glasgow Prognostic Score is a good predictor of treatment outcome in patients with unresectable pancreatic cancer. Chemotherapy 56(6):501–506
Ishizuka M, Kubota K, Kita J et al (2011) Usefulness of a modified inflammation-based prognostic system for predicting postoperative mortality of patients undergoing surgery for primary hepatocellular carcinoma. J Surg Oncol 103(8):801–806
Li C, Wen TF, Yan LN et al (2015) Postoperative neutrophil-to-lymphocyte ratio plus platelet-to-lymphocyte ratio predicts the outcomes of hepatocellular carcinoma. J Surg Res 198(1):73–79
Peng W, Li C, Wen TF et al (2014) Neutrophil to lymphocyte ratio changes predict small hepatocellular carcinoma survival. J Surg Res 192(2):402–408
Mano Y, Shirabe K, Yamashita Y et al (2013) Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: a retrospective analysis. Ann Surg 258(2):301–305
Yamamura K, Sugimoto H, Kanda M et al (2014) Comparison of inflammation-based prognostic scores as predictors of tumor recurrence in patients with hepatocellular carcinoma after curative resection. J Hepatobiliary Pancreat Sci 21(9):682–688
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shimoda, M., Tago, K., Shiraki, T. et al. Risk Factors for Early Recurrence of Single Lesion Hepatocellular Carcinoma After Curative Resection. World J Surg 40, 2466–2471 (2016). https://doi.org/10.1007/s00268-016-3529-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-016-3529-7