Abstract
The study aims at developing a process to evaluate the impacts of the accidental emission of hazardous chemicals. The proposed process consists of four basic steps: i) identifying risks/hazards; ii) development of the worst-case scenario; iii) simulating the emission and dispersion of the toxic chemicals; and iv) assessing the severity of the impact to the people and the surroundings. It makes use of different techniques including accidental release source term, atmospheric dispersion modeling and results in the concentration and extent of the toxic chemicals in the atmosphere for either the direct evaporation of toxic chemicals as a primary emission or the dispersion of toxic chemicals as a domino effect of a fire or explosion accident. This process has been applied in a contrived case study in Ho Chi Minh City, Vietnam. In a suppositious accident of p–xylene spill from a pesticide factory, the assessment for the worst-case scenario showed that p–xylene concentration in the atmosphere could reach up to 8,882,381 µg/m3, that is higher than Protective Action Criteria for Chemicals–level 2 but far lower than the level 3. p–Xylene from the accident could disperse more than 20 km from the site, to a highly populated area with a large number of sensitive social economic object. The results of this assessment provide helpful information for the development of accidental response plan in the practical cases or supports the training for accident prevention and responses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chemical accident is defined as “any unplanned event involving hazardous substances that causes, or is liable to cause, harm to health, the environment, or property. This excludes any long-term events (such as chronic pollution)” (OECD 2013). The major hazards of chemical accidents can be classified as fire, explosion, and toxic release (DMI 2010). Of these major hazards, an occurrence of the chemical fire and explosion are generally associated with the serious consequences to people and property, whereas an accidental release of hazardous chemicals can affect directly the site and possibly a large-populated area, the emergency plan can be unresponsive once chemical has dispersed into the atmosphere and resulting in an unpredictable consequence in the case of toxic chemical release.
In an overview of the massive hazardous chemical accidents (HCAs) that have occurred within Organization for Economics–operation and Development (OECD) member countries from 1974–2013 (OECD 2013), release of HCAs account for a majority. One of the most outstanding release of HCAs is the emission of toxic and corrosive chemicals containing phenols, sodium hydroxide, 2,3,7,8-tetrachlorodibenxo–pdioxin (TCPPs) in Seveso, Italy on 10 July 1976 leading to a large number of cases of chloracne, chemical burns, livestock killed and a widespread contamination of the surrounding countryside. On 3 December 1984 a deadly cloud of methy isocyanate from a pesticide plant in Bhopal, India directly resulted in more than 3000 killed immediately, 15,000 deaths in the days following, plus more than 100,000 with injuries and illnesses. A fire at a pesticide storage facility in Schweizerhalle, Switzerland on 1 November 1986 resulted in widespread ecological damage, with pollution of the River Rhine for more than 500 km impacting countries along the route. The statistical data of 1632 hazardous chemical accidents occurring in China during 2006–2010 (Zhang and Zheng 2012) shows that releases of HCAs account for 48.4% of the fixed facility HCAs and 79.6% of transportation HCAs and were often the cause of subsequent explosions or fires as domino affects. On the other hand, releases of HCAs lead to more human deaths and major injuries than chemical fires due to the fact that hazardous chemical releasing immediately lead to poisoning and suffocation, and it is difficult for people on–site to escape quickly.
It has been found that most of the studies in the field of health or environmental risk assessment found in the literature focus on industrial fire and explosion accidents, especially for petrochemical and chemical process industry. I et al. (2009) employed computational fluid-dynamics fire and explosion model to evaluate the hazard severity of different worst-case scenarios within a petrochemical plant fire/explosion. The result of the model, the mortality rate of the target area, is then used in the simulation of the 3D individual risk value. In a study by Van der Voort et al. (2007), a dust explosion model was developed in combination of BLAST 3D blast and flame effect model to evaluate the consequence of a dust explosion accident in industrial plants. Many other studies also developed simulation software to evaluate the fire and explosion accidents in industrial facilities. These studies focused on the evaluation of the consequences due to the thermal radiation and overpressure, with rare concern on the dispersion of the toxic substances as a domino effect of the HCAs.
A number of studies had developed methods and techniques for estimating the impacts of the hazardous chemical releases as a sole accident. Mitchel Scientific Inc. and RTI International (2007) provided guidance on the assessment of the impacts of hazardous chemical emission in the chemical facilities. Policastro et al. (1994) studied on the accident scenarios for hazardous waste storage facility, including the accidental release of hazardous chemicals. United States Environmental Protection Agency (US EPA) (1997) provided specific instructions on the selection and assessment of hazardous chemical emission for different accident scenarios in chemical industry. Truong et al. (2016) applied the American Meteorological Society and Environmental Protection Agency Regulatory Model (AERMOD) to assess the risk caused by an accidental release and dispersion of the toxic chemical benzene in the vicinity of a highly populated urban area.
Simulation software have also been developed to support the analysis of the emission of HCAs. Stenzel and Baumann–Stanzer (2010) analyzed and compared several hazardous chemical accidental release models including: ALOHA (Areal Location of Hazardous Atmospheres, developed by studied and US EPA, and National Oceanic and Atmospheric Administration (NOAA) (Jones et al. 2013), MEMPLEX (Keudel av–Technik GmbH), Breeze (Trinity Consulting), SAFER, SAM (Engineering Office Lohmeyer) by using these models to analyze the same chemical accident scenarios. Evaluation showed that the dispersion result of the models varied significantly due to the differences in the input parameters and hypothetical conditions of the models.
In Vietnam, the occurrences of release of HACs have increased in recent years, some of them were considered as major chemical accidents for example: a massive chemical fire at a printing ink factory in Binh Duong Province on 18 September 2014 leading to the release of many hazardous substances to the atmosphere and water bodies; a fire accident at a chemical storage at Ho Chi Minh City on 17 April 2014, causing many injures and a release of 500 tons of chemicals to the nearby river; or the leakage of SO2 and H2SO4 chemicals from a zinc production plant in Thai Nguyen province to the surrounding populated areas on 20 July 2006, and so forth. However, studies on HACs in Vietnam are still comparatively small, fragmented, and lack of a holistic approach (Do et al. 2016). On the other hand, the experimental application of the existing chemical accident simulation software usually underestimates the consequence of the HACs in the real practices in Vietnam due to the fact that they make use of the simple user–specific meteorological conditions and default topographic data for a single model run. They are likewise unable to analyze the releases of toxic substances as a domino effect, which occurs very frequently in the HCAs (Do 2017).
For these reasons, in this study, a four–stage assessment process has been developed to analyze the risk, scope, and effects of the accidental release of hazardous chemicals as a primary consequence or a domino effect of a hazardous chemical accident. The process is expected to support the development of a feasible and practical chemical accident prevention and response plan.
Research Methods
The procedure for assessment of an accidental release of hazardous chemicals includes four steps as presented in Fig. 1.
Identification of the Risks/Hazards
According to the Technical guidance for risk assessment of hazardous chemical release in industrial sectors (VN EPA 2014), the potential accidental activities include: usage, storage, and transportation. The hazards are listed and analyzed to identify the hazards of highest concern (mainly based on the quantity of on–site chemical storage and use, storing, and process facilities). The highest risks/hazards identified in this step will be further considered for the development of the worst-case scenarios in the following steps.
Development of a Worst-Case Scenario
The risk of highest potential occurrence and consequence of the accident will be taken into consideration in this step. A worst-case scenario providing the hypothetical case of the release rate of toxic substances, potential consequence (dispersion as a primary emission or a domino effect of a fire or explosion) will be developed based on an actual investigation of the site.
Simulation
The hypothetical parameters of the worst-case scenario will be included in the mathematic models to identify the scope and level of the impacts. This step makes use of two different models: 1) the emission model to identify the quantity of chemical release, evaporation, and emission rate of toxic chemical on the mathematical basis of thermodynamics; and 2) the dispersion model to analyze the extent and concentration of toxic substances in the atmosphere as a consequence of the chemical release.
The emission model
The chemical emission rate during a hazardous chemical accident will be estimated quantitatively based on the physical–chemical parameters of the typical toxic substance. Two instinct cases of toxic substances release are developed in the following mathematical models:
Direct evaporation of toxic substances (primary emission)
In this case, the evaporation rate of the toxic substance in the absence of timely response activities is calculated according to the method of accidental release source term (Mitchell Scientific Inc. and RTI International (2007)). The toxic substance evaporates due to the difference between the chemical pressure and the atmospheric pressure calculated in the following equation:
In which:En−1: Evaporation rate (g/h), Mi: Molecular weight of the volatile substance (g/mol), Ki: Mass transfer coefficient (m/h), A: Area of evaporation (m2). A is calculated based on the amount of the chemical spill and a width of the road under the guidance of Mitchell Scientific Inc. and RTI International (2007), R: Ideal gas constant, TL: Absolute temperature of the liquid (˚K), Pisat: Saturated solvent vapor pressure (mmHg)
Emission of toxic substance as a domino effect of a fire or explosion accident (secondary emission).
In several cases, the burning of chlorinated hydrocarbons may lead to the formation of very toxic substances. In this study the emission of several toxic substances, especially phosgene, TCPPs, polychlorinated dibenzofurans (PCDFs), polychlorinated dibenzo–p–dioxins (PCDDs), the highly toxic substances that can be formed in an incomplete combustion of chlorinated hydrocarbons are taken into consideration.
The potential formation of phosgene as a product of incomplete combustion of chlorinated hydrocarbons could be evaluated based on the following parameters (US EPA 1997):
Phosgene formation is likely when a ≥ 4. The phosgene emission rate could be estimated from the following equation:
In which:rPG: Phosgene emission rate (kg/s), xTCE: Phosgene formation rate (kg/kg chlorinated hydrocarbons). Formation rate of phosgene from the combustion of several chlorinated hydrocarbons can be found. \(\frac{{\mathrm{dm}}}{{\mathrm{dt}}}\): Mass burning rate per unit area (kg/m2.s), A: liquid pool are of the chlorinated hydrocarbons release (m2).
The formation of TCDDs, PCDDs, and PCDFs as the products of incomplete chlorinated hydrocarbon combustion during a hazardous chemical accident is still under development.
The dispersion model
A small pilot study has been conducted in a study by Do (2017) to compare some air pollution emission models and define the most appropriate one for the simulation of accidental release of HACs. The models under consideration of this study are AERMOD model (American Meteorological Society/Environmental Protection Agency Regulatory Model) developed by American Meteorological Society/Environmental Protection Agency Regulatory Model Improvement Committee (AERMIC 2015); and TAPOM model (Transport and Photochemistry Mesoscale Model) developed by LPAS–EPEL (Junier 2004); and ALOHA developed by US EPA and NOAA (Jones et al. 2013). It has been found that ALOHA as a chemical accident simulation software underestimates the consequence of the HACs in some case studies due to the fact that they make use of the simple user–specific meteorological conditions and default topographic data for a single model run. Whereas TAPOM resulted in an overestimate of the atmospheric concentration of toxic substances, since it is a long-range dispersion model.
For the these reasons, AERMOD was chosen to describe the dispersion of hazardous chemical releases in the atmosphere. AERMOD is a regulatory steady state plume modeling system with three separate components: AERMOD (AERMIC Dispersion Model), AERMAP (AERMOD Terrain Preprocessor), and AERMET (AERMOD Meteorological Preprocessor) which are developed by American Meteorological Society (AMS) and US EPA from 1991.
A series of steps taken in using AERMOD model is described in Fig. 2.
Implementation process of simulating air dispersion from the accident (adapted from AERMIC (2015))
Module AERMET accepts surface meteorological data, upper air soundings, and optionally, data from on–site instrument towers. It then calculates atmospheric parameters needed by the dispersion model, such as atmospheric turbulence characteristics, mixing heights, friction velocity, Monin–Obukov length, and surface heat flux. Surface data are from hourly observation for mixing height, which can be obtained from the charged service of the National Center for Environmental Information, NOAA. In this study, TAPM (The Air Pollution Model) was used instead of AERMET to obtain the surface and upper air meteorology data from the Commonwealth Scientific and Industrial Research Organization at ftp://ftp.csiro.au/TAPM/SYNOPTIC_DATA/.
AERMAP whose main purpose is to provide a physical relationship between terrain features and the behavior of the air pollution plumes. It generates the location and height data for each receptor location. It also provides information that allows the dispersion model to simulate the effects of air flowing over the hills or splitting to flow around the hills. Finally, AERMOD will run and generate the output results in 2D and 3D, export data into Google Earth map that help user to define the affected area of the pollutants.
These models were modified to match the condition of Vietnam. The TAPM was calibrated and validated by Ho (2015) shows that the R2 for temperature, wind speed, wind direction ranging from 0.7 to 0.88 (Fig. 3). The calibration and validation of AERMOD was carried out in the study of Nguyen (2013) for simulating the dispersion of NOx and CO for a number of factories at Thi Vai area in Southeast Vietnam. The results of the mean average percent errors of NOx and CO on 06/06/2014 were 12 and 14%, respectively, which are smaller than the required value of ± 15%, demonstrating the acceptable simulation of AERMOD.
Assessing the Severity of the Impact
The atmospheric concentration of the toxic chemicals obtained from the dispersion simulation are then compared with Vietnamese regulations or the technical guidelines on toxicity assessment and impact thresholds for analyzing the endpoints and the severity of the impact. The Toxic Level of Concerns (LOCs) in the database of CAMEO (ORR 2016) and the Vietnam national technical standards are considered as the most important criteria for the evaluation.
Results and Discussion
Evaluating the Impacts of p–Xylene Spill During Onsite Storage
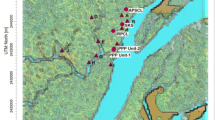
The procedure is supposed to apply internationally, and was validated in a case study of p–xylene accidental emission from a pesticide factory in Ho Chi Minh City of Vietnam. The plant currently stores and makes use of a variety of chemicals for the production and trade. Of those chemicals, p–xylene is one of the most common and largest hazardous chemicals in use with an average annual consumption of 415 tons. p–Xylene is a liquid chemical of high flammability and toxicity to human and aquatic life. Therefore, p–xylene spill was chosen for the worst-case chemical accident scenario of the plant. It is assumed that a failure during storage causes p–xylene to spill into the surrounding. The volume of spilled p–xylene is assumed to be 16 m3, which is 80% of the total capacity of the storage tank (Table 1).
The formation of phosgene is unlikely in this case study since p–xylene is not a chlorinated hydrocarbon. Due to the pressure difference, xylene will evaporate causing toxic and fire threats. In order to calculate the evaporation rate of p–xylene from the accident, two different atmospheric temperature scenarios were used in the emission model based on the highest and lowest values of the statistical temperature within 2013–2015 of the Tan Son Hoa meteorological station (Ho Chi Minh City Statistical Office 2016).
The results showed that the evaporation rates of p–xylene in the two scenarios were not significantly different (less than 1%), the atmospheric concentrations of p–xylene from the dispersion model is predicted to be significantly identical. Therefore, the high-emission scenario (the temperature is 30.7 °C) were used. As a result, the evaporation rate of p–xylene in this scenario was 767.9 g/s.
The above scenario was used in the AERMOD model to determine the ground level, p–xylene concentration and the area under threaten of p–xylene exposure and fire risk. To identify the worst meteorology conditions which support the highest dispersion, we run the model for the whole year 2015 year and selected the maximum of 1 h and maximum during 24 h average p–xylene concentration for the worst-case. Figure 4 demonstrates that the highest 1-h mean concentration of p–xylene dispersed from the accident for all the wind directions within 2015 incident.
Dispersion modeling results show that p–xylene spread to a large area depending on the wind direction. Within the simulation area of 40 km in diameter, the highest hourly mean concentration of p–xylene in 2015 is higher than Vietnam National technical regulation on hazardous substances in ambient air QCVN 06:2009/BTNMT (hourly concentration limit of 1000 µg/m3). Compared to Protective Action Criteria for Chemicals (PACs) (EMI SIG 2012), the area of highest hourly mean concentration of p–xylene exceeding PAC-1 (low level, no obviously health impact –130 p.p.m., equal to 554,852 µg/m3), preferred to as PAC-1 zone, has a diameter of 7 km, focusing on District 7 and Nha Be district of Ho Chi Minh City, industrial and cultivated land with low-density of population. The area of highest hourly mean p–xylene concentration exceeding PAC-2 (irreversible or other serious health effects that could impair the ability to take protective action –920 p.p.m., equal to 3,926,645 µg/m3), preferred to as PAC-2 zone, is significantly smaller, which is about 1.5 km in diameter and focusing around the accident site. The PAC-3 zone where hourly concentration of p–xylene higher than PAC-3 (life–threatening health effects –2500 p.p.m., equal to 10,670,231 µg/m3) does not exist in this case study.
The worst-case scenario occurs at 1 a.m. on 20th February 2015. In this case (as shown in Fig. 5), p–xylene would spread out in the Southwest direction up to 3.73 km from the source point. The highest down-wind concentration located 400 m from the accident site, reaching 8,882,382 µg/m3, which is 8882 times higher than QCVN 06:2009/BTNMT, 16 times higher than PAC-1 and 2.3 times higher than PAC-2, but far lower than PAC-3.
The highest 24-h concentration of p–xylene at ground level was 1,058,400 µg/m3, which is still higher than QCVN 06:2009/BTNMT and PAC-1 but lower than PAC-2 (presented in Fig. 6). Compared to Occupational Safety and Health Administration permissible exposure limit for the industry action level (OSHA PEL, 8-h time-weight average of 100 p.p.m., equal to 435,000 µg/m3) (OSHA 2012), p–xylene would exceed this standard in an area with diameter of 1000 m.
Discussion
p–Xylene from the accident could disperse more than 20 km from the site, thus potentially affect the center of Ho Chi Minh City, a highly populated area with a large number of sensitive social economic objects. In the worst-case scenario, the highest hourly mean p–xylene concentration at the ground level is lower than PAC-3, the threshold value for irreversible toxic effects in term of exposure in air, as well as the lower explosive limit (9000 p.p.m.)–the minimum concentration of p–xylene necessary to support its combustion in air. Thus p–xylene cloud released from the accident is unlikely to cause death or fire. However, p–xylene concentration could exceed PAC-2 level in an area of 1.5 km diameter surrounding the accident site, covering a part of Hiep Phuoc industrial zone and the nearby cultivation land. The impacts of the accident could therefore be mitigated in terms of scale and severity, with the effected population being mostly the workers in the industrial zone. The area where the highest hourly mean p–xylene concentration exceeding PAC-1 is wide–ranging over 7 km in diameters, covering a part of the suburban Ho Chi Minh City.
Vietnam National technical regulation on hazardous substances in ambient air QCVN 06:2009/BTNMT stipulates that the highest hourly mean p–xylene concentration in the ambient air must not exceed 1000 µg/m3. The xylene limit concentration of this standard is far lower than that of PAC-1 and OSHA PEL. Thus, it could fail to support the development of a feasible and efficient response plan for a xylene spill accident due to the large uncertain effected area and population.
To develop an effective evacuation and emergency response plan, we propose to determine the potential effected areas based on PAC-2 and PAC-3 instead of QCVN 06:2009/BTNMT. In order to minimize the risk of wind direction change, which could happen anytime, the area of evacuation and isolation should be confined inside the PAC-2 zone, and PAC-3 zone should be set as the strictly protected area where the rescue team and authorized persons must be fully equipped with protective clothing and equipment.
Since it was adopted as a preferred regulatory model of US EPA, AERMOD could produce more comprehensive and accurate simulation results than many other modeling software available in the market. However AERMOD fails to simulate the air pollution sources, which change its emission load in time. It can only generate the immediate dispersion areas and areas of concentration of the pollutants released during the accident, rather than the atmospheric lifetime of the pollutants. For this reason, it is unable to determine the endpoint time of the impacts from the accidents and difficult to support an efficient and feasible accident prevention and response plan.
Conclusion
In this study, a consistent method has been developed for the assessment of the scope and severity of the impact from an accidental release of hazardous chemicals. The proposed process consists of four basic steps and makes use of a variety of techniques including accidental release source terms, air dispersion modeling, and GIS to determine the impacts of an accidental hazardous chemical release. The process is applicable for either the direct evaporation of toxic substances (primary emission) or the emission of toxic substance as a domino effect of a fire or explosion accident (secondary emission). Several other toxic substances such as TCDDs are still under development for the secondary emission scenarios.
The case study of p–xylene spill from the storage tank of a pesticide plant in Ho Chi Minh City, Vietnam showed that the proposed procedure can be very useful to support the determination of the potential impact areas and population of the accidents and provide helpful cautionary information for the development of the feasible and practical chemical accident response plan or support the rehearsal of an accident response plan. Still, the application of the emission and dispersion models need further development to support the evaluation of domino accidents and the endpoint of the chemical existence in the atmosphere.
References
AERMIC (2015) AERMOD Modeling System. American Meteorological Society/Environmental Protection Agency Regulatory Model Improvement Committee (AERMIC), North Carolina, USA
DMI (2010) Theme−10 Guide to the District Collector: What I Must Know as District Collector? and Why? industrial Disaster Risk Management. Disaster Management Institute (DMI), Bhopal, India
Do TTH (2017) Development of an assessment process for environmental incidents from the emission of hazardous chemicals, case study in Ho Chi Minh City. Institute for Environment and Resources, Vietnam National University–Ho Chi Minh City, Ho Chi Minh City, Vietnam
Do TTH, Nguyen TM, Ho QB, Ly TBT (2016) Evaluation of environmental impacts from an accidental release of hazardous chemical in industry. In: Nguyen VP, Ho MD (eds) Proceeding of the International Conference 2016 ICENR–ILTER–EAP. Ecological Insights and Environmental Protection for Sustainable Development under Climate Changes in East–Asia and Pacific Region. Institute for Environment and Resources, Vietnam National University, Ho Chi Minh City, Vietnam, pp 235–242
EMI SIG (2012) Protective Action Criteria for Chemicals–Including AEGLs, ERPGs, & TEELs. Emergency Management Issues Special Interest Group, USA. http://www.atlintl.com/DOE/teels/teel.html. Accessed 3 Nov 2014
Ho Chi Minh City Statistical Office (2016) Ho Chi Minh City Statistical Yearbook 2015. Statistical Publishing House, Ho Chi Minh City, Vietnam
Ho QB (2015) Estimation of air pollutants dispersion from Thanh Cong Textile Company, Ho Chi Minh City. Institute for Environment and Resources, Vietnam National University, Ho Chi Minh City, Vietnam
I Y-P, Shu C-M, Chong C-H (2009) Applications of 3D QRA technique to the fire/explosion simulation and hazard mitigation within a naphtha-cracking plant. J Loss Prev Process Ind 22:506–515. https://doi.org/10.1016/j.jlp.2009.04.002
Jones R, Lehr W, Simecek-Beatty D, Bañares-Alcántara R (2013) ALOHA® (Areal Locations of Hazardous Atmospheres) 5.4.4: Technical Documentation. Emergency Response Division, NOAA, Washington, D.C, USA
Junier M (2004) Gas phase chemistry mechanisms for air quality modeling: generation and application to case studies. Dissertation, École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland
Mitchell Scientific Inc., RTI International (2007) Methods for estimating air emissions from chemical manufacturing facilities. In: Emission Inventory Improvement Program, US Environmental Protection Agency, USA
Nguyen TC (2013) Study on the impact of air pollution on the surrounding areas of Thi Vai river basin. Master thesis, Institute for Environment and Resources, Vietnam National University, Ho Chi Minh City, Vietnam
OECD (2013) 25 years of chemical accident prevention at OECD-History and Outlook. Organization for Economic Co-operation and Development (OECD), Paris, France
ORR (2016) Toxic Level of Concerns. Office of Response and Restoration (ORR), USA, http://response.restoration.noaa.gov/oil-and-chemical-spills/chemical-spills/resources/toxic-levels-concern.html Accessed 1 Jul 2016
OSHA (2012) Chemical Sampling Information/Xylene. United State Department of Labor–Occupational Safety and Health Administration (OSHA). https://www.osha.gov/dts/chemicalsampling/data/CH_276400.html. Accessed 11 Jan 2017
Policastro A, Roglans-Ribas J, Marmer D et al (1994) Hazardous waste storage facility accident scenarios for the U.S. Department of Energy Environmental Restoration and Waste Management Programatic Environmental Impact Statement. In: WM’94 Conference. Arizona, USA
Stenzel S, Baumann-Stanzer K (2010) Dispersion modeling of accidental toxic gas releases–A model comparison study. In: HARMO13, Paris, France
Truong SCH, Lee M-I, Kim G et al (2016) Accidental benzene release risk assessment in an urban area using an atmospheric dispersion model. Atmos Environ 144:146–159. https://doi.org/10.1016/j.atmosenv.2016.08.075
US EPA (1997) Volume VII: Accident analysis: selection and assessment of potential release scenarios. U.S. Environmental Protection Agency (US EPA), Ohio, USA
van der Voort MM, Klein AJJ, de Maaijer M et al (2007) A quantitative risk assessment tool for the external safety of industrial plants with a dust explosion hazard. J Loss Prev Process Ind 20:375–386. https://doi.org/10.1016/j.jlp.2007.04.024
VN EPA (2014) Technical guidance for risk assessment of hazardous chemical release in industrial sectors. Vietnam Environmental Protection Agency (VNEPA), Ha Noi, Vietnam
Zhang H-D, Zheng X-P (2012) Characteristics of hazardous chemical accidents in China: A statistical investigation. J Loss Prev Process Ind 25:686–693. https://doi.org/10.1016/j.jlp.2012.03.001
Acknowledgements
This research is funded by Vietnam National University of Ho Chi Minh City (VNU–HCM) under grant number C2016-24-03. It is also a preliminary study for the project number 48/2017/HĐ–SKHCN entitled “Development of environmental accident preparedness and response plan for the industrial zones and export processing zones in Ho Chi Minh City” funded by the Department of Science and Technology of Ho Chi Minh City, Vietnam.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Huyen, D.T.T., Tram, L.T.B. Development of a Procedure for Evaluating the Impacts of the Accidental Emission of Hazardous Chemicals, Case Study in Ho Chi Minh City, Vietnam. Environmental Management 63, 486–494 (2019). https://doi.org/10.1007/s00267-017-0979-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00267-017-0979-0