Abstract
Non-native species are often major drivers of the deterioration of natural ecosystems. The common carp Cyprinus carpio are known to cause major changes in lentic systems, but may not be solely responsible for large scale changes in these ecosystems. We used data from extensive collection efforts to gain insight into the importance of carp as drivers of ecosystem change in Lake Patzcuaro, Mexico. We compared the structure (fish density, biomass, diversity, and evenness) of fish assemblages from six Lake Patzcuaro sites with different habitat characteristics. Intersite comparisons were carried out for both wet and dry seasons. We explored the relationships between non-carp species and carp; and studied multivariate interactions between fish abundance and habitat characteristics. From a biomass perspective, carp was dominant in only four of six sites. In terms of density, carp was not a dominant species in all sites. Further, carp density and biomass were not negatively related to native species density and biomass, even when carp density and biomass were positively correlated to water turbidity levels. Carp dominated fish assemblages in the shallowest sites with the highest water turbidity, plant detritus at the bottom, and floating macrophytes covering the lake surface. These results suggest that the effect of carp on fish assemblages may be highly dependent on habitat characteristics in Lake Patzcuaro. Watershed degradation, pollution, water level loss, and other sources of anthropogenic influence may be more important drivers of Lake Patzcuaro degradation than the abundance of carp.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Introducing species beyond their native range is one of the most important human impacts on aquatic ecosystems (Cucherousset and Olden 2011). The common carp Cyprinus carpio (L. 1758) (Pisces: Cyprinidae) causes many alterations to freshwater ecosystems (Zambrano et al. 1999; Miller and Crowl 2006; Zambrano et al. 2006). Carp can modify entire habitats which can lead to a loss of local (native) biodiversity (Britton et al. 2010). Water transparency decline and water turbidity increase can result from benthic feeding activity by carp (Vitousek et al. 1996; Zambrano et al. 2001; Leung et al. 2002; Scheffer et al. 2003; Ozbay 2008). Numerous studies suggest that impacts of carp on native fish communities and their environment are indirect (Zambrano and Macías-García 2000; Wolfe et al. 2009; Weber and Brown 2011), density dependent, and often localized on littoral zones of lentic systems (Miller and Crowl 2006). As omnivorous, fast-growing fish, carp can inhabit a variety of habitats, including shallow lentic systems with high water turbidity, low transparency, and low dissolved oxygen levels (Zambrano et al. 1999, 2001; Koehn 2004).
Introduction of carp to reservoirs and lakes in Mexico has been widely documented because the species has become one of the most important aquaculture species (Zambrano et al. 1999; Zambrano and Macías-García 2000; Hinojosa-Garro and Zambrano 2004). Carp were introduced to nearly all natural, lentic water bodies in Central Mexico, including Lago de Chapala, Lago de Cuitzeo, and Lago Xochimilco, some of which harbor endemic fish species (Zambrano and Macías-García 2000). Carp was introduced into Lake Patzcuaro in 1974 and spread throughout the lake after the accidental release of fish from aquaculture pens (Rosas 1976).
Lake Patzcuaro native fishes include species in the genera Goodea, Allophorus, Allotoca, Algansea, and Chirostoma (Rosas 1976; Berlanga-Robles et al. 2002; Miller et al. 2005), all endemic to central Mexico. These genera have been the focus of numerous studies (e.g., Peresbarbosa-Rojas et al. 1994; Espinosa-Huerta et al. 1996; Mendoza-Garfias et al. 1996; Pérez-Ponce de León et al. 2000; Palacios et al. 2007) but, to date, there is only one published study that documents fish abundance in the lake based on information from commercial catches (Berlanga-Robles et al. 2002). Prior to this work, the structure of Lake Pátzcuaro fish assemblages had never been documented from standardized, comprehensive scientific collections. Lake Patzcuaro is a shallow lake (Max depth: <13 m) with marked differences among distinct areas of the lake in terms of physical and chemical water properties and surrounding land use (Alcocer and Bernal-Brooks 2002). Such differences include variations in water transparency, water turbidity, anthropogenic activity, and physical habitat characteristics (e.g., water depth, bottom type, and floating vegetation cover) (Huerto-Delgadillo and Amador-García 2011; Sánchez-Chávez et al. 2011; Ramírez-Herrejón et al. 2013). This habitat diversity makes Lake Patzcuaro an interesting model system to study the role of common carp in the structure of fish assemblages under a variety of environmental conditions.
Observations from artificial ponds (Roberts et al. 1995; Zambrano et al. 1999; Parkos et al. 2006), large mesocosms (Wolfe et al. 2009), and cages in littoral areas of large lakes (Miller and Crowl 2006) have linked high water turbidity to high density of carp. Increases in water turbidity, alteration of submerged vegetation associations including the disappearance of Potamogeton foliosus (Potamogetonaceae), declines in endemic salamander Ambystoma dumerilii and invertebrate populations, and potential resource competition with native fishes attributed to carp have all been speculated in Lake Patzcuaro (Arroyo-Quiroz et al. 2014).
There are several other lines of evidence about how carp and other impacts have altered Lake Patzcuaro. Carp production has increased relative to native fish production (Alaye 2006). Currently, carp supports one of the most important fisheries in the lake (60 % of the capture, ~10 t of fish annually) (Orbe-Mendoza et al. 2002; Zambrano et al. 2011), while fisheries for native species are declining (Berlanga-Robles et al. 2002; Berry et al. 2011). Water transparency of the lake has decreased in the last 30 years (post-carp establishment), going from >3 m to <30 cm on average (Orbe-Mendoza et al. 2002; Sánchez-Chávez et al. 2011). Lake Patzcuaro has a marked north-to-south increasing turbidity gradient, related positively to carp distribution (Ramírez-Herrejón et al. 2013). However, carp may not be solely responsible for turbidity changes as Lake Patzcuaro presents daily wind-induced sediment re-suspension, high habitat heterogeneity, and seasonal water level fluctuations (Bernal-Brooks et al. 2002). The lake has also become shallower in recent years (>5 m) as a potential consequence of shoreline alterations, water extraction, and watershed deforestation (Orbe-Mendoza et al. 2002; Sánchez-Chávez et al. 2011). Further, boating activity and input of untreated sewage have also altered shoreline habitats. All these various lines of evidence suggest that synergistic impacts have all affected the native fish community and the environmental characteristics of the lake. To further document the current status of fish communities in Lake Patzcuaro, this study tests whether (1) carp density and biomass are negatively related to native species density and biomass (expecting lower diversity and evenness in sites with carp), (2) carp density and biomass are positively related to water turbidity, and (3) carp is the dominant species regardless of habitat characteristics. To address these hypotheses, we use fish community structure data from sites with different water and habitat characteristics in Lake Patzcuaro.
Materials and Methods
Study Area
Lake Patzcuaro (Fig. 1) is part of an enclosed drainage basin in the highlands of central Mexico (19°32′N–19°42′N, 101°32′W–101°42′W) (State of Michoacán) (Bernal-Brooks et al. 2002). The lake was originated by tectonic and volcanic events that insulated ancient Lerma River inflowing to create a closed basin (De Buen 1944). The lake has a maximum surface area of 116 km2 (Gómez-Tagle et al. 2002), and a maximum recorded depth of 12.2 m. Average depth is 4.9 m. Ephemeral streams (Arroyo San Gregorio and Arroyo Santa Fe) occasionally feed the northern zone of the lake during the summer. One perennial stream (Arroyo Chapultepec) feeds the southern part of the lake. Water volume in the lake is determined by precipitation, evapotranspiration, and water input from springs at the bottom of the lake (Bernal-Brooks et al. 2002). The highest recorded water level was 2041 m.a.s.l. (during the 1970s) and the lowest was 2035 m.a.s.l. (during the 2000s), with an annual variation of ~1.2 m.a.s.l. (Bernal-Brooks et al. 2002). There are 26 main towns (with populations from ~300 to ~55,298) surrounding the lake and one densely populated island (Isla Janitzio, >3000 people) in the southern part of the lake. Although there are at least two wastewater treatment plants in the City of Pátzcuaro and Janitzio Island, wastewaters are still entering the lake with little treatment. The lake watershed covers 9340 km2 and is used for agriculture (~25 % of the watershed area, mostly corn), raising livestock (~20 %), forestry (~30 %), and urban activities (~25 %) (Bravo-Espinosa et al. 2006). Soil erosion from agriculture and ranching in parts of the basin is reflected in sedimentation rates in the lake (Gómez-Tagle et al. 2002); around 100,000 m3 of sediments have been received by the lake each year for more than 20 years (Rodríguez-Arteaga and Zarazúa-Sánchez, 2009). Accelerated decline of forested area, increasing desertification, and partial disappearance of native plants and animals are signs of Lake Patzcuaro watershed alteration (Bravo-Espinosa et al. 2006). Native fish populations of Lake Patzcuaro, including the Pátzcuaro allotoca Allotoca diazi (Meek 1902), the Bumblebee allotoca Allotoca dugesii (Bean 1887), the Bulldog goodeid Alloophorus robustus (Bean 1892), the Pátzcuaro chub Algansea lacustris (Steindachner 1895), the Blackfin goodea Goodea atripinnis (Jordan 1880), and the salamander Ambystoma dumerilii (Dugès), have population declines that reach over 90 % of density (Orbe-Mendoza et al. 2002; Ramírez-Herrejón et al. 2014b). Populations of non-native Largemouth Bass Micropterus salmoides (Lacepède 1802), which was introduced in the 1930s, have also declined for over 99 % (Orbe-Mendoza et al. 2002; Ramírez-Herrejón et al. 2014b). All fisheries in the lake including native and exotic species have declined from ~2500 tons per year (t/year) (1988) to <50 t/year (2007). Pike silverside Chirostoma estor fishery has declined from >130 t/year (1981) to <1 t/year (2010). Pátzcuaro chub fishery has diminished from >650 t/year (1988) to <1 t/year (2010). Goodea atripinnis fishery has declined from ~258 t/year (1989) to <1 t/year (2010) (Ramírez-Herrejón et al. 2014b).
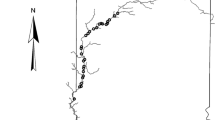
Geographic location of Lake Patzcuaro (Central Mexico) and study sites. Limnological zones defined by Alcocer and Bernal-Brooks (2002) are indicated by lines crisscrossing the lake area. (1) San Jerónimo (SAJ) (19°40′40.4″N, 101°36′16.9″W); (2) La Pacanda (PAC) (19°36′38.1″N, 101°39′2.7″W); (3) Napízaro (NAP) (19°35′20.8″N, 101°40′12.7″W); (4) Ihuatzio (IHU) (19°35′35.1″N, 101°40′45.2″W); (5) Embarcadero (EMB) (19°33′0.6″N, 101°37′30.7″W); (6) Ucasanastacua (UCA) (19°35′51.7″N, 101°37′58.5″W). Black rectangles show an approximation of the collection areas in each study site (500 m × 100 m, approximately). This map was taken from Ramírez-Herrejón et al. 2013 (reproduced with permission from Revista de Biologia Tropical)
Alcocer and Bernal-Brooks (2002) and Ramírez-Herrejón et al. (2013) described the spatial differences in Lake Patzcuaro limnology. A northern zone of the lake has the highest water transparency (0.43 ± 0.06 m, Secchi disk transparency), the highest water conductivity (924.7 ± 25.7 μS cm−1), and the lowest concentration of suspended solids (32.7 ± 1.9 mg L−1). A zone located between the northern and southern basins of the lake has water transparency of 0.3 ± 0.05 m (Secchi disk transparency), water conductivity values of 794.7 ± 125.7 μS cm−1, and suspended solids of 50.1 ± 13.8 mg L−1. The southern basin has the lowest transparency (0.19 ± 0.09 m, Secchi disk transparency), and conductivity (566.5 ± 276 μS cm−1), and also the highest concentration of suspended solids (128.2 ± 78.7 mg L−1). A small inlet in the southern portion of the lake, where human activity is concentrated, has high nutrient concentration (e.g., nitrogen concentration 922.2 ± 774.3 μg L−1 and total phosphorus concentration of 225.5 ± 84.5 μg L−1). Based on these physical and chemical water properties of the different areas of the lake, six collection sites were chosen (Fig. 1). Sites included shallow (<2 m) and deep (>4 m) sections of littoral (<25 m from the shore) and limnetic (>200 m from the shore) lake zones (Table 1). Site San Jerónimo (SAJ) is a shallow littoral area located in the northern portion of the lake. Isla Pacanda (PAC) is located in a deep littoral area in the middle section of the lake. Ucasanastacua (UCA) is a shallow littoral area in the middle section of the lake. Napízaro (NAP) is a shallow limnetic area in the southern part of the lake. Ihuatzio (IHU) is a shallow littoral area in the southern part of the lake. Embarcadero (EMB) is a shallow littoral area in the southern part of the lake near the main wharf for the city of Pátzcuaro.
Characterization of Habitat and Sampling
Sites were sampled during September and November 2009 (wet season) and February and June 2010 (dry season). Before collecting fishes at each site, a series of water and habitat characteristics were recorded. Water physical–chemical parameters were measured with a multimeter (Hach Hydromet Quanta, Loveland, Colorado, USA), and included dissolved oxygen (mg L−1), total dissolved solids (mg L−1), water transparency (m), and water turbidity (NTU). Depth (m) was measured with a depth meter at the initial point of a seine haul (see fish collections, below). Aquatic vegetation (mainly water hyacinth Eichhornia crassipes [Mart.] Solms) coverage (% of sampling site) was estimated using a visual technique (Barbour et al. 1999). Sediment samples at each site were collected with an Ekman dredge and the dominant bottom type was described as rock, mud, and degraded organic matter using Bouyoucos (1936).
Fish collections were carried out using two seines, a silverside seine (SS) and a carp seine (CS). To avoid using multiple fishing techniques at sites repeatedly, we used SS and CS on two different days, allowing for at least 120 h between samples. The two seines provided a representative sample of the fish community, capturing individuals from <1 cm to 45 cm standard length (SL). Small-sized fish (<10 cm SL) were preferentially captured with the SS. Large fish (>10 cm SL) were preferentially captured using the CS. The SS (75 m × 7 m, 10 mm mesh) leads and floats were separated by 10 m and 1 m of net, respectively. The CS (150 m × 9 m, 40 mm mesh) leads and floats were separated every 3 m and 1 m of net, respectively. Both seines were always deployed from a fishing boat. Nets were always deployed so that they would form a circle when set; this allowed the straightforward calculation of the collection area for each net. Total collection area per seine haul was approximately 447.6 m2 for the SS and approximately 1790.5 m2 for the CS. Each seine was deployed three times at each site, for a total of 36 sets per sampling and 144 sets in the entire study. An approximate area of 50,000 m2 was defined at each site to avoid overlapping of net sets. The area defined by the site is represented with black rectangles in Fig. 1, and each set was randomly located within this area.
Captured Fish
Captured fish were kept in ice and transported to the “Javier Alvarado Díaz” Laboratory of Aquatic Biology (Universidad Michoacana de San Nicolás de Hidalgo, Morelia, Michoacán). Specimens were identified to species except for Oreochromis spp. and Chirostoma spp.; at least two species of Oreochromis were introduced to Lake Patzcuaro in the 1970s, blue tilapia O. aureus (Steindachner 1864), and Nile tilapia O. niloticus (L. 1758) (Berlanga-Robles et al. 2002; Gaspar-Dillanes et al. 2006). Much hybridization has occurred between these two species, making positive identification to species level difficult. Taxonomic differences between four native species of Chirostoma, bigeye silverside C. grandocule (Steindachner 1894), Pátzcuaro silverside C. patzcuaro (Meek 1902), slender silverside C. attenuatum (Meek 1902), and pike silverside C. estor (Jordan 1880), and one introduced species, shortfin silverside C. humboldtianum (Valenciennes 1835) are currently in dispute (Barriga-Sosa et al. 2002; Bloom et al. 2009). Thus, silversides were identified only to the genus level and considered a native taxon. Specimens were separated by taxonomic group (species or genus) and seine set. Individuals in each taxonomic group and seine net were counted, measured (mm), and weighed (g).
Data Analysis
Non-parametric Kruskal–Wallis rank sums tests were used to detect differences among sites (though not among seasons) for water physical–chemical parameters, fish density (individuals/m2), and fish biomass (g/m2). Data from September and November (2009) were averaged to represent the wet season. Data from February and June (2010) were averaged to represent the dry season. Separate analyses were done for wet and dry seasons. If significant differences were found, multiple comparisons were made using the Tukey–Kramer honest significant difference (HSD) post hoc test (Zar 1999). Both analyses were executed using JMP 3.1.6.2 software (SAS Institute 1995).
To assess the importance of different species in the fish community, the category of each species, from the most common and abundant to the rarest species (dominant, abundant, frequent, or rare), was determined using a modified Olmstead-Tukey’s association procedure (Sokal and Rohlf 1969). In this procedure, species were ranked according to their relative abundance (above and below the average density or biomass of all species), and their frequency of occurrence (above and below the average frequency of occurrence of all species). This allowed for categorizing species as: dominant (density or biomass and frequency above the average for all species), abundant (density or biomass above the average, but frequency below the average), frequent (density or biomass below the average, but frequency above the average), or rare (density or biomass and frequency below the average).
We examined the fish community diversity with a Shannon index (H′, log base 10) and community evenness with a Pielou index (J′) for each site during the wet and dry seasons. Inter-site diversity and evenness variation in both seasons was calculated using the Kruskal–Wallis rank sums-test and the Tukey–Kramer HSD post hoc test following Magurran (2004).
Spearman rank order correlation analysis (Zar 1999), performed using Statistica 6.0 (StatSoft, Tulsa, OK) software, was used to estimate (1) the relationship between turbidity, and density and biomass of each fish species and (2) the relationship between density and biomass of each (non-carp) fish species and carp. This correlation analysis was carried out to assess if increases in water turbidity or carp density or biomass could be associated with decreases of native fish density or biomass.
Distance-based redundancy analysis (db-RDA, Legendre and Anderson 1999) was used to identify multivariate interactions between fish density and biomass spatial patterns, and habitat characteristics (water physical–chemical parameters, aquatic vegetation coverage, and bottom type) in the wet and dry seasons. This analysis was selected because a detrended correspondence analysis (not presented) showed species turnover was <2 standard deviation units, which is the recommended criterion for choosing linear versus uni-modal ordination models (Lepš and Šmilauer 2003). The Bray-Curtis distance measure was used between samples (McArdle and Anderson 2001). The db-RDA was carried out on the correlation matrix, and a forward selection procedure was used to determine significant explanatory variables at P < 0.05 (Magalhães et al. 2002). Statistical significance was determined using Monte Carlo tests with 499 permutations. Density and biomass values were transformed logarithmically and those environmental variables that failed a normality test were square root-transformed. CANOCO 4.5 (ter Braak and Smilauer 2002) software was used to run the db-RDA analysis.
Finally, a similarity percentage analysis (SIMPER Clarke and Warwick 2001) was used to identify if particular species discriminated among sites. The SIMPER procedure compares the average density or biomass between species at each site and examines the contribution of each species to average Bray–Curtis dissimilarity.
Results
A total of 92,054 specimens (160.6 kg) were collected. Six species, eight genera, and six families were identified. Four were native fish taxa: Algansea lacustris (Cyprinidae) (7 ind., 0.0025 kg), Alloophorus robustus (Goodeidae) (10 ind., 0.075 kg), G. atripinnis (Goodeidae) (252 ind., 3.4 kg), and Chirostoma spp. (Atherinopsidae) (83,209 ind., 41.4 kg). Four were introduced fish taxa: Carp (Cyprinidae) (1721 ind., 96.5 kg), Lerma livebearer Poeciliopsis infans Woolman 1894 (Poecilliidae) (3679 ind., 1.6 kg), M. salmoides (Centrarchidae) (2 ind., 0.0026 kg), and Oreochromis spp. (Cichlidae) (3174 ind., 17.4 kg). Three native Goodeidae species known for Lake Patzcuaro (Berlanga-Robles et al. 2002), Olive skiffia Skiffia lermae Meek 1902, A. dugesii, and Allotoca diazi were absent from the samples.
Characterization of Habitats
Turbidity and other habitat parameters differed among sites in both seasons. A turbidity gradient occurred in both seasons (Kruskal–Wallis rank sum test, χ 2 = 29.82, DF = 5, P < 0.001; χ 2 = 24.27, DF = 5, P < 0.002; respectively) (Table 1). Sites SAJ and PAC had the lowest turbidity, IHU had medium turbidity values, and EMB had the highest turbidity during both seasons. Sites SJ and EMB had lower dissolved oxygen than IHU and UCA during the wet season (Kruskal–Wallis rank sum test, χ 2 = 23.31, DF = 5, P < 0.003) but not during the dry season. Dissolved oxygen did not differ between sites during the dry season. Lowest total dissolved solids occurred at EMB during the wet season (Kruskal–Wallis rank sum test, χ 2 = 22.66, DF = 5, P < 0.004). There were no differences in total dissolved solids between sites sampled during the dry season (Table 1).
Highest transparency occurred at SAJ and PAC during the wet season (Kruskal–Wallis rank sum test, χ 2 = 27.92, DF = 5, P < 0.001). SAJ and EMB had similar transparency during the dry season (Kruskal–Wallis rank sum test, χ 2 = 22.75, DF = 5, P < 0.004). PAC was the deepest site, followed by UCA, NAP, and IHU; the shallowest sites were SAJ and EMB (Kruskal–Wallis rank sum test, χ 2 = 26.09, DF = 5, P < 0.001 during the wet season, and χ 2 = 29.55, DF = 5, P < 0.001 during the dry season). Average water hyacinth cover varied in a pattern similar to turbidity during the wet and dry seasons. SAJ and PAC had the least floating macrophytes cover (<5 %), and EMB had the highest cover (>90 %). EMB had >95 % of plant detritus (plant remains) at the bottom (Table 1).
Structure of Fish Assemblages
In terms of density, carp was rare at SAJ and PAC, and frequent at UCA, NAP, IHU, and EMB. Chirostoma spp. was the dominant taxa at all sites during both seasons. Algansea lacustris, A. robustus, M. salmoides, and Oreochromis spp. were absent or rare at most sites. Oreochromis spp. was dominant only at EMB during the wet season. Poeciliopsis infans was consistently a frequent species in all sites but was also dominant at EMB during both seasons. Goodea atripinnis was frequent only at EMB during the dry season, and was rare at the other sites in both wet and dry seasons.
In terms of biomass, carp was classified as dominant species at NAP, IHU, and EMB in both seasons and at UCA during the wet season. It was rare in SAJ and PAC during both seasons, and it was absent at UCA during dry season. Chirostoma spp. was dominant at SAJ, PAC, UCA, and EMB in the wet season and at all sites during the dry season. Oreochromis spp. was a dominant species at EMB, and a rare species in the rest of the sites. Carp shared dominance with Chirostoma spp. and Oreochromis spp. at EMB during both seasons. Poeciliopsis infans was a frequent species at all sites during both seasons. Algansea lacustris, A. robustus, and M. salmoides were absent or rare at all sites.
Carp occurred >90 % of the time at NAP, IHU, and EMB. At sites with relatively low turbidity (SAJ and PAC), this species did not exceed the average frequency of all fishes. Chirostoma spp. was present at all sites in both seasons. Micropterus salmoides was collected only at EMB during the dry season. Alloophorus robustus was captured at SAJ, PAC, UCA, and NAP (wet season only), and A. lacustris was captured at NAP (dry season only), IHU (wet season only), and EMB (both seasons).
Carp was most dense at EMB in both seasons (Kruskal–Wallis rank sum test for wet and dry seasons (χ 2 = 25.82, DF = 5, P < 0.001; χ 2 = 30.48, DF = 5, P < 0.001, respectively). Carp and Oreochromis spp. were most concentrated at EMB, the site with the highest turbidity. Also, biomass of carp was highest at EMB in both seasons (Kruskal–Wallis rank sum test, χ 2 = 19.27, DF = 5, P < 0.01 [wet season]; χ 2 = 27.02, DF = 5, P < 0.001 [dry season]). Density of Chirostoma spp. was higher at UCA during the wet season (Kruskal–Wallis rank sum test, χ 2 = 12.5, DF = 5, P < 0.028). During the dry season, density of Chirostoma spp. was similar among sites; its biomass was similar among sites during both seasons (Table 2). Density of Poeciliopsis infans was similar at all sites during the wet season. However, P. infans density was highest at EMB during the dry season (Kruskal–Wallis rank sum test, χ 2 = 21.52, DF = 5, P < 0.006); its biomass was highest at EMB (Kruskal–Wallis rank sum test, χ 2 = 21.52, DF = 5, P < 0.006 in the wet season, and χ 2 = 18.37, DF = 5, P < 0.002 during the dry season). Goodea atripinnis, A. lacustris, A. robustus, and M. salmoides did not have density differences among sites in either season (Table 2).
Density and biomass of carp (Spearman’s rank correlation, r s = 0.70, P < 0.05; r s = 0.58, P < 0.05; respectively) and the native A. lacustris (Spearman’s rank correlation, r s = 0.38, P > 0.05; r s = 0.33, P < 0.05; respectively) and the introduced Oreochromis spp. (Spearman’s rank correlation, r s = 0.61, P < 0.05; r s = 0.60, P < 0.05; respectively) were positively related to water turbidity values. Introduced P. infans was positively related to turbidity only in terms of density (Spearman’s rank correlation, r s = 0.25, P < 0.05). Chirostoma spp. was the only taxon related negatively to turbidity by biomass (Spearman’s rank correlation, r s = −0.41, P < 0.05). Allophorus robustus, G. atripinnis, and M. salmoides were not related to water turbidity (N = 72) either by density or biomass. Among all species, density and biomass of native G. atripinnis (Spearman’s rank correlation, r s = 0.41, P < 0.05; r s = 0.36, P < 0.05; respectively) and exotic Oreochromis spp. (Spearman’s rank correlation, r s = 0.70, P < 0.05; r s = 0.55, P < 0.05; respectively) had a significant positive relationship with carp. Algansea lacustris (Spearman’s rank correlation, r s = 0.23, P < 0.05) and P. infans (Spearman’s rank correlation, r s = 0.40, P < 0.05) were positively related to carp only in terms of density (N = 72). Carp density and biomass was not negatively related to native species abundance.
The forward selection procedure in the db-RDA analysis revealed a significant effect of plant detritus at the bottom and transparency on species density (P = 0.02 and P = 0.01, respectively). Both variables contributed to the significant association between density of fish species and environmental characteristics (Monte Carlo test; P = 0.03). The first two db-RDA axes accounted for 92.4 % of variability in species abundance-environment relations and 84.6 % of the variation in species data (Table 3). The ordination diagram, defined by the first two db-RDA axes, mainly reflected a plant detritus gradient in the first axis (Fig. 2a). Distribution of site scores in this ordination space reflected a clear break between EMB and the rest of the sites. This break was due mainly to densities of Oreochromis spp., carp and Poeciliopsis infans.
Distribution-based Redundancy Analysis (db-RDA) ordination diagram of species density (a), species biomass (b) and habitat parameters (gray arrows) for six sites in Lake Patzcuaro. Fish species are indicated with black triangles. Sites are indicated with gray circles. Principal coordinate axes are indicated with black arrows. Acronyms for fish species are as in Table 2: (CC) Cyprinus carpio; (CH) Chirostoma spp.; (GA) Goodea atrippinis; (PI) Poeciliopsis infans; (OX) Oreochromis spp. Acronyms for environmental variables are (tra) transparency; (tds) total dissolved solids; (de) depth; (om) organic matter; (av) aquatic vegetation; and (tur) turbidity. Acronyms for site and season are (sw) San Jerónimo wet season; (pw) La Pacanda wet season; (uw) Ucasanastacua wet season; (nw) Napízaro wet season; (hw) Ihuatzio wet season; (ew) Embarcadero wet season: (sd) San Jerónimo dry season; (pd) La Pacanda dry season; (ud) Ucasanastacua dry season; (nd) Napízaro dry season; (hd) Ihuatzio dry season; and (ed) Embarcadero dry season
The forward selection in db-RDA revealed an effect of water transparency and water turbidity on fish biomass (P = 0.002 and P = 0.02, respectively). The relationship between fish species and environmental characteristics was significant (Monte Carlo test; P = 0.006). The first two db-RDA axes accounted for 85.7 % of variability in the species-environment relations, and 79.2 % of the variation in species data (Table 3). The ordination diagram showed that water transparency and water turbidity gradients are related to the first axis (Fig. 2b).
Similarity percentage analyses (SIMPER), based on estimates of fish density, showed that Chirostoma spp., P. infans, and Oreochromis spp. represented more than 90 % of the fish community during both seasons at EMB. At the other five sites, Chirostoma spp. contributed >95 % of the fish community (Table 4). Based on biomass, Chirostoma spp. contributed >90 % of the fish community at SAJ, PAC, and UCA during both seasons. Carp and Chirostoma spp. contributed >90 % of the fish community at NAP and IHU during both seasons. At EMB, carp, Oreochromis spp., and Chirostoma spp. contributed >90 % during both seasons (Table 4).
Diversity varied from 0.01 to 0.5 decits/ind in terms of density and from 0.02 to 0.5 decits/ind in terms of biomass. Evenness varied from 0.02 to 0.5 in terms of density and from 0.1 to 0.6 in terms of biomass (Fig. 3). In terms of density, diversity and evenness were higher at the most turbid sites (IHU and EMB) during the wet season (Kruskal–Wallis rank sum test, H′, χ 2 = 20.80, DF = 5, P < 0.009; J′, χ 2 = 20.85, DF = 5, P < 0.009) (Fig. 3a). During the dry season, EMB had the highest diversity and evenness (Kruskal–Wallis rank sum test, H′, χ 2 = 18.60, DF = 5, P < 0.002; J′, χ 2 = 18.97, DF = 5, P < 0.001) (Fig. 3b). The pattern of diversity in terms of biomass is shown in Fig. 3c, d. In terms of biomass, diversity and evenness were highest at EMB and lowest at PAC (Kruskal–Wallis rank sum test, H′, χ 2 = 11.18, DF = 5, P = 0.047 in the wet season and χ 2 = 23.94, DF = 5, P < 0.002 in the dry season; J′, χ 2 = 11.75, DF = 5, P = 0.038 in the wet season and χ 2 = 23.45, DF = 5, P < 0.003 in the dry season).
Fish diversity (H’) and evenness (J’) in six study sites in Lake Patzcuaro. Fish density is presented for wet (I) and dry seasons (II). Fish biomass is presented for wet (III) and dry (IV) seasons. Columns show the mean ± SD. Superscripts a, b, c refer to among-site differences (Tukey–Kramer honest significant difference [HSD] post hoc test, P < 0.05)
Discussion
Invasive species cause economic or environmental harm and degrade ecosystem integrity, altering nutrient flows and species interactions (NISC 2006; Weber et al. 2010). Carp are described as one of the most detrimental invasive species affecting aquatic ecosystems (Koehn 2004; Lowe et al. 2004; Miller and Crowl 2006). In Mexico, they are considered one of the greatest threats to biodiversity in shallow lakes (Zambrano et al. 2001). Carp are a known threat to native fish fauna in Lake Patzcuaro (Arroyo-Quiroz et al. 2014), and significant federal and state monetary efforts are being directed to control their population in the lake. Our analyses have shed light into how native fish communities might respond to these efforts. By comparing the structure of the fish community in situations with different carp abundances and biomass, we can suggest potential scenarios resulting from efforts to reduce carp population in the lake. Importantly, native fish communities and lake turbidity or other environmental conditions may not respond linearly to reductions in carp abundance.
Our analyses led to three main conclusions. (1) Native species density and biomass did not show negative relationships with carp abundance. Furthermore, carp density and biomass were highest in sites with the greatest diversity and evenness. (2) Although density and biomass of carp were positively related to water turbidity, so were these population aspects for other native and introduced species. (3) Carp were dominant only in terms of biomass during the dry season, at the most turbid sites with plant detritus at the bottom, a variety of aquatic vegetation forms, and water surface covered mainly by water hyacinth. Carp were rare at deep and shallow sites with rocky substrates and scarce aquatic vegetation.
Assessing the status and effects of carp in Lake Patzcuaro requires a review of three key issues: (1) the interactions between carp and other species, (2) the potential impact from carp behavior on habitat, and (3) the adaptation of carp to ecosystem change. In the following lines, we explore these issues incorporating our findings to larger scale management issues in the lake.
Interactions Between Carp and Other Species
Carp affect aquatic ecosystems by indirectly restructuring communities (Weber and Brown 2009) and reducing the abundance of native fishes (Weber and Brown 2011). We hypothesized that carp would be the dominant species at all sites in Lake Patzcuaro regardless of habitat characteristics, and that its abundance would be negatively related to native species abundance. Contrary to our expectations, the contribution (in terms of biomass) of carp to the fish community was relevant only in a few sites and their density and biomass was frequently equaled or surpassed by those of other taxa (Chirostoma spp., P. infans [adults of both species are >5 times shorter and >50 times lighter than carp adults] and Oreochromis spp.). Further, we found, as did Berlanga-Robles et al. (1997, 2002) that native species density and biomass were independent of carp density and biomass. These authors analyzed gillnet (12–16 mm mesh size) Lake Patzcuaro commercial fisheries, and found that Chirostoma spp. were the most abundant taxa (92 % of their catch) and carp were rare (0.1 % of their catch). Further, they found no evidence for negative impacts of carp on native fish species, as had been found by Zambrano and Macías-García (2000), Miller and Crowl (2006), and Weber and Brown (2011) for non-natural systems.
The current biomass (<20 kg ha−1) (Zambrano et al. 2011) of common carp in Lake Patzcuaro is small compared to that in other systems such as Lake Naivasha in Africa (>370 kg ha−1) (Britton et al. 2007), South Lancashire Lake in United Kingdom (~380 kg ha−1) (Linfield, 1980) and Hennepin and Hopper Lakes in USA (~250 kg ha−1) (Bajer et al., 2009). Carp biomass in the system may decline further in coming years. In Lake Patzcuaro, all fisheries have declined from ~2500 t in 1988 to <50 t in 2007 and carp catches declined from >600 t in 1988 (Gaspar-Dillanes et al. 2006) to <20 t in 2007 (Diario Oficial de la Federación 2010) and <10 t in 2009 (Zambrano et al. 2011). These data suggest that the carp fishery (Ramírez-Herrejón et al. 2014b) in Lake Patzcuaro; along with those for other fishes, are dwindling. Thus, the observed ecosystem-wide fisheries decline may not be attributable to the effect of a single species.
Vegetation removal is one of the ways carp is known to impact the littoral zone of lakes (Scheffer et al. 2003; Ozbay 2008). The extent of damage depends on the size and density of carp and the type of aquatic vegetation in the system (Zambrano et al. 2001; Miller and Crowl 2006; Adámek and Maršálek 2013). Our data from Lake Patzcuaro, showed site EMB had the greatest density and biomass of carp, and several aquatic vegetation forms: rooted and submerged, rooted with floating leaves, rooted and emergent, and floating (Huerto-Delgadillo and Amador-García 2011). The southern zone of Lake Patzcuaro (where EMB is located) presents more diversity and biomass of aquatic vegetation than the rest of lake (Huerto-Delgadillo and Amador-García 2011). High quantities of macrophyte biomass can increase plant detritus at bottom and primary productivity in lakes (Carpenter and Lodge 1986). Hence, we contend that the density and biomass of common carp in Lake Patzcuaro is likely positively related to vegetation debris in the lake bottom, which is also located in turbid habitats. This is similar to the findings of Britton et al. (2007), who described a positive relationship between the presence of macrophytes and greater carp abundance in Lake Naivasha, Kenya.
Potential Impact from Carp Behavior on Habitat
Bioturbation caused by fish impacts the functioning of aquatic ecosystems, mainly by increasing nutrient levels, turbidity and seston particle dynamics, which in turn affect the development of phytoplankton and submersed macrophytes (Qin and Threlkeld 1990). In experimental ponds, benthic feeding of confined carp resuspends sediments and increases water turbidity and nutrients (Breukelaar et al. 1994; Roberts et al. 1995; Lougheed et al. 1998; Kloskowski 2011). Studies of artificial systems conclude that unconfined carp populations cause the same effects in shallow lakes (Zambrano et al. 1999, 2001; Scheffer et al. 2003; Miller and Crowl 2006; Parkos et al. 2006; Wolfe et al. 2009). However, the impact is density dependent; higher biomass of carp (476 kg ha−1) had a greater effect on nutrients, turbidity, and suspended solids than a low biomass (174 kg ha−1; Roberts et al. 1995).
Water turbidity levels and habitat characteristics (e.g., depth, bottom type, aquatic vegetation cover) in Lake Patzcuaro have been affected by several factors: watershed deforestation and increased erosion for over 30 years (Chacón-Torres 1993); increased nutrient loading since the 1940s leading to phytoplankton blooms (Berry et al. 2011); direct discharge of untreated waste for over 30 years (Rosas et al. 1985); organic matter buildup from water hyacinth removal efforts for over 20 years (Orbe-Mendoza et al. 2002); and dredging of navigation channels for over 10 years (Rodríguez-Arteaga and Zarazúa-Sánchez 2009). Further, Gómez-Tagle et al. (2002) found that nutrient loading and sedimentation in Lake Patzcuaro are consequences of watershed degradation; Alcocer and Bernal-Brooks (2002) showed that the main cause of sediment re-suspension is wind action; and Sánchez-Chávez et al. (2011) argued that Lake Patzcuaro experiences climatic influences that have led to a decrease in water volume, increased nutrient concentrations, and facilitation of sediment re-suspension. The latter also found that the quantity of chlorophyll a often exceeds the hypertrophic limit (25 µg/L) and that the water turbidity gradient could be explained by primary productivity and particle sedimentation. Finally, Alcocer and Bernal-Brooks (2002) argued that increased human activities on the watershed and near Lake Patzcuaro are positively associated with water turbidity, nutrient loading, and sedimentation. All these lines of evidence, in addition to our results lead us to propose that water turbidity and habitat change in Lake Patzcuaro cannot be directly and solely caused by carp. It is possible that carp are a synergistic factor in promoting increased turbidity locally within the lake, but their relatively low dominance throughout the system suggests that other impact sources might carry greater weight in causing increased turbidity levels in the lake.
Adaptation of Carp to Ecosystem Change
Habitats generated as a consequence of severe environmental alterations can favor resistant species (Byers 2002). Carp is considered a tolerant species, capable of resisting anthropogenic changes (Maceda-Veiga and De Sostoa 2011). It lives under a wide range of abiotic and biotic conditions (Weber and Brown 2009, 2011). Its ability to survive is related to adaptability to water turbidity variations which is an advantage over other fish inhabiting degraded systems (Zambrano et al. 1999; Zambrano and Macías-García 2000; Zambrano et al. 2001; Koehn 2004). Our results show that the main abundance and dominance of carp occurs at EMB, a shallow habitat with detritus at bottom and a surface covered by floating aquatic vegetation. This zone of the lake is characterized by high levels of pollution associated to wastewater discharges (Sánchez-Chávez et al. 2011). Based on our findings, we propose that carp can take advantage of impacted habitats in Lake Patzcuaro, and makes comparatively little use of less impacted areas where food resources may be limiting. It is possible that this shallow lake, with large inputs of plant detritus, provides many opportunities to benthic species such as carp. We found that the density of carp was comparatively low at sites with mud or rock on the bottom. According to Britton et al. (2007), in Lake Naivasha, numerous food resources in the benthos were underutilized by native species, and introduced carp can make use of these niches.
The resistance of carp to anthropogenic environmental alterations is not directly related with its dominance on the structure of the fish assemblages. The dominance of carp in Lake Patzcuaro can be dissembled from a diverse array of biotic and abiotic factors and anthropogenic impacts occurring simultaneously in the system. This agrees with the findings by Light and Marchetti (2006) who mention that the size of the ecosystem, habitat complexity, biological composition and structure, and location are factors that affect the outcome of an invasion of non-native species. Mitchell and Knouft (2008) argue that, in freshwater systems, abiotic characteristics can restrict ecological impacts by invasive species on fish community structure. Further, the trophic ecology of all fish taxa in Lake Patzcuaro have revealed a reduction in length and complexity of trophic web (Ramírez-Herrejón et al. 2014a), which is considered an ecosystem degradation symptom more alarming than the potential effect of carp on the fish community, trophic structure, and trophic web.
While we have provided strong evidence about the role of carp in today’s Lake Patzcuaro fish communities and how they may be impacting the ecosystem, we recognize that our data are limited in space and time. We sampled only six sites in the lake; while these sites are representative of habitat conditions in other areas of the system, it is still possible that fish community data from additional sites could improve our understanding of community dynamics and spatial habitat use. Our analysis is also based on data from only two sampling seasons in 2 years. Better understanding of community dynamics would have resulted from analyses of long-term datasets that could have helped our understanding of interannual variability. These data are unfortunately not available. The only data that can be interpreted over time for Lake Patzcuaro are that from fishery landings; but these data have their own set of limitations. We believe that our sampling efforts offer good evidence to aid management activities in the lake.
Our data and other evidence discussed above suggest that degraded environmental conditions in Lake Patzcuaro cannot be solely attributable to carp. With over 40 years of presence in the ecosystem, this species is now integrated to trophic processes in the lake, and is exposed, along with other native and non-native fishes, to numerous environmental challenges throughout the lake. We recognize that carp have probably had some effect over time on Lake Patzcuaro fisheries. They remain an exotic species which must be monitored to prevent future damages to certain areas of the lake or native species.
Carp Control Initiative or Better Habitat Restoration Management
At least two strategies had been implemented to control carp in the lake, the provision of fishing gear specific to this species and failed attempts to subsidize its marketing (Arroyo-Quiroz et al. 2014). Unfortunately, for more than 10 years there was no interaction between fishers and federal agencies responsible for the regulation of fishing, and such strategies did not work (Huerto pers. comm. 2014). Although it is fundamental not to neglect the potential detrimental effect from carp, enhanced by the eutrophic condition of the lake, it is perhaps more important to address other problems that threaten the permanence of the entire ecosystem.
Recommendations
Our results suggest that management and conservation efforts in Lake Patzcuaro should focus on restoration and protection of habitat, natural ecosystem processes, and ecosystem services, and perhaps focus less on eradicating common carp to reinstate species of conservation concern. This does not mean that there is a lack of restoration initiatives for the lake. Several management proposals have been established, and one of the most important was structured in the year 2000 (Plan Pátzcuaro 2000: Investigación multidisciplinaria para el desarrollo sosteniso; Plan Pátzcuaro 2000: Multidisciplinary research for sustainable development, Fundación Friedrich Ebert). Solving Lake Patzcuaro’s problems requires the involvement and coordination of different federal and academic institutions with producers and local inhabitants of the region. It also requires the efficient allocation of resources following a sequential implementation plan.
General Conclusions
In our study, the presence and abundance of carp was not related with native fish habitat loss, aquatic vegetation destruction, loss of diversity, or negative effects on native fish abundance. The abundance of carp was dependent on plant detritus at the bottom and aquatic vegetation cover. Our analyses provided evidence that water turbidity in Lake Patzcuaro is caused mainly by wind-driven sediment resuspension and primary productivity.
Currently, all commercial fisheries of Lake Patzcuaro are collapsed, and two exotic species (Nile tilapia and carp) represent the most important subsistence fishery. However, the remaining population of carp, dependent on large inputs of plant detritus, may decline further in coming years.
Lake Patzcuaro shows functional degradation signs such as the loss of trophic web structure, which represents a more urgent problem, than the effect of common carp.
References
Adámek Z, Maršálek B (2013) Bioturbation of sediments by benthic macroinvertebrates and fish and its implication for pond ecosystems: a review. Aquacult Int 21:1–17. doi:10.1007/s10499-012-9527-3
Alaye N (2006) Actualización de la información técnica para el manejo pesquero del Lago de Pátzcuaro y actividades relativas a la ejecución del Plan de Manejo. CRIP-Pátzcuaro. INAPESCA, Mexico
Alcocer-Durand J, Bernal-Brooks FW (2002) Spatial and temporal heterogeneity of physical and chemical variables for an endorheic, shallow water body: Lake Patzcuaro, México. Arch Hydrobiol 155:239–253
Arroyo-Quiroz I, Flores-Armillas VH, Huerto-Delgadillo R, Pérez Gil-Salcido R (2014) Estrategia para apoyar la recuperación de peces nativos en el lago de Pátzcuaro a través del manejo de especies exóticas: propuestas para el manejo de la carpa común Cyprinus carpio. In: Huerto-Delgadillo R, Vargas-Velázquez S (eds) Estudio ecosistémico del lago de Pátzcuaro: aportes en gestión ambiental para el fomento del desarrollo sustentable. Instituto Mexicano de Tecnología del Agua, Comisión Nacional del Agua, Secretaría de Medio Ambiente y Recursos Naturales, México, pp 137–174
Bajer PG, Sullivan G, Sorensen PW (2009) Effects of a rapidly increasing population of common carp on vegetative cover and waterfowl in a recently restored Midwestern shallow lake. Hydrobiologia 632:235–245. doi:10.1007/s10750-009-9844-3
Barbour MT, Gerritsen J, Zinder BD, Stribling JB (1999) Rapid bioassessment protocols for use in streams and wadeable rivers: periphyton, benthic macroinvertebrates and fish. U.S. Environmental Protection Agency, Office of Water, Washington, DC
Barriga-Sosa I, Ibáñez-Aguirre AL, Arredondo-Figueroa JL (2002) Morphological and genetic variation in seven species of the endangered Chirostoma “humboldtianum species group” (Atheriniformes: Atherinopsidae). Rev Biol Trop 50:199–216
Berlanga-Robles C, Madrid VJ, Ruiz A (2002) Fish abundance and trophic structure from the commercial catch in Lake Patzcuaro, Mexico. Hydrobiologia 467:117–122. doi:10.1023/A:1014965504486
Bernal-Brooks FW, Gómez TR, Alcocer J (2002) Lake Patzcuaro (Mexico): a controversy about the ecosystem water regimen approached by field references, climatic variables, and GIS. Hydrobiologia 467:187–197. doi:10.1023/A:1014919032228
Berry JP, Lee E, Walton K, Wilson A, Bernal-Brooks F (2011) Bioaccumulation of microcystins by fish associated with a persistent cyanobacterial bloom in Lago de Pátzcuaro (Michoacan, Mexico). Environ Toxicol Chem 30:1621–1628
Bloom DD, Piller KR, Lyons J, Mercado-Silva N, Medina-Nava M (2009) Systematics and biogeography of the silverside tribe Menidiini (Teleostomi: Atherinopsidae) based on the mitochondrial ND2 gene. Copeia 2:408–417. doi:10.1643/CI-07-151
Bouyoucos G (1936) Directions for making mechanical analyses of soils by the hydrometer method. Soil Sci 42:225–230
Bravo-Espinosa M, Fregoso-Tirado LE, Medina-Orozco LE (2006) Parámetros de erosionabilidad del modelo WEPP para andosoles con uso pecuario en la cuenca del Lago de Pátzcuaro, Michoacán. Téc Pecu Méx 44:129–141
Breukelaar AW, Lammens EHRR, Breteler JGPK, Tatrai I (1994) Effects of benthivorous bream (Abramis brama) and carp (Cyprinus carpio) on sediment resuspension and concentrations of nutrients and chlorophyll a. Fresh Biol 32:113–121. doi:10.1111/j.1365-2427.1994.tb00871.x
Britton JR, Boar RR, Grey J, Foster J, Logonzo J, Harper DM (2007) From introduction to fishery dominance: the initial impacts of the invasive carp Cyprinus carpio in Lake Naivasha, Kenya, 1999 to 2006. J Fish Biol 71:239–257. doi:10.1111/j.1095-8649.2007.01669.x
Britton JR, Cucherousset J, Davies GD, Godard MJ, Copp GH (2010) Non-native fishes and climate change: predicting species responses to warming temperatures in a temperate region. Freshw Biol 55:1130–1141. doi:10.1111/j.1365-2427.2010.02396.x
Byers JE (2002) Impact of non-indigenous species on natives enhanced by anthropogenic alteration of selection regimes. Oikos 97:449–458. doi:10.1034/j.1600-0706.2002.970316.x
Carpenter SR, Lodge DM (1986) Effects of submersed macrophytes on ecosystem processes. Aquat Bot 26:341–370. doi:10.1016/0304-3770(86)90031-8
Chacón-Torres A (1993) Lake Patzcuaro, Mexico: watershed and water quality deterioration in a tropical high-altitude Latin American lake. Lake Reserv Manage 8:37–47. doi:10.1080/07438149309354457
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation. Primer-E, Ivybridge
Cucherousset J, Olden JD (2011) Ecological impacts of non-native freshwater fishes. Fisheries 36:215–230. doi:10.1080/03632415.2011.574578
De Buen F (1944) Los lagos michoacanos. II. El lago de Patzcuaro. Rev Soc Mex Hist Nat 5:99–125
Diario Oficial de la Federación (2010) Carta nacional pesquera. Mexico City
Espinosa-Huerta E, García-Prieto L, Pérez-Ponce de León G (1996) Helminth community structure of Chirostoma attenuatum (Osteichthyes: Atherinidae) in two Mexican Lakes. Southwest Nat 41:288–292
Gaspar-Dillanes MT, Rojas P, Fernández JI, Díaz-Rubin MP (2006) El Lago de Pátzcuaro. Sustentabilidad y pesca responsable en México. Instituto Nacional de la Pesca (INP), Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación (SAGARPA), México, pp 391–418
Gómez-Tagle AF, Bernal-Brooks FW, Alcocer J (2002) Sensitivity of Mexican water bodies to regional climatic change: three study alternatives applied to remote sensed data of Lake Patzcuaro. Hydrobiologia 467:169–176. doi:10.1023/A:1014962831319
Hinojosa-Garro D, Zambrano L (2004) Interactions of common carp (Cyprinus carpio) with benthic crayfish decapods in shallow ponds. Hydrobiologia 515:115–122. doi:10.1023/B:HYDR.0000027323.77213.39
Huerto-Delgadillo R, Amador-García A (2011) Evaluación y análisis de la vegetación acuática y bases para su control. In: Huerto-Delgadillo R, Vargas-Velázquez R, Ortíz-Paniagua CF (eds) Estudio ecosistémico del Lago de Pátzcuaro. Aportes en gestión ambiental para el fomento del desarrollo sustentable. Instituto Mexicano de Tecnología del Agua, Universidad Autónoma del Estado de Morelos, Universidad Michoacana de San Nicolás de Hidalgo, México, pp 49–86
Kloskowski J (2011) Differential effects of age-structured common carp (Cyprinus carpio) stocks on pond invertebrate communities: implications for recreational and wildlife use of farm ponds. Aquacult Int 19:1151–1164
Koehn JD (2004) Carp (Cyprinus carpio) as a powerful invader in Australia waterways. Freshw Biol 49:882–894. doi:10.1111/j.1365-2427.2004.01232.x
Legendre P, Anderson MJ (1999) Distance-based redundancy analysis: testing multispecies responses in multifactorial ecological experiments. Ecol Monogr 69:1–24. doi:10.1890/0012-9615(1999)069[0001:DBRATM]2.0.CO;2
Lepš J, Šmilauer P (2003) Multivariate analysis of ecological data using CANOCO. Cambridge University Press, Cambridge
Leung B, Lodge DM, Finnoff D, Shogren JF, Lewis MA, Lamberti G (2002) An ounce of prevention or a pound of cure: Bioeconomic risk analysis of invasive species. Proc R Soc Lond 269:2407–2413. doi:10.1098/rspb.2002.2179
Light T, Marchetti MP (2006) Distinguishing between invasions and habitat changes as drivers of diversity loss among California’s freshwater fishes. Conserv Biol 21:434–446. doi:10.1111/j.1523-1739.2006.00643.x
Linfield RSJ (1980) Catchability and stock density of common carp, Cyprinus carpio L. in a Lake fishery. Aquacult Res 11:11–22. doi:10.1111/j.1365-2109.1980.tb00277.x
Lougheed V, Crosbie B, Chow-Fraser P (1998) Predictions on the effect of common carp (Cyprinus carpio) exclusion on water quality, zooplankton, and submergent macrophytes in a Great Lakes wetland. Can J Fish Aquat Sci 55:1189–1197. doi:10.1139/f97-315
Lowe S, Browne M, Boudjelas S, De Poorter M (2004) 100 of the world’s worst invasive alien species: a selection from the Global Invasive Species Database. The Invasive Species Specialist Group, World Conservation Union, Auckland
Maceda-Veiga A, De Sostoa A (2011) Observational evidence of the sensitivity of some fish species to environmental stressors in Mediterranean rivers. Ecol Indic 11:311–317. doi:10.1016/j.ecolind.2010.05.009
Magalhães MF, Beja P, Canas C, Collares-Pereira MJ (2002) Functional heterogeneity of dry-season fish refugia across a Mediterranean catchment: The role of habitat and predation. Freshw Biol 47:1919–1934. doi:10.1046/j.1365-2427.2002.00941.x
Magurran AE (2004) Measuring biological diversity. Blackwell Publishing, London
McArdle BH, Anderson MJ (2001) Fitting multivariate models to community data: A comment on distance-based redundancy. Ecology 82:290–297. doi:10.1890/0012-9658(2001)082
Mendoza-Garfias B, García-Prieto L, Pérez-Ponce de León G (1996) Helmintos de la “acúmara” Algansea lacustris en el Lago de Pátzcuaro, Michoacán, México. An Inst Biol Univ Nac Auton Mex Ser Zool 67:77–88
Miller SA, Crowl TA (2006) Effects of common carp (Cyprinus carpio) on macrophytes and invertebrate communities in a shallow lake. Freshw Biol 51:85–94. doi:10.1111/j.1365-2427.2005.01477.x
Miller R, Minckley WL, Norris SM (2005) Freshwater fishes of Mexico. The University of Chicago Press, Chicago
Mitchell AL, Knouft JH (2008) Non-native fishes and native species diversity in freshwater fish assemblages across the United States. Biol Invasions 11:1441–1450. doi:10.1007/s10530-008-9352-9
National Invasive Species Council (NISC) (2006) Invasive species definition clarification and guidance white paper. National Invasive Species Information Center, U.S. Department of Agriculture. http://www.invasivespeciesinfo.gov/laws/execorder.shtml#sec1. Accessed 31 July 2014
Orbe-Mendoza A, Acevedo-Mendoza J, Lyons J (2002) Lake Patzcuaro fishery management plan. Rev Fish Biol Fisher 12:207–217. doi:10.1023/A:1025087705940
Ozbay H (2008) An enclosure experiment to test the effects of common carp on the water quality in a shallow Turkish soda lake. Fresen Environ Bull 17:2078–2082. http://www.psp-parlar.de/details_feb_afs_.asp?typ=feb&datum=15.12.2008&jahr=2008
Palacios E, Racotta IS, Aparicio B, Arjona O, Martínez-Palacios CA (2007) Lipid classes and fatty acids during embryogenesis of captive and wild silverside (Chirostoma estor estor) from Patzcuaro Lake. Fish Physiol Biochem 33:81–91. doi:10.1007/s10695-006-9119-0
Parkos JJ, Santucci VJ, Wahl DH (2006) Effectiveness of a plastic mesh substrate cover for reducing the effects of common carp on aquatic ecosystems. N Am J Fish Manag 26:861–866. doi:10.1577/M06-020.1
Peresbarbosa-Rojas E, Pérez-Ponce de León G, García-Prieto L (1994) Helmintos parásitos de tres especies de peces (Goodeidae) del Lago de Pátzcuaro, Michoacán. An Inst Biol Univ Nac Auton Mex Ser Zool 65:201–204
Pérez-Ponce de León G, García-Prieto L, León-Règagnon V, Choudhury A (2000) Helminth communities of native and introduced fishes in Lake Patzcuaro, Michoacán, México. J Fish Biol 57:303–325. doi:10.1111/j.1095-8649.2000.tb02174.x
Qin JG, Threlkeld ST (1990) Experimental comparison of the effects of benthivorous fish and planktivorous fish on plankton community structure. Arch Hydrobiol 119:121–141
Ramírez-Herrejón JP, Castañeda-Sam LS, Moncayo-Estrada R, Caraveo-Patiño J, Balart EF (2013) Trophic ecology of the exotic Lerma livebearer Poeciliopsis infans (Cyprinodontiformes: Poeciliidae) in the Lago de Pátzcuaro, Central Mexico. Rev Biol Trop 61:1289–1300
Ramírez-Herrejón JP, Balart-Páez EF, Caraveo-Patiño J et al (2014a) Trophic interrelationships between invasive common carp (Cyprinus carpio) and fish community in the Lago de Pátzcuaro, Central Mexico. Acta Ichthyol Piscat 44:45–58. doi:10.3750/AIP2014.44.1.06
Ramírez-Herrejón JP, Zambrano L, Mercado-Silva N et al (2014b) Long term changes in the fish fauna of Lago de Pátzcuaro in Central Mexico. Lat Am J Aquat Res 42:137–149. doi:10.3856/vol42-issue1-fulltext-11
Roberts J, Chick A, Oswald L, Thompson P (1995) Effect of carp, Cyprinus carpio L., an exotic benthivorous fish, on aquatic plants and water quality in experimental ponds. Mar Freshw Res 46:1171–1180. doi:10.1071/MF9951171
Rodríguez-Arteaga JM, Zarazúa-Sánchez R (2009) Rehabilitación y mantenimiento del Lago de Pátzcuaro. Fisheries Commission Michoacán State Government (Technical report)
Rosas M (1976) Datos biológicos de la ictiofauna del lago de Pátzcuaro, con énfasis en la alimentación del sus especies. Memorias del Simposio sobre Pesquerías en Aguas Continentales. Tuxtla Gutiérrez, Chiapas, pp 299–436
Rosas I, Mazari M, Saavedra J, Báez P (1985) Benthic organisms as indicators of water quality in Lake Patzcuaro, México. Water Air Soil Pollut 25:401–414
Sánchez-Chávez J, Bravo-Inclán L, Tomasini-Ortíz C, Bernal-Brooks F (2011) Calidad del agua del Lago de Pátzcuaro. In: Huerto-Delgadillo R, Vargas-Velázquez R, Ortíz-Paniagua CF (eds) Estudio ecosistémico del Lago de Pátzcuaro. Aportes en gestión ambiental para el fomento del desarrollo sustentable. Instituto Mexicano de Tecnología del Agua, Universidad Autónoma del Estado de Morelos, Universidad Michoacana de San Nicolás de Hidalgo, México, pp 29–48
SAS Institute (1995) JMP, Statistic Mode Visual Version, 3.1.6.2. SAS Institute, USA
Scheffer M, Portielje R, Zambrano L (2003) Fish facilitate wave resuspension of sediment. Limnol Oceanogr 48:1920–1926
Sokal RR, Rohlf FJ (1969) Biometría. Ed. Blume, Barcelona
Ter Braak CJF, Smilauer P (2002) Canoco reference manual and CanoDraw for Windows user’s guide: software for canonical community ordination. Version 4.5. Microcomputer Power, NY, USA
Vitousek PM, D’Antonio CM, Loope LL, Westbrooks R (1996) Biological invasions as global environmental change. Am J Sci 84:468–478
Weber MJ, Brown ML (2009) Effects of common carp on aquatic ecosystems 80 years after “carp as a dominant”: ecological insights for fisheries management. Rev Fish Sci 17:524–537. doi:10.1080/10641260903189243
Weber MJ, Brown ML (2011) Relationships among invasive common carp, native fishes and physicochemical characteristics in upper Midwest (USA) lakes. Ecol Fresh Fish 20:270–278. doi:10.1111/j.1600-0633.2011.00493.x
Weber MJ, Brown ML, Willis DW (2010) Spatial variability of common carp populations in relation to lake morphology and physicochemical parameters in the upper Midwest United States. Ecol Fresh Fish 19:555–565. doi:10.1111/j.1600-0633.2010.00436.x
Wolfe MD, Santucci VJ, Einfalt LM, Wahl DH (2009) Effects of common carp on reproduction, growth, and survival of largemouth bass and bluegills. Trans Am Fish Soc 138:975–983. doi:10.1577/T08-115.1
Zambrano L, Macías-García C (2000) Impact of introduced fish for aquaculture in Mexican freshwater systems. In: Claudi R, Leach JH (eds) Non-indigenous freshwater organisms: vectors, biology and impacts. Lewis Publishers, USA, pp 113–124
Zambrano L, Perrow MR, Macías-García C, Aguirre-Hidalgo V (1999) Impact of introduced carp (Cyprinus carpio) in subtropical shallow ponds in Central Mexico. J Aquat Ecosyst Stress Recovery 6:281–288. doi:10.1023/A:1009958914016
Zambrano L, Scheffer M, Martínez-Ramos M (2001) Catastrophic response of lakes to benthivorous fish introduction. Oikos 94:334–350. doi:10.1034/j.1600-0706.2001.940215.x
Zambrano L, Martinez-Meyer E, Menezes N, Towsend P (2006) Invasive potential of common carp (Cyprinus carpio) and Nile tilapia (Oreochromis niloticus) in American fresh water systems. Can J Fish Aquat Sci 63:1903–1910. doi:10.1139/f06-088
Zambrano L, Cordova-Tapia F, Ramírez-Herrejón JP et al (2011) Las especies exóticas en el lago de Pátzcuaro, Michoacán, México. In: Huerto-Delgadillo R, Vargas-Velázquez R, Ortíz-Paniagua CF (eds) Estudio ecosistémico del Lago de Pátzcuaro. Aportes en gestión ambiental para el fomento del desarrollo sustentable. Instituto Mexicano de Tecnología del Agua, Universidad Autónoma del Estado de Morelos, Universidad Michoacana de San Nicolás de Hidalgo, México, pp 133–156
Zar JH (1999) Biostatistical analysis. Prentice Hall, New Jersey
Acknowledgments
The authors thank members of the Aquatic Biology Laboratory “Javier Alvarado Díaz” of Universidad Michoacana de San Nicolás de Hidalgo (UMSNH); M. M. Herrejón-Almanza, and J. J. Ramírez-Becerra for their logistical support; UMSNH students A. Torres, L. S. Castañeda, L. A. García, B. Vital, D. Montejo, C. E. Díaz, and A. F. Mar; R. Quirino, B. Quirino, and A. Quirino for assistance in fish collections; L. H. Escalera-Vázquez provided assistance with statistical analysis; I. Fogel of Centro de Investigaciones Biológicas del Noroeste (CIBNOR) provided editorial services and C. Silva-Bejarano for technical support. This work was funded by Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO) project GN049 and CIBNOR. R.M.E. is supported by the Comisión de Operación y Fomento de Actividades Académicas (COFAA) and Estimulo al Desempeño Académico (EDI). J.P.R.H. is grateful to Consejo Nacional de Ciencia y Tecnología (CONACYT) and the Universidad Autónoma de Querétaro (UAQ) for the facilities provided for the development of this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramírez-Herrejón, J.P., Mercado-Silva, N., Balart, E.F. et al. Environmental Degradation in a Eutrophic Shallow Lake is not Simply Due to Abundance of Non-native Cyprinus carpio . Environmental Management 56, 603–617 (2015). https://doi.org/10.1007/s00267-015-0524-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00267-015-0524-y