Abstract
Due to their multipurpose use, leguminous trees are desirable for the restoration of degraded ecosystems. Our aim was to investigate seed germination of the leguminous tree Prosopis chilensis in response to salinity, one of the major abiotic challenges of desertified soils. Germination percentages of seed from 12 wild P. chilensis populations were studied. Treatments included four aqueous NaCl concentrations (150, 300, 450, and 600 mM). In each population, the highest germination percentage was seen using distilled water (control), followed closely by 150 mM NaCl. At 300 mM NaCl or higher salt concentration, germination was progressively inhibited attaining the lowest value at 450 mM NaCl, while at 600 mM NaCl germination remained reduced but with large variation among group of samples. These results allowed us to allocate the 12 groups from where seeds were collected into three classes. First, the seeds from Huanta-Rivadavia showed the lowest percent germination for each salt condition. The second group was composed of moderately salt-tolerant seeds with 75 % germination at 300 mM NaCl, followed by 50 % germination at 450 mM NaCl and 30 % germination at 600 mM NaCl. The third group from Maitencillo and Rapel areas was the most salt tolerant with an impressive seed germination level of 97 % at 300 mM NaCl, 82 % at 450 mM NaCl, and 42 % at 600 mM NaCl. Our results demonstrate that P. chilensis seeds from these latter localities have an increased germination capability under saline stress, confirming that P. chilensis is an appropriate species to rehabilitate desertified soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Desertification is defined as “the land degradation in arid, semiarid and dry sub-humid areas resulting from various factors, including climatic variations and human activities” (UNEP 1992). In Chile, 49.1 % of the territory underwent some degree of soil erosion and in particular, the Coquimbo Region has 84 % of its soil affected by erosion, with 65.3 % of it categorized as extremely or severely degraded (CIREN 2011). The afforestation and reforestation program proposal using plant species adapted to arid and semiarid environments has been recognized as the best option to restore social, economic, and ecological value of impoverished lands (Koohafkan 1996; Ondrasek et al. 2011).
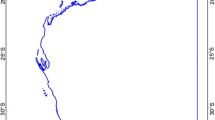
Among plants identified in affected lands, Algarrobo, or Chilean mesquite (Prosopis chilensis (Mol.) Stuntz), a tree belonging to Fabaceae family found naturally in dry regions of Peru, Bolivia, Chile, and Argentina (Galera 2000) is a good alternative for reclamation of degraded ecosystems, due to its nutritional value, capacity to fix nitrogen, resistance to drought, and ability to grow in saline soils (Serra 1997; Montandon et al. 2011). In Chile, wild Algarrobo forests are found in arid/semiarid regions from Copiapo River (Atacama Region; 27°S) to Maule River (Maule Region, 37°S) (Santibáñez et al. 2008). Unfortunately, current populations have been decimated to few fragmented forests located between marginal areas of the Intermediate Depression and Andean foothills (Fig. 1). Luebert and Pliscoff (2006) reported that the original forest of this species has been reduced from 3425 to 1316 km2 (62 % loss). In man-occupied sites, trees are dispersed or form small patches, exposed to several abiotic stresses such as poor soil structure, human pressure exerted by agricultural activities, grazing, use of biomass as fuel, and one of the highest solar irradiances of the planet (Chapin et al. 2001; Peñuelas and Filella 2001; Parmesan and Yohe 2003; CNE 2009). In view of this lack of recruitment observed across the original habitat (personal observation) and recent evidence that current adult trees are in senescent stage with chances to disappear (Valdivia and Romero 2013), the prospect for survival is grim. Moreover, according to a recent report on the effect of global climate change on Chilean biodiversity, it is suggested that the Mediterranean Spiny Forest formed by Acacia caven and Prosopis chilensis species would be one of the three most affected ecosystems by 2050 (PAN 2014). Accordingly, P. chilensis is currently classified as a vulnerable species (Squeo et al. 2007; Avilés 2012).
Geographical distribution of Prosopis chilensis sampled in this study. The green area in the map (left) shows the natural distribution of P. chilensis in Chile. This area is part of the arid/semiarid climate in Chile. The zoomed map section (upper center) shows the geographical location of the 12 areas evaluated in this study. Pictures on the right show two of these 12 populations, corresponding to Maitencillo (up) and Matancilla (down), respectively (Color figure online)
Spontaneous recruitment of P. chilensis in this habitat is associated with the occurrence of El Niño Southern Oscillation (ENSO) phenomenon (Holmgren 2006a; Squeo et al. 2007). During the ENSO period, intense rainfalls triggered by anomalous heating of Pacific Ocean surface in the Northern coast of Chile (Rutland and Fuenzalida 1991; Montecinos and Aceituno 2003) increase soil water availability that stimulates germination of dormant seeds (Vidiella and Armesto 1989). The last ENSO event occurred in 1997 and, at least, 10 of these events happened during the last century (Holmgren and Scheffer 2001). Following ENSO rainfall period, strong sun irradiance increases soil evaporation and decreases water availability. As a consequence, salts deposited in deeper layers diffuse to the surface, induce ion accumulation, and exacerbate the Na+:Ca2+, Na+:K+, Ca2+:Mg2+, and Cl−:NO3 ratios, thus triggering salt stress to plants (Munns and Tester 2008). At seed level, the redistribution of toxic Na and Cl ions affects the embryo and impairs germination, and creates an osmotic imbalance affecting root growth and overall performance of new born plants (Grattan and Grieve 1999; Almansouri et al. 2001; Denby and Gehring 2005; Araújo et al. 2006; Adolf et al. 2013). In response to saline soils, germinating seeds modify their physiology to avoid the deleterious effects of toxic ions (e.g., Na+ and Cl−) (Gul and Khan 2002; Munns 2005; Läuchi and Grattan 2007). Meanwhile, salinity may decrease seed germination and seedling growth efficiency among halophytes (P. chilensis) tolerant to salinity (Felker et al. 1981; Cazebonne et al. 1999). In addition, leguminous plants are affected by saline stress reducing the ability to grow and fix nitrogen during subsequent developmental stage (Bekki et al. 1987; Dupont et al. 2012).
P. chilensis is an allogamous plant (Pasiecznik et al. 2001) with high genetic variability (Mottura et al. 2005; Verga and Greogorius 2007) that can express different phenotypes in response to biotic or abiotic stress. In this context, the soil salt content must induce adaptive responses in seeds and/or seedlings of P. chilensis when facing salt stress (Blun 1998; IRRI 2006; Läuchi and Grattan 2007). We hypothesized that this adaptive response must be conserved but with some variability along its entire distribution range in Chile. This paradigm is supported by data from Plaza (2010) who reported by microsatellite analysis on P. chilensis across the Elqui, Limarí, and Choapa valleys (Coquimbo Region) the existence of allelic differences. Therefore, to use P. chilensis in afforestation or reforestation programs on desertified lands, a prior selection of seeds that can successfully withstand possible abiotic stress is appropriate .
This study aimed to compare the germination of P. chilensis seeds from Chilean arid and semiarid areas to evaluate their response to saline stress. The data do not represent a comprehensive study among populations due to the scarceness of this species in their habitat; therefore, we provide a partial account of the germination rates of P. chilensis from 12 sites distributed along a 450 km transect that represents 80 % of its current distribution in Chile. We evaluated the seed germination response of these samples at different NaCl concentrations and discuss how these responses varied along its Chilean distribution and how this information could be useful to restore marginal zones with this species and as a model to implement sustainable agroforestry. We believe that our results can be used as a reference guide by the National Forestry Agency (CONAF) and/or private companies pursuing to restore marginal or desertified lands using seeds from the most salt-tolerant sites identified in this study.
Methods
Climatic Conditions and Soil Prevailing in the Area of Study
P. chilensis (Mol. (Stuntz)) trees naturally growing in the hinterlands between Coquimbo Region (29°S) to Metropolitan Region (33°S) were used in this study (Table 1). This area is located south of the Atacama Desert and spans an arid climate in Coquimbo to semiarid conditions in the Metropolitan Region (Luebert and Pliscoff 2006; Santibáñez 2014). A recent climatic study predicts that by 2050, the Metropolitan area will become an arid zone (Santibáñez 2014). The area of study is characterized by 8–11 months of a warm dry period, with relative humidity always below 78 % (Galera 2000). The rainy season is short and occurs during the winter period (May–August) (Santibañez et al. 2008). The annual rainfall averages 226 mm varying from 74–108 mm in the Elqui province (Coquimbo Region) to 344–378 mm in the Chacabuco province (Metropolitan Region) (Santibáñez 2014). A shortage of precipitation is frequent in the Coquimbo Region and under such condition evapotranspiration exceeds precipitation most of the year. Plants growing in this scenario frequently undergo hydric stress (Luebert and Pliscoff 2006). The annual mean temperature of the area of study is 17.6 °C. During winter (June–August), the minimum temperature varies from 4.9° C in the Elqui province (Coquimbo) to 4.0 °C in Chacabuco province (Metropolitan Region). In summer (January–March), the maximum temperature reaches 32 °C in the Elqui province (Coquimbo) and 30.4 °C in the Chacabuco province (Metropolitan Region). Another characteristic of P. chilensis habitat is the high prevalence of clear days which can sum up to 300 days per year and accumulate between 1600 and 1700 degree days (Santibañez et al. 2008). This high luminosity has been reported as necessary to obtain a good development of this species (Contreras 1983). The soils in areas where Algarrobo grows are aridisols, with high presence of salts (such as sulfates and carbonates) or sodium, which in some places can form a petrocalcic horizon at the first meter (Luzio 1994; Casanova et al. 2004; CAAP 2010). These soils present limitations in salinity, sodicity, water availability, organic material, and compactivity limiting their arability without irrigation (Roshetko and Gutteridge 1996; Galera 2000; Casanova et al. 2004; Doussolin and Quezada 2006). Normally this tree occupies stony fluvial plain soils (Fig. 1) between 500 and 1500 masl with groundwater availability (Pasiecznik et al. 2001). It probably prefers mild alkaline soils (pH 7.6–8.9) since it is being proposed that acidic soils are a limiting factor for its distribution (Pasiecznik et al. 2001).
Seed Collection
In 2011, seeds of P. chilensis were collected from eight locations in Coquimbo Region (29°S–31°S), two locations from Valparaíso Region (32°S), and two locations from Metropolitan Region (33°S) (Table 1). In each locality, pods from several trees were collected and then seeds pooled in one sample. Seeds were placed in polypropylene tubes and stored at 4 ± 2 °C, as recommended by Pompelli et al. (2010) for a minimum of 2 months. Seed masses from each locality are presented in Supplemental Table 1.
Soil Collection
At least five samples from the top 25 cm of soil were collected in each locality and soil samples from the same locality were pooled into one sample. The soil salinity, determined as electrical conductivity, (EC total concentration of ionized solutes in aqueous sample; Rhoades et al. 1999) was measured at the Centro Tecnológico de Suelos y Cultivos, Universidad de Talca, Chile. Content of Na, Ca, Mg, K, Cl, P, N–NO3, HCO3, and SO4 was also determined (see Supplemental Table 4).
Preliminary Seed Germination Assay
As in many others salt-stress studies, we used NaCl as a surrogate for saline conditions (Felker et al. 1981; Katembe et al. 1998; Khasa et al. 2001; Khan and Gulzar 2003). The experimental design adopted from a preliminary study incorporated six concentrations of NaCl from 100 to 600 mM, using seeds, with or without sulphuric acid. Results of this preliminary study are presented in Supplemental Tables 2 and 3. Initially, seeds from healthy adult specimens in 18 localities were collected, but trees near villages, highly transited routes, seeds heavily infected by bruquids or planted trees were excluded. In the end, 6 of these sites were excluded and the study pursued at the remaining 12 sites. The scarification protocol was evaluated after separation of pooled seeds into two groups, (treated with H2SO4 vs. non-treated) followed by soaking in water. The germination observed on day 21 (Supplemental Table 2) indicates that non-treated seeds afforded lower germination (22 %) than those subjected to acid scarification. Consequently, subsequent germination experiments included an acid scarification step. Next, seeds from each locality were pooled into one sample and evaluated using six NaCl concentrations (100, 200, 300, 400, 500, and 600 mM) during a 14-day interval. Since no germination was observed above 400 mM NaCl (data not shown), the assay was replicated during a 21-day interval, as shown in Supplemental Table 2. Using this condition, similar germination percentages occurred at 100 and 200 mM, and also at 500 and 600 mM NaCl, as confirmed by one-way ANOVA (Supplemental Table 3). Based on these data, we selected an intermediate salt concentration (150 mM) in lieu of the 100 and 200 mM NaCl conditions. Also, because no statistical germination differences were seen between 300 mM NaCl and 400 mM NaCl, but, a difference was evident between 300 and 500 mM NaCl (Supplemental Table 3), we kept the 300 mM NaCl condition and adjust the second concentration to 450 mM NaCl (between 400 and 500 mM NaCl). In short, the adopted protocol included a sulphuric acid scarification procedure and four saline concentrations (150, 300, 450, and 600 of NaCl). Two germinations assays were carried out independently.
Seed Germination Assay
In germination assays, 30 seeds from each locality were scarified in concentrated H2SO4 (Miranda et al. 2011) for 3 min and rinsed thoroughly with distilled water for 2 min. Then, the seeds were dried out on tissue paper, placed into polypropylene tubes, and stored at 4 ± 2 °C for 24 h. Then, the seeds were placed into three Petri dishes (10 seeds/plate) covered with sterile filter paper (Whatmann 603) moistened with distilled water (control, 100 % humidity) and incubated in a growth chamber at 21 °C, 50–60 % relative humidity and photoperiod of 16 h day/8 h night using 120 μE/m2/s (where E = 1 mol of photons) provided by Sylvania cool-white fluorescent lamps. Germination was monitored every 24 h and at the same time (midday) for 21 days. A seed was considered “germinated” when its radicle was ≥2 mm (Cazebonne et al. 1999; Miranda et al. 2011). The salt treatment included four conditions: 150 mM (ψ = −0.99 MPa), 300 mM (ψ = −1.64 MPa), 450 mM (ψ = −2.33 MPa) and 600 mM (ψ = −3.11 MPa) NaCl. Sea water concentration is ~500 mM; 30,000 mg/L (Kim et al. 2010). The NaCl concentration that reduced seed germination by 50 % at the end of the assay (21th day) was termed as LD50 (Djanaguiraman et al. 2006; Delatorre-Herrera and Pinto 2009; Li et al. 2013). The LD50max term represents the same value but obtained in the most tolerant population (Delatorre-Herrera and Pinto 2009). In summary, a total of 30 seeds from each locality were germinated using four saline concentrations plus a water control, totaling 1800 samples. The complete germination assay was repeated twice. Then, the average of these two independent germination assays (3600 seeds in total) was compared and analyzed following a logistic regression model analysis.
Statistical Analysis
Statistical analysis is based on a multiple logistic regression model, considering the germination status at day 21 as response, and both accession and saline treatment as predictor variables. Model selection was based on likelihood ratio tests and Akaike information criterion. Final conclusions are based on an additive model, considering Huanta-Rivadavia accession and control treatment as reference cells. A baseline shift parameter was considered to adjust for any difference between the first and second experiment. Results are expressed as odds ratios (OR) with 95 % confidence intervals. The OR is defined as the quotient between the probability that any event occurs and the probability that it does not occur (Agresti 2002). In this case, the event is “germination in response to salinity”. The OR compares the “odds” between two populations, typically exposed to two different treatments. Normally, it is considered as an approximation to the relative risks, it means, the probability of an event to occur in a population compared to another event. All statistical analysis was performed with R version 2.15.3 (R 2013).
Results
Soils Analysis
None of the soil samples gathered at the same sites where seeds were collected had EC higher than 4 dS/m (equivalent to 40 mM NaCl, Table 1, Supplemental Table 4), a value considered as low for saline soils (Munns 2005). pH averaged 7.86, varying from 8.16 (Peldehue site, Metropolitan Region) to 7.15 (Ruta Internacional site, Los Andes province) (Supplemental Table 4). It means that at levels sampled during 2011, seeds were not exposed to salt levels or pH levels considered detrimental for germination in the field. Nevertheless, no single seedling was found in any of the locations where soils were collected.
Germination Potential of Prosopis chilensis Seeds
Regardless of the location, acid-scarified seeds exhibited the largest germination (92.9 % in average; P < 0.0001), (Table 2). This successful germination demonstrated that seed dormancy was properly broken by the stratification and scarification protocol and that embryo viability was not affected by this treatment.
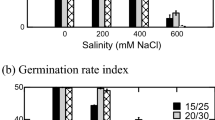
Also, regardless of the location, germination was impaired by various concentrations of NaCl (P < 0.0001). This adverse effect was marginal at 150 mM NaCl (72–100 % range, 91 % mean), minor to moderate at 300 mM NaCl (47–100 % range, 88 % mean), moderate to significant at 450 mM (22–95 % range, 67 % mean), and robust at 600 mM NaCl (6.7–57 % range, 34 % mean), (Table 2). At 150 mM NaCl, seed germination maxima occurred on day 5 similar to the control without salt treatment (Fig. 2). Germination was delayed until day 9 in seeds incubated with 300 mM NaCl. At 450 mM NaCl and 600 mM NaCl, maximal germination was observed on day 15th, with large variability within each population (Fig. 2).
Cumulative mean germination in Prosopis chilensis influenced by NaCl concentration. Each curve is a mean of two independent seed germination assays and it represents the mean of the cumulative germination observed in the 12 populations during a 21-day period. Each curve represents the performance of 760 seeds (n = 60 seeds per population) at each NaCl concentration, totalling 3600 seeds analyzed. Error bars show 25 and 75 % percentiles
Influence of Geographical Location on Seed Germination in Response to Saline Conditions
Overall, no large differences in germination were observed among seeds from different localities. The exception was the seeds from Huanta-Rivadavia, the germination of which was decreased at 150 and 300 mM NaCl (Table 2). At 450 mM NaCl, seeds from Huanta-Rivadavia and Chacabuco showed germination below 50 % (21.7 and 48.3 %, respectively), while seed germination from remaining locations remained above 50 %. At 600 mM NaCl, the overall germination processes was affected, however, four populations were less affected, as their germination level remained above 50 % (Matancilla, Rapel, Maitencillo and Ruta Internacional I) (Table 2). Assuming that germination levels correlate with the competence of seeds, we establish the LD50 as germination set at 50 %. The LD50 doses for seeds from Huanta-Rivadavia was 300 mM NaCl, for Chacabuco seeds was 450 mM NaCl and for the remaining locations was 600 mM NaCl. The exception was Maitencillo and Rapel whose germination percentages remained above 50 % (56.7 and 55 %, respectively, Table 2). Consequently, the most salt-tolerant population were Matancilla (Choapa Region, 56.7 %) followed by Rapel (55 %).
Probability of Seed Germination in Response to Salinity
In this study, we applied a model that determined the OR of germination in P. chilensis seeds exposed to four NaCl conditions from several populations and compare them with one population used as reference. In this case, Huanta-Rivadavia was chosen as reference because it showed the lowest germination level throughout different conditions. Analysis of every population included in this study was highly significant (Table 3). The Matancilla population with an OR of 33.3 (19.4; 19.1) (95 % CI) and the sample from Ruta Internacional I with an OR of 16.4 (10.1; 26.5) showed the highest germination probability relative to the reference population. In other words, Matancilla seeds afforded approximately 33-fold higher chance of germination than seeds from Huanta-Rivadavia. In contrast, seeds from Río Hurtado (OR of 3.5) and Peldehue (OR of 4.0) had a 3.5- and 4-fold higher chance of germinating compared to Huanta-Rivadavia, respectively.
When comparing each salt concentration with the control condition, all ORs were <1 indicating that every salt condition impaired the probability of germination relative to the control (Table 3). Nevertheless, the OR at 150 mM NaCl is 0.7 (0.5; 1.1) which is barely significant (P < 0.08). Above 300 mM NaCl, the reduction in germination was statistically significant with “odds” of 48, 11, and 2 %, for 300, 450, and 600 mM NaCl, respectively.
Discussion
Seeds Viability and Lack of Recruitment
The initial evaluation of seed viability using sulphuric acid to break physical dormancy (Catalán and Balzarri 1992) demonstrates that scarification is required to stimulate germination in P. chilensis (Supplemental Table 2). All the seeds treated with sulphuric solution attained 100 % of germination by the third day, while 17 % of non-scarified seeds germinated during the same interval and increased to 22 % by the end of the assay on day 21 (Supplemental Table 2). In summary, using the sulphuric acid protocol, germination (48 h for radicle emergence) was similar to that reported by Cony and Trione (1998) and Villagra (1995) in other Prosopis species.
Like in many Fabaceas, P. chilensis seeds are released by endozoic dispersion following animal ingestion. Animal digestion weakens the wax endocarps that protect seeds from a number of environmental factors facilitating germination when excreted on soils with appropriate water supply (Campos and Ojeda 1997; Peinetti et al. 1993). This strategy aids P. chilensis seeds to gain temporal and spatial opportunity for germination in adverse conditions, similar to what has been reported in other Prosopis species (Villagra et al. 1995). It is assumed that grazing herbivores ate tree pods and spread the seeds, as signs of depredation were detected on visited sites, although no new plants were detected across the sampled area. Excess of grazing after ENSO events can also be another fact putatively effecting establishment of new plants. The overall mean germination was 93 %, with extreme values between 82 % in Huanta-Rivadavia to 100 % in Montepatria, Matancilla, and Peldehue sites. This yield is considerably higher than that reported in other woody plants (Khasa et al. 2001; Beritognolo et al. 2007; Li et al. 2008) but it is similar to that described in other species of the genus Prosopis (Rohdes and Felker 1988; Cazebonne et al. 1999; El-Keblawy and Al-Rawai 2005; Villagra and Cavagnaro 2006). In this case, the unaffected viability of P. chilensis seeds was confirmed during germination assays when the physical coat-imposed dormancy had been interrupted by acid treatment. Therefore, the absence of recruitment in almost every site searched along the latitudinal transect (personal observation) is not a consequence of dysfunctional germination imposed by defective embryo development, but it is a result of forced dormancy in seeds where the presence of rearing herbivores feeding on plant pods is inefficient to warrant adequate germination that could contribute to ameliorate afforestation. Another factor that may be influencing the lack of recruitment is allied to the abiotic stress imposed mainly by water deficit. It is a fact that a diminishing of precipitation and increment in the index of aridity has occurred in north and central regions of Chile since 1950 (Santibáñez and Santibáñez 2007; PAN 2014; UDP 2012; Santibáñez 2014), and that 17 out of 18 of these dry periods (3–6 years of extension) occurred in the area where P. chilensis is distributed (Meza et al. 2010). This aridness is influenced by the less frequent ENSO phenomenon (Wang et al. 2012; PAN 2014) whose precipitation events are vital to recruit new plants (Holmgren et al. 2006b; Squeo et al. 2007). Lack of recruitment worsen by unrestrained animal breeding and/or wild animal predation (Valdivia and Romero 2013), poor soil structure, and reduced organic matter, with less P and other essential minerals that are vital for facilitating germination and establishment of new plants (Miranda et al. 2013).
Effects of NaCl on Germination Response
As seen in many plant species, stress impairs germination and several other physiological traits (Hu and Schmidhalter 2005; Beritognolo et al. 2007; Ríos-Gómez et al. 2010; Zolla et al. 2010; Mane et al. 2011; Bui 2013; Jamil et al. 2014). Prosopis chilensis is not the exception but interestingly, seeds of this tree are capable of germinating under adverse conditions when seeds from other woody plants fail (Fang et al. 2006; Li et al. 2008; Hosseini et al. 2012). In fact, it is demonstrated here that germination of P. chilensis was not affected at 150 mM NaCl and became marginally affected at 300 mM NaCl (CI ≥ 99 %) (Fig. 2; Table 2). The salt tolerance of P. chilensis confirms observations by Villagra and Cavagnaro (2005) in seeds of Prosopis argentina (80 % at 300 mM), Al-Ansari (2002) for Prosopis tamarugo (57 % at 18,000 ppm of salts), and Sosa et al. (2005) for Prosopis strombulifera (60 % at −1.2 MPa). These germination efficacies contrast with those observed in glycophytes like Arabidopsis (Vallejo et al. 2010), Lupinus albus (Jeschke 1984), and Solanaceas (Bojovic et al. 2010) where seeds hardly germinate at concentrations higher than 200 mM NaCl. The inability to germinate in these latter cases is being linked to an increase of reactive oxygen species (ROS) triggered by toxic levels of NaCl that deplete the activity of relevant antioxidant enzymes such as catalase (CAT), peroxidase (POX), and polyphenol oxidase (PPO) (Dash and Panda 2014) leading to severe damage of proteins and nucleic acid (Miller et al. 2010).
At higher salt concentrations, P. chilensis delays germination, a similar finding was observed during the culture of quinoa (Adolf et al. 2013); however, following this delay germination remains successful (Fig. 2). In fact, germination above 450 mM NaCl is remarkable for plants and not many of them succeed (Munns 2002; Villagra and Cavagnaro 2005). For instance, a wild wheat species considered resistant to abiotic stress (Aegilops spp.) held as gene reservoir with potential to improve commercial wheat (Triticum) species, attains LD50 at 450 mM NaCl, becoming unsuitable for saline soils (Yildiz et al. 2006). At a similar NaCl concentration, we observed a mean germination of 67 %, for the large majority of studied samples (Table 2). Finally, at 600 mM NaCl, we obtained a sizable germination mean of 34 % (Fig. 2; Table 2). This percentage is similar to that obtained in other Prosopis species incubated at 600 mM NaCl, such as P. flexuosa (40 %, Catalán et al. 1994) and P. tamarugo (85 %, Arce and Balboa 1988) and approximates the germination of extreme halophytes such as some species of Chenopodium, Atriplex, and Casuarina (Rhodes and Felker 1988; Cushman 2001; Khan et al. 2001; Araújo et al. 2006; Kachout et al. 2009; Zurita-Silva et al. 2014). Adolf et al. (2013) analyzed the germination capability and seedling establishment of quinoa genotypes populating the Andes Altiplano arid environment. They proposed that tolerance of quinoa to salt stress at germination is the result of a significant gradient limiting the distribution of potential toxic (Na+ and Cl−) ions and essential ions such as K+, Mg+2, Ca+2, PO4 2−, and SO4 2−, across the protecting seed coat and to a reallocation of elements in the embryo. Hariadi et al. (2011) suggested that seed viability of quinoa was dependent on its ability to exclude toxic Na+ from the developing embryo to avoid ion toxicity. In summary, it seems that the high salt tolerance of quinoa seeds is associated to structural and physiological protective features in the seeds. It is possible that P. chilensis adopted a similar strategy to avoid salt toxicity. This notion gain supports now following the observation that high salt concentrations in P. chilensis delayed seed germination (Fig. 2), similar to what has been observed in quinoa genotypes (González and Prado 1992; Prado et al. 2000; Delatorre-Herrera and Pinto 2009).
Seed Germination in Different Locations
The results show that P. chilensis seeds display enhanced germination especially under salt levels considered as toxic (Table 2). The results also show that seeds from the central section encompassing Limarí and Choapa provinces (see Table 1 for georeference details) displayed increased germination under saline conditions relative to seeds collected at bordering sites (Elqui Valley and Chacabuco province, see Table 1 for georeference details). A possible explanation for this unexpected difference comes from Plaza (2010) who found that P. chilensis trees growing in Choapa valley display five “rare” alleles only found in these populations. In addition, in Elqui populations they described two specific alleles, one of them in locus Mo05 (213 bp) and the second in locus Mo13 (236 bp), while in the Limarí populations a specific allele in locus Mo09 (214 bp) was detected. In summary, eight polymorphic sites were described, two in Elqui valley, one for Limarí valley, and the remaining five alleles found in algarrobos growing in Choapa valley. Based on these genetic differences, it can be argued that the P. chilensis populations along its geographical distribution display specific genetic determinants involved in tolerance to saline stress. While the altered expression of several genes has been associated with abiotic stress, in leguminous species the enzyme betaine aldehyde dehydrogenase responsible for the synthesis of the osmotic regulator glycine betaine appears as candidate to tolerate stress (Yu et al. 2014). Because of the limited number of samples collected due to insufficiency of available trees and because the sampling procedure represented a single year of collection (2011), new assays circumventing these limitations are required. Under similar conditions, Cazebonne et al. (1999) reported 68 % seed germination at 500 mM NaCl using seeds collected in 1993 at the same location (Peldehue) we reported in this study. After eighteen years (2011), we observed a 13 % reduction in germination (55 %, Table 2) at 450 mM NaCl. In view of these data, it is suggested that the germination capacity at Peldehue site has declined during this period. Since we have no explanation that accounts for this difference, it is felt that further studies are required to confirm this difference and to investigate possible factor (s) influencing the decreased seed competence.
Finally, it is remarkable that in this study two populations of P. chilensis (Matancilla and Rapel) successfully withstand salt stress, as their germination rate remains superior to the LD50 (Table 2) at 600 mM NaCl (sea water salt concentration is ~500 mM; 30,000 mg/L, Kim et al. 2010). For the purpose of seed selection, seeds from these two places are good candidates to be used during recovery of degraded or marginal zones, and it would be of interest to learn if seedlings from these two localities tolerate irrigation with sea water. More importantly, the utilization of P. chilensis for afforestation becomes desirable considering countless experiences where afforestation in degraded soils using non-native species caused undesirable effects such as uncontrolled propagation, loss of biological diversity, changes in composition and structure of wild vegetation, poisonous fodder for livestock and wild animals among others (Mc Neely 2001; Andrade et al. 2009). In the Chilean situation, local programs should encourage enrichment of P. chilensis reforestation due to the imminent process of extinction recently detected (Valdivia and Romero 2013) and for recovery of marginal lands thus contributing to the development of a more sustainable world.
Conclusion
The results showed that the absence of recruitment of Prosopsis chilensis observed along its natural Chilean habitat is a consequence of physical seed dormancy, a prolonged drought affecting north and central Chile since 1950 and inefficacious herbivory unable to release the protective dormancy of seed. Also, it is demonstrated that germination of P. chilensis seeds is highly tolerant to salt stress and that this adaptation remains effective in most naturally growing trees in Chile. We found that seeds from Limarí and Choapa valley remain able to germinate when challenged by extreme salt stress. The effort to screen the Chilean P. chilensis populations represents a valid approach not only to find candidate locations to collect seeds, but also to perform further detailed studies aimed to recover degraded and marginal soils in arid and semiarid regions.
References
Adolf VI, Jacobsen S-E, Shabala S (2013) Salt tolerance mechanisms in quinoa (Chenopodium quinoa Willd.). Environ Exp Bot 92:43–54
Agresti A (2002) Categorical data analysis, 2nd edn. John Wiley and Sons, New York, p 710
Almansouri M, Kinet J-M, Lutts S (2001) Effect of salt and osmotic stresses on germination in durum wheat (Triticum durum Desf.). Plant Soil 231(2):243–254
Al-Ansari F (2002) Effects of underground saline water of the UAE on seed germination, seedling growth and chemical constituents of Prosopis tamarugo. Pak J Appl Sci 2(11):1018–1021
Andrade L, Fabricante J, Oliveira F (2009) Invasão biológica por Prosopis juliflora (Sw.) DC.: impactos sobre a diversidade e a estrutura do componente arbustivo-arbóreo da caatinga no estado do Rio Grande do Norte, Brasil. Acta Botanica Brasilica 23:935–943
Araújo SA, Silveira JAG, Almeida TD, Rocha I, Morais DL, Viégas RA (2006) Salinity tolerance of halophyte Atriplex nummularia L. grown under increasing NaCl levels. Revista Brasileira de Engenharia Agrícolae Ambiental 10:848–854
Arce P, Balboa O (1988) Some aspects of the biology of Prosopis growing in Chile. In: Habit MA (ed) The current state of knowledge on Prosopis juliflora. Roma, FAO, pp 313–322
Avilés R (2012) Acta Sesión N° 8. Novenos proceso clasificación. Ministerio de Medio Ambiente. 17 p
Bekki A, Trinchant J-C, Rigaud J (1987) Nitrogen fixation (C2H2 reduction) by Medicago nodules and bacteroids under sodium chloride stress. Physiol Plant 71(1):61–67
Beritognolo I, Piazzai M, Benucci S, Kuzminsky E, Sabatti M, Scarascia Mugnozza G, Muleo R (2007) Functional characterization of three Italian Populus alba L. genotypes under salinity stress. Trees 21:465–477
Blun A (1998) Plant breeding for stress environments. CRC Press, Boca Raton
Bojović B, Đelić G, Topuzović M, Milan Stanković (2010) Effects of NaCl on seed germination in some species from families Brassicaceae and Solanaceae. Kragujev J Sci 32:83–87
Bui EN (2013) Soil salinity: a neglected factor in plant ecology and biogeography. J Arid Environ 92:14–25
CAAP (2010) Capítulo 5—Suelos. In: Estado del Medio Ambiente en Chile 2008. Instituto de Centros Públicos. Centro de Análisis de Políticas Públicas. Gobierno de Chile/PNUMA/CEPAL, p 255
Campos C, Ojeda R (1997) Dispersal and germination of Prosopis flexuosa (Fabaceae) seeds by desert mammals in Argentina. J Arid Environ 35:707–714
Casanova M, Vera W, Salazar O, Luzio W (2004) Edafología, Guía de clases prácticas. Departamento de Ingeniería y Suelos, Facultad de Ciencias Agronómicas, Universidad de Chile, Santiago, p 74
Catalán L, Balzarini M (1992) Improved laboratory germination conditions for several arboreal Prosopis species: P. chilensis, P. flexuosa, P. nigra, P.alba, P. caldenia and P. affinis. Seed Sci Technol 20:293–298
Catalán L, Balzarini M, Talesnik E, Sereno R, Karlin U (1994) Effects of salinity on germination and seedling growth of Prosopis flexuosa (D.C.). For Ecol Manag 63:347–357
Cazebonne C, Vega A, Varela D, Cardemil L (1999) Salinity effects on germination and growth of Prosopis chilensis. Revista Chilena Hist Nat 72:83–91
Chapin FS III, Sala OE, Huber-Sannwald E (2001) Global biodiversity in a changing environment: scenarios for the 21st century. Springer-Verlag, New York, p 376
CIREN (2011) Determinación de la erosión actual y potencial del territorio de Chile. http://www.ciren.cl/cirenxml/noticias/default.asp?a=5&id=647. Accessed 25 Feb 2013
Comisión Nacional de Energía (CNA) (2009) Modelación del Recurso Solar y Eólico en el Norte de Chile. Gobierno de Chile, p 21
Contreras B (1983) Diversidad Morfológica en poblaciones de algarrobo (Prosopis chilensis (Mol) Stuntz ) en la IV Región. Dissertation, Universidad de Chile, Chile, p 105
Cony MA, Trione SO (1998) Inter and intraespecific variability in Prosopis flexuosa and P. chilensis: seed germination under salt and moisture stress. J Arid Environ 40:307–317
Cushman JC (2001) Osmoregulation in plants: implications for agriculture. Am Zool 41(4):758–769
Dash M, Panda SK (2014) Salt stress induced changes in growth and enzyme activities in germinating Phaseolus mungoo seeds. Biol Plant 44(4):587–589
Delatorre-Herrera J, Pinto M (2009) Importance of ionic and osmotic componentes of salts stress on the germination of four quinua (Chenopodium quinoa Willd.) selections. Chilean. J Agric Res 69(4):477–485
Denby K, Gehring C (2005) Engineering drought and salinity tolerance in plants: lessons from genome-wide expression profiling in Arabidopsis. Trends Biotechnol 23(11):547–552
Djanaguiraman M, Sheeba JA, Shanker AK, Devi DD (2006) Rice can acclimate to lethal level of salinity by pretreatment with sublethal level of salinity through osmotic adjustment. Plant Soil 284:363–373
Doussolin E, Quezada C (2006) Introducción al problema de los suelos de las zonas áridas, 1st edn. Departamento de suelos y recursos naturales, Facultad de Agronomía, Universidad de Concepción, Concepción
Dupont L, Alloing G, Pierre O, El Msehli S, Hopkins J, Hérouart D, Frendo P (2012) The Legume Root Nodule: From Symbiotic Nitrogen Fixation to Senescence, Senescence, Dr. Tetsuji Nagata (Ed.), ISBN: 978-953-51-0144-4, InTech. http://www.intechopen.com/books/senescence/the-legume-root-nodule-from-symbiotic-nitrogen-fixation-tosenescence
El-Keblawy A, Al-Rawai A (2005) Effects of salinity, temperatura and light on germination of invasive Prosopis juliflora (Sw.) D.C. J Arid Environ 61:555–565
Fang S-Z, Song L-Y, Fu X-X (2006) Effects of NaCl stress on sewed germination, leaf gas exchange and seedling growth of Pteroceltis tatarinowii. J For Res 17(3):185–188
Felker P, Clark PR, Laag AE, Fratt PF (1981) Salinity tolerance of the tree legumes: mesquite (Prosopis glandulosa var. torreyana, P. velutina and P. articulata) algarrobo (P. chilensis), kiawe (P. pallida) and tamarugo (P. tamarugo) grown in sand culture on nitrogen-free media. Plant Soil 61:311–317
Galera MF (2000) Las especies del género Prosopis (algarrobos) de América Latina con especial énfasis en aquellas de interés económico. Ed. FAO. Córdoba, Argentina. ISBN 987-43-2577-1. http://www.fao.org/docrep/006/ad314s/ad314s00.HTM. Accessed 29 March 2013
González J, Prado F (1992) Germination in relation to salinity and temperature in Chenopodium quinoa (Willd.). Agrochimica 34(1-2):101–108
Grattan SR, Grieve CM (1999) Mineral nutrient acquisition and response by plants grown in saline environments. In: Pessarakli AM (ed) Handbook of plant and crop stress. Marcel Dekker, New York, pp 203–229
Gul B, Khan MA (2002) Seed germination of halophytes exposed to high salinity and temperature in the seed bank. In: Liu X, Liu M (eds) Halophytes utilization and regional sustainable development of agriculture. Meteorological Press of China, Beijing, pp 69–76
Hariadi Y, Marandon K, Tian Y, Jacobsen S-E, Shabala S (2011) Ionic and osmotic relations in quinoa (Chenopodium quinoa Willd.) plants grown at various salinity levels. J Exp Bot 62:185–193
Holmgren M, Scheffer M (2001) El Niño as a window of opportunity for the restoration of degraded arid ecosystems. Ecosystems 4:151–159
Holmgren M, Stapp P, Dickman CR, Gracia C, Graham S, Gutiérrez JR, Hice C, Jaksic F, Kelt DA, Letnic M et al (2006a) Extreme climatic events shape arid and semiarid ecosystems. Front Ecol Environ 4:87–95
Holmgren M, López BC, Gutiérrez JR, Squeo FA (2006b) Herbivory and plant growth rate determine the success of ENSO-driven tree establishment in semi-arid South America. Glob Chang Biol 12:2263–2271
Hosseini S, Parsakhoo A, Naghavi H, Kiane S, Koohi S (2012) Effect of salt stress on germination and seedlings growth of Prosopis juliflora (Sw.). New For 43:45–55
Hu Y, Schmidhalter U (2005) Drought and salinity: a comparison of their effects on mineral nutrition of plants. J Plant Nutr Soil Sci 168:541–549
IRRI (2006) International Rice Research Institute. Stress and disease tolerance. http://www.knowledgebank.irri.org/ricebreedingcourse/Breeding_for_salt_tolerance.htm. Accessed 29 March 2013
Jamil M, Rehman S, Rha ES (2014) Response of growth, PSII photochemistry and chlorophyll content to salt stress in four brassica species. Life Sci J 11(3):139–145
Jeschke WD (1984) K+-Na+ exchange at cellular membranes, intracellular compartmentation of cations, and salt tolerance. In: Staples RC (ed) Salinity tolerance in plants: strategies for crop improvement. Wiley, New York, pp 37–66
Kachout SS, Mansoura AB, Jaffel K, Leclerc JC, Rejeb MN, Ouerghi Z (2009) The effect of salinity on the growth of the halophyte Atriplex hortensis (chenopodiaceae). Appl Ecol Environ Res 7(4):319–332
Katembe WJ, Ungar IA, Mitchell JP (1998) Effect of salinity on germination and seedling growth of two Atriplex species (Chenopodiaceae). Ann Bot 82:167–175
Khan MA, Gulzar S (2003) Light, salinity, and temperature effects on the seed germination of perennial grasses. Am J Bot 90(1):131–134
Khan MA, Gul B, Weber DJ (2001) Seed germination characteristics of Halogeton glomeratus. Can J Bot 79:1189–1194
Khasa PD, Hamblingb B, Kernaghanb G, Fungc M, Ngimbib E (2001) Genetic variability in salt tolerance of selected boreal woody seedlings. For Ecol Manag 165:257–269
Kim SJ, Ko SH, Kang KY, Han J (2010) Direct seawater desalination by ion concentration polarization. Nat Nanotechnol 5:297–302
Koohafkan AP (1996) Desertification, drought and their consequences. Sustainable Development Department (SD), Food and Agriculture Organization of the United Nations (FAO). http://www.fao.org/waicent/faoinfo/sustdev/EPdirect/EPan0005.htm. Accessed 10 March 2013
Läuchi A, Grattan SR (2007) Plant growth development under salinity stress. In: Jenks MA et al (eds) Advances in molecular breeding toward drought and salt tolerant crops. Springer, The Netherlands, pp 1–32
Li W, An P, Liu X, Khan MA, Tsuji W, Tanaka K (2008) The effect of light, temperature and bracteoles on germination of polymorphic seeds of Atriplex centralasiatica Iljin under saline conditions. Seed Sci Technol 36:325–338
Li Z-Y, Zhou J-H, Liang Y-M (2013) Dose-effect correlation of chloride de-icing salt on Euonymus japonicas. For Sci Pract 15(3):238–245
Luebert F, Pliscoff P (2006) Sinopsis bioclimática y vegetacional de Chile. Editorial Universitaria, Santiago de Chile, p 316
Luzio W (1994) Suelos, una vision actualizada del recurso. Publicaciones misceláneas agrícolas n° 38. Segunda Edición. Universidad de Chile. http://mazinger.sisib.uchile.cl/repositorio/lb/ciencias_agronomicas/miscelaneasagronomicas38/. Accessed 29 December, 2014
Mane AV, Deshpande TV, Wagh VB, Karadge BA, Samant JS (2011) A critical review on physiological changes associated with reference to salinity. Int J Environ Sci 1(6):1–25
McNeely J (2001) Invasive species: a costly catastrophe for native biodiversity. Land Use Water Resour Res 1(2):1–10
Meza L, Corso S, Soza S (2010) Gestión del riesgo de sequía y otros eventos climáticos extremos en Chile. Organización de la Naciones Unidas para la Agricultura y la Alimentación (FAO). Documento técnico, p 128
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33(4):453–467
Miranda RQ, Oliveira MTP, Correia RM, Almeida-Cortez JS, Pompelli MF (2011) Germination of Prosopis juliflora (Sw) DC seeds after scarification treatments. Plant Species Biol 26:186–192
Miranda M, Vega-Gálvez A, Martínez E, López J, Marín R, Aranda M, Fuentes F (2013) Influence of contrasting environments on seed composition of two quinoa genotypes: nutritional and functional properties. Chil J Agric Res 73(2):108–116
Montandon G, Silva A, Carneiro E, Miana S, Boddey RM, Schmidt S (2011) Nitrogen-fixing legume trees species for the reclamation of severely degraded lands in Brazil. Tree Physiol 31(2):139–149
Montecinos A, Aceituno P (2003) Seasonality of the ENSO-related rainfall variability in central chile and associated circulation anomalies. J Clim 16:281–296
Mottura M, Finkeldey R, Verga A, Gailing O (2005) Development and characterization of microsatellite markers for Prosopis chilensis and Prosopis flexuosa and cross-species amplification. Mol Ecol Notes 5:487–489
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Munns R (2005) Genes and salt tolerance: bringing them together. New Phytol 167:645–663
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Ondrasek G, Rengel Z, Veres S (2011) Soils salinisation and sal stress in crop production, Chapter 8. In: Shanker A, Venkateswarlu B (eds) Abiotic stress in plants—mechanism and adaptions. INTECH, Rijeka
PAN (2014) Plan Nacional de Adaptación al Cambio Climático. Oficina de Cambio Climático. Ministerio de Medioambiente. Santiago, p 56
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42
Pasiecznik NM, Felker P, Harris PJC, Harsh LN, Cruz G, Tewari JC, Cadoret K, Maldonado KJ (2001) The Prosopis juliflora—Prosopis pallida complex: a monograph. HDRA, Coventry 170 p
Peinetti R, Pereyra M, Kin A, Sosa A (1993) Effects of cattle ingestion on viability and germination rate of caldén (Prosopis caldenia) seeds. J Range Manag 46:483–486
Peñuelas J, Filella I (2001) Phenology: responses to a warming world. Science 294:793–795
Plaza D (2010) Análisis de la diversidad genética del Algarrobo (Prosopis chilensis (Molina) Stuntz) en la Región de Coquimbo y su correlación con la composición proximal de sus frutos maduros. Dissertation, Universidad de La Serena, Chile, p 83
Pompelli M, Ferreira D, Cavalcante P, Salvador T, Hsie B, Endres L (2010) Environmental influence on the physico-chemical and physiological properties of Jatropha curcas seeds. Aust J Bot 58:421–427
Prado F, Boero C, Gallardo M, González J (2000) Effect of germination, growth, and soluble sugar content in Chenopodium quinoa Willd. seeds. Bot Bull Acad Sin 41:27–34
Rhoades JD, Chanduvi F, Lesch S (1999) Soils salinity assessment. Methods and interpretation of electrical conductivity measurements. FAO, Irrigation and Drainage Document. p 165
Rhodes D, Felker P (1988) Mass screening of Prosopis (mesquite) seedlings for growth at seawater salinity. For Ecol Manag 24:169–176
Ríos-Gómez R, Salas-García CE, Monroy-Ata A, Solano E (2010) Salinity effect on Prosopis laevigata seedlings. Terra latinoamericana 28(2):99–107
Roshetko J, Gutteridge R (1996) Nitrogen fixing trees for fodder production—a field manual. Winrock, Morrilton
Rutllant J, Fuenzalida H (1991) Synoptic aspects of the central Chile rainfall variability associated with the Southern oscillation. Int J Climatol 11:63–76
R Core Team (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. http://www.R-project.org/. Accessed 3 March 2013
Santibáñez F (2014) Atlas de cambio climático de la zona semiárida de Chile. Fondo de Protección Ambiental del Ministerio de Medioambiente. p 145
Santibáñez F, Santibáñez P (2007) Trends in land degradation in Latin America and the Caribbean, the role of climate change. In: Climate and land degradation World Meteorological Organization. Springer Verlag, pp 65–81
Santibáñez F, Roa P, Santibáñez P (2008) El medio físico. Capítulo I. In: Rovira J, Ugalde J, Stutzin M (eds) Biodiversidad en Chile. CONAMA, Santiago de Chile, p 639
Serra MT (1997) Prosopis chilensis. In: Especies arbóreas y arbustivas para las zonas áridas y semiáridas de América Latina. Serie: Zonas Áridas y Semiáridas, Organización de las Naciones Unidas para la Agricultura y la Alimentación, Programa de las Naciones Unidas para el Medio Ambiente 12:215–225
Sosa L, Llanes A, Reinoso H, Reginato M, Luna V (2005) Osmotic and specific ion effects on the germination of Prosopis strombulifera. Ann Bot 96:261–267
Squeo F, Holmgren M, Jiménez M, Albán L, Reyes J, Gutiérrez J (2007) Tree establishment along an ENSO experimental gradient in the Atacama desert. J Veg Sci 18:193–200
UDP (2012) Unidad de Diagnóstico Parlamentario. La Desertificación en Chile. Departamento de Evaluación de la Ley. Valparaíso, p 20
UNEP (1992) Managing fragile ecosystem: combating desertification and drought. Agenda 21, chapter 12. United Nations Environment Programme. http://www.unep.org/Documents.Multilingual/Default.asp?DocumentID=52&ArticleID=60 Accessed 05 March 2013
Valdivia C, Romero C (2013) En la senda de la extinción: el caso del algarrobo Prosopis chilensis (Fabaceae) y el bosque espinoso en la Región Metropolitana de Chile central. Gayana Botanica 70(1):57–65
Vallejo A, Yanovsky M, Botto J (2010) Germination variation in Arabidopsis thaliana accessions under moderate osmotic and salt stresses. Ann Bot 106:833–842
Verga A, Gregorius H (2007) Comparing morphological with genetic distances between populations: a new method and its application to the Prosopis chilensis—P. flexuosa complex. Silvae Genet 56(2):45–51
Vidiella P, Armesto J (1989) Emergence of ephemeral plant species from the north-central Chilean desert in response to experimental irrigation. Revista Chilena Hist Nat 62:99–107
Villagra P (1995) Temperature effects on germination of Prosopis argentina and P. alpataco (Fabaceae, Mimosoideae). Seed Sci Technol 23:639–646
Villagra P, Cavagnaro J (2005) Effects of salinity-soil type interactions on the establishment, growth and water relations of Prosopis argentina and P. alpataco seedlings. Implications for their ecological success. Austral Ecol 30:325–335
Villagra P, Cavagnaro J (2006) Water stress effects on the seedling growth of Prosopis argentina and Prosopis alpataco. J Arid Environ 64:390–400
Wang C, Deser C, Yu J-Y, DiNezio P, Clement A (2012) El Niño Southern Oscillation (ENSO): a review. In: Coral reefs of the eastern Pacific. Springer, p 46
Yildiz M, Terzi H, Arikan ES (2006) Seed germination of populations of wild wheat species, Aegilops biuncialis and Ae. triuncialis: effects of salinity, temperature and photoperiod. Pak J Biol Sci 9:1299–1305
Yu HQ, Wang YG, Yong TM, She YH, Fu FL, Li WC (2014) Heterologous expression of betaine aldehyde dehydrogenase gene from Ammopiptanthus nanus confers high salt and heat tolerance to Escherichia coli. Gene 549:77–84
Zolla G, Heimer YM, Barak S (2010) Mild salinity stimulates a stress-induced morphogenic response in Arabidopsis thaliana roots. J Exp Bot 61(1):211–224
Zurita-Silva A, Fuentes F, Zamora P, Jacobsen S-E, Schwember A (2014) Breeding quinoa (Chenopodium quinoa Willd.): potential and perspectives. Mol Breed 34(1):13–30
Acknowledgments
The authors are grateful for the valuable comments of Dr Julio Gutiérrez and three anonymous reviewers. C. Ibáñez acknowledges Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) Grant No. 1110831 (CONICYT—Chile) and Ministerio de Educación (MINEDUC)—Convenio de Desempeño para la Educación Superior, Grant No. ULS-1401 for financial support. C. Westphal is supported by “Programa de Formación de Capital Humano Avanzado de CONICYT, Becas para Estudios de Doctorado Nacional 2011, Grant No. 21120460”.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Westphal, C., Gachón, P., Bravo, J. et al. The Potential of Algarrobo (Prosopis chilensis (Mol.) Stuntz) for Regeneration of Desertified Soils: Assessing Seed Germination Under Saline Conditions. Environmental Management 56, 209–220 (2015). https://doi.org/10.1007/s00267-015-0490-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00267-015-0490-4