Abstract
The aim of this study was to describe the impact of re-vegetation on the restoration of microbial community structure and soil microbiological properties in sand dunes that had been affected by mining activity. Soil samples were collected during the dry and rainy seasons from a chronosequence (1, 9, 21 years) of re-vegetated dunes using a single preserved dune as a reference. The composition of the fatty acid methyl esters and soil microbial properties were evaluated. The results showed that the changes in microbial community structure were related to seasonal variations: biomarkers of Gram-positive bacteria were higher than Gram-negative bacteria during the dry season, showing that this group of organisms is more tolerant to these stressful conditions. The microbial community structure in the natural dune was less affected by seasonal variation compared to the re-vegetated areas, whereas the opposite was observed for microbiological properties. Thus, in general, the proportion of saprobic fungi was higher in the natural dune, whereas Gram-negative bacteria were proportionally more common in the younger areas. Although over time the re-vegetation allows the recovery of the microbial community and the soil functions, these communities and functions are different from those found in the undisturbed areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mining activity impacts on an increasing number of natural areas. In addition to affecting soil stability and decreasing its nutrient and organic content, mining promotes significant losses in biodiversity above and below ground (Claassens et al. 2012; Izquierdo et al. 2005). These disturbances alter the functioning and the structure of microbial communities, energy transfer, nutrient cycling, and the re-establishment of plants (Mummey et al. 2002).
The re-composition of edaphic characteristics is directly related to the establishment of plant communities and the biological activity of the soil (Claassens et al. 2005). In this way, the developments of strategies that help the restoration process over time, such as reforestation and the use of topsoil before planting, are important because they promote the growth and development of soil microbiota. The most important goals of the rehabilitation process are to stabilize the ecosystem and to restore its ability to resist further disturbances (Chodak et al. 2009). Monitoring the rehabilitation process involves measuring important physical, chemical, and biological soil quality indicators. Besides evaluating soil functioning, these indicators also guide the progress of the restoration of soils over time (Ramsey et al. 2012; Silva et al. 2012).
Microorganisms and their activities are among the indicators that have been used to monitor the processes of disturbance and/or recovery in soils (Chaer and Tótola 2007; Hinojosa et al. 2004; Huang et al. 2011). The microbial activity or microbial functioning can be indirectly evaluated by soil enzyme activity, being useful to monitor edaphic changes (Fernandes et al. 2011; Mendes et al. 2012).
The ester-linked fatty acid methyl esters (EL-FAME) analysis is a technique utilized to access the soil microbial community. The fatty acid methyl esters are extract of the soil and used as microbial taxonomic biomarkers of microorganisms. This technique has been also used to investigate response of the microbial community structure related to changes occurred in the soil, mainly due to degradation or recuperation of these environments (Chaer et al. 2009; Fernandes et al. 2011; Vallejo et al. 2012). The application of this technique linked to enzyme assays allows microbial communities to be characterized in terms of structure and function (Bardgett et al. 1999; Claassens et al. 2008; Hahn and Quideau 2013).
The main aim of the present study was to assessing the effect caused by re-vegetation of mining-polluted land dunes on the restoration of autochthonous microbial community of soil. Considering the low variability of the soil type, landscape position, and climatic differences between areas selected in the present study, changes in response variables must be primarily related to the timing of vegetation. Thus, this study is based on an observational, not an experimental, investigation. This study was designed to test the following hypotheses: (i) that, over a period of 20 years, the re-vegetation of mined areas with seedlings of native plants allows the restoration of the structure and activity of the microbial community to its original condition and (ii) that communities in non-degraded areas are more stable against seasonal variations. With these hypotheses in mind, changes in the composition of fatty acid methyl esters (FAMEs) and in soil microbial properties were assessed in three areas of mined coastal dunes at different stages in the recovery process (one, nine, and 21 years) and compared to a dune with native vegetation. These measurements were made during the dry and rainy seasons.
Materials and Methods
Sampling Sites
Soil samples were taken from dune areas in Brazil owned by the Millennium Mineração Ltda., a Cristal Global company, located in the municipality of Mataraca, in the north of the State of Paraíba (6°28′20″–6°30′00″S, 34°55′50″–34°57′10″W). The local climate is tropical wet (Köppen Am type) with average annual temperature of 25.5 °C, ranging from 23.7 °C in July to 26.8 °C in December, and annual average rainfall of 1795 mm, with 80 % of the rainfall concentrated from April to August. The soil in the collection areas is of type quartzarenic neosols. The predominant geological formation is composed of sandy clay, sedimentary rocks overlain by fixed dunes, where minerals of economic interest, such as ilmenite (FeO·TiO2), zirconium (ZrO2·SiO2), and rutile (TiO2), are found (Souza et al. 2010).
During the process of mining, the dunes are disassembled and the ores are separated from the sand by density. The tailings (quartzite sand) are deposited on the side of the mined areas, and the surface layer (30 cm) of soil is transported and subsequently used to cover areas that will be replanted (Souza et al. 2010). The planting of seedlings in all areas was done in pits of approximately 70 × 20 × 20 cm, with a distance of 2 m between plants and between lines. Only the areas of 1 and 9 years of age were fertilized at planting with an organic blend of sugarcane bagasse and cattle manure (pH H2O = 7.3; N = 1.4 g kg−1; C = 147.2 g kg−1; P = 0.4 g kg−1; and K = 1.5 g kg−1) per pit.
Re-vegetation of the studied areas was carried out using native plant species, represented mainly by the families Leguminosae, Anacardiaceae, Bignoniaceae, Rhamnaceae, Myrtaceae, Rubiaceae, Chrysobalanaceae, Annonaceae, Malvaceae, and Sapotaceae. However, floristic surveys of these places, carried out by the company, indicate that there is a gradual change of vegetative cover over time. Arboreal species such as Leguminosae, Anacardiaceae, Bignoniaceae, Rhamnaceae, Myrtaceae, Rubiaceae, Annonaceae, Malvaceae, Chrysobalanaceae, and Sapotaceae are more common in the older areas, whereas in the younger ones, herbaceous species from the families Leguminosae, Poaceae, Rubiaceae, Tiliaceae, Lamiaceae, Passifloraceae, Loganiaceae, Turneraceae, Euphorbiaceae, Violaceae, and Asteraceae predominate.
Sampling
Soil samples were collected in March (dry season) and July (rainy season) of 2010 at a depth of 0-10 cm. The study was conducted in three areas of mined dunes with 1, 9, and 21 years of re-vegetation. A dune with preserved arboreal restinga vegetation (natural dune) was used as a reference. In each area, four plots of 5 × 20 m were drawn, and in each plot, six samples were collected to form a composite sample. The soil samples were placed in plastic bags, taken to the laboratory, and stored under refrigeration (4 °C) until the biochemical and microbiological analyses were conducted.
Analyses of the Biological Properties of the Soil
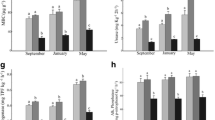
The carbon content of the microbial biomass (MBC) was estimated by the fumigation-extraction method (Vance et al. 1987). Fumigation was conducted with ethanol-free chloroform in 10 g of soil, followed by carbon extraction with potassium sulfate (0.5 M) and oxidation with potassium dichromate (0.66 mM) in concentrated acid medium. Carbon quantification was carried out by titration with ferrous ammonium sulfate (0.033 N), using 1 % diphenylamine as an indicator (De-Polli and Guerra 1997). The MBC values were calculated by the difference of carbon in fumigated and non-fumigated soil samples, considering K C = 0.41 (Babuija et al. 2010). Basal respiration (RES) was estimated from the incubation of soil samples (50 g) in a closed system with 10 mL of NaOH (0.5 M), for seven days. The CO2 captured by the NaOH solution (0.5 M) was measured by titration with 0.1 N HCl (Alef and Nannipieri 1995). The activities of the enzymes β-glucosidase (BETA) and arylsulfatase (ARYL) were determined according to Tabatabai and Bremner (1970), except for the use of toluene, which was excluded during the assays. Acid phosphatase activity (PHOS) was determined according to Tabatabai and Bremner (1969), omitting the toluene. The activity of these enzymes is based on colorimetric determination of p-nitrophenol released in 1 g of soil samples after incubation for 1 h at 37 °C. For each enzyme, specific substrates were used in buffered solutions. One-mL solutions of p-nitrophenyl-β-d-glucoside (25 mM), p-nitrophenyl-sulfate (0.05 M), and p-nitrophenyl-phosphate (0.05 M) were used for the activity of β-glucosidase, arylsulfatase, and phosphatase, respectively. The p-nitrophenol released was extracted by filtration and determined colorimetrically at 400 nm.
The activity of dehydrogenase (DEHY) was determined according to the methodology of Casida et al. (1964). In short, soil samples (3 g) were incubated with 3 mL of 1 % TTC (2,3,5 triphenyltetrazolium chloride) in a water bath (37 °C) for 24 h. After this period, the reaction was stopped with 10 mL of methanol and the TTF (triphenylformazan), formed by the action of dehydrogenase in reduction of TTC, was measured in a spectrophotometer (485 nm). Urease activity was measured by determining the ammonia released after incubation of 2.5 g of soil in urea solution (0.2 M) at 37 °C for 2 h, and the reading of the samples was done in a spectrophotometer (690 nm) (Kandeler and Gerber 1988). Fluorescein diacetate (FDA) hydrolysis was estimated according to Swisher and Carrol (1980) in 5 g of soil samples incubated with 20 mL of potassium phosphate buffer (66 mM; pH 7.6) and 200 μL of fluorescein diacetate solution (0.02 g 10 mL−1 acetone) for 30 min. After this period, the reaction was stopped with the addition of 20 mL of acetone, and the readings were done in a spectrophotometer (490 nm).
Extraction of Fatty Acids Methyl Esters (EL-FAME)
The structure of the microbial community was investigated using the technique of ester-linked fatty acid profiles extracted from soil according to Schutter and Dick (2000). In summary, 3 g of soil samples under field capacity were weighed in glass centrifuge tubes (35 mL) with Teflon-lined cap. Fifteen mL of KOH solution (0.2 M) prepared in methanol was added to the soil, and the tubes were agitated for 15 s on a vortex-type agitator and incubated for 1 h at 37 °C. For every 10 min during the incubation period, the bottles were shaken in a vortex to increase the efficiency of extraction of fatty acids and formation of their methyl forms (methyl esters of fatty acids, FAME). After incubation, 3 mL of acetic acid (1 M) was added and the samples were homogenized. Ten mL of hexane was added to the tubes to promote organic and aqueous phase partition. The samples were centrifuged for 10 min (480 × g). The organic phase with hexane, which contains the FAMEs, was transferred by pipette to test tubes and dried in an ultrapure N2 atmosphere at a temperature of 37 °C. After drying, the FAMEs were re-suspended in hexane and transferred to amber chromatography tubes (GC vials). One-μL samples were injected into the gas chromatograph equipped with a capillary column (5 % biphenyl–95 % dimethylpolysiloxane, 25 M) and flame ionization detector (FID). The equipment was programed to allow an increase of 4 °C min−1, from 120 to 270 °C. The temperatures of the injector and detector were 250 °C and 280 °C, respectively (Fernandes et al. 2011). The chromatographic peaks were identified on the basis of a comparison of the microbial FAME retention times of samples and commercial standards (BAME and FAME 37, Supelco). Changes in microbial community structure were interpreted by the analysis of concentration of FAMEs characteristic of each group relative to the total of these microbial compounds in the samples. The following microbial groups, with their respective biomarkers identified in parentheses, were analyzed: Gram-positive bacteria (15:0i + 15:0a + 16:0i + 17:0i + 17:0a), Gram-negative bacteria (16:1ω7c + 18:1 ω7c + 17:0cy + 19:0cy), actinomycetes (16:0 10 Me), arbuscular mycorrhizal fungi (AMF) (16:1 ω5c), and saprobic fungi (18:2ω6c + 18:1ω9c). The relationship between biomarkers of fungus and bacteria (F/B) was calculated by the ratio between the mass of the FAMEs of fungal (18:2ω6c + 18:1ω9c) (Kaiser et al., 2010) and bacterial origin (15:0i + 15:0a + 16:0i + 17:0i + 17:0a + 17:0cy, 18:1ω7c + 19:0cy) (Frostegård and Bååth 1996). The ratio between the 19:0cy and its precursor (18:1ω7c) was used to assess the state of microbial stress of the bacterial community (Guckert et al. 1986).
Data Analysis
Multivariate analyses were used to evaluate the effects of re-vegetation time on the structure and function of the microbial community during the dry and rainy seasons. Differences in the microbial community structure were represented graphically using the NMS (non-metric multidimensional scaling) ordination technique (Sokal 1979; McCune and Grace 2002), using the Sørensen distance measure, a statistic used for comparing the similarity of two multivariate samples.
Before data analysis began, the principal matrix containing data about the microbial community was relativized in order to reduce the impact of differences in the total quantities of fatty acids obtained in the samples and focus the analysis on the relative composition of the FAMEs within the various groups of organisms in the microbial community. FAMEs that occurred in amounts smaller than 2 % of the fatty acid 16:0 were excluded from the data matrix.
The ordinations obtained were submitted to a rotation procedure so that samples from the natural dune were always on the left side of axis 1. Changes in the structure of the microbial communities were characterized using Pearson correlation coefficients (r) between the percentage contribution of the FAMEs of microbial groups in the samples and the scores of the ordination axes by NMS. Analyses of Pearson correlation were also used to describe the relationships between biological variables and the microbial community structure (scores of samples on the ordination axes for NMS) and individually with the biomarkers of the microbial groups.
Statistical differences in the structure of microbial communities at different stages of re-vegetation and sampling seasons were assigned using the multi-response permutation procedure (MRPP) based on Sørensen distance (P < 0.05). A matrix consisting of the data of nine microbiological variables (MBC, RES, PHOS, BETA, ARYL, FDA, DEHY, URE, and the ratio 19:0cy/18:1ω7, descriptive of bacterial stress), relativized by their respective totals in the columns, was used to describe the differences between the samples regarding the functioning of microbial communities.
All multivariate tests were conducted using the statistical program PC-ORD, version 6.0 (McCune and Mefford 2011).
Results
Soil Properties
The chemical characteristics of the soil are presented in Table 1. The mean and coefficient of variation (CV) of the soil moisture and soil biological properties are shown in Table 2. The sand content in these soils ranged from 94.3 to 96.5 %, and silt and clay contents were less than 5.5 %. All soils had a similar texture, being classified as sandy soils.
Seasonal Influence on Microbial Community Structure
A 2D graph of ordination by NMS represented 81 % of the total variability of the original data regarding the structure of the microbial communities investigated by the ester-linked fatty acid technique (Fig. 1). Most of the variation in the data (52 %) was associated with the axis 2, while axis 1 represented 29 % of the data variability (Fig. 1).
Differences between the structure of the microbial community in soils of a native dune and re-vegetated sand dunes in response to season and different periods of re-vegetation, in accordance with NMS ordination. The symbols represent the centroid of the samples under each condition of re-vegetation and sampling time (n = 4). The horizontal and vertical bars indicate standard deviation of the samples in relation to their centroids along axes 1 and 2, respectively. ND natural dune, DP dry period, RP rainy period, year (s): time of re-vegetation
Between sampling periods, the differences in microbial community structure were strongly differentiated along axis 2, with data points from the dry season positioned in the upper part of the graph and those from the rainy season at the bottom (Fig. 1). Positive correlations were observed between the scores of this axis and the biomarkers of Gram-positive bacteria 15:0i (r = 0.74; P < 0.001) and 16:0i (r = 0.82; P < 0.001), indicating a relative enrichment of these microorganisms in samples taken during the dry season. In contrast, Gram-negative bacteria were favored during the rainy season, as indicated by the negative correlations between two of their biomarkers, 16:1ω7c (r = −0.63; P < 0 .01) and 17:0cy (r = −0.59; P < 0.05), and axis 2 scores.
According to the MRPP technique, the communities established during the dry and rainy seasons differed from each other in the re-vegetated areas (P < 0.001). Although these differences were observed, they were less marked than in the natural sand dune, suggesting a greater stability in relation to these communities found in recomposed areas (Table 3).
Microbial Community Structure in a Chronosequence
Due to the strong seasonal influence on Gram-positive and Gram-negative bacteria, the dry and rainy seasons were analyzed separately.
The ordinations of microbial communities in both seasons were very similar with respect to the percentage of the total variability of the fatty acid data represented by the two axes and by the partition of this variability between them. In both cases, the variability represented along axis 1, 55 % in the dry season and 56 % in the rainy season, was significantly greater than axis 2, where the dry season represented 32 % and the rainy season 30 % of the data variation. Independent of the season evaluated, the structures of microbial communities of areas re-vegetated for nine and 21 years did not differ between themselves, but were distinct from those established on the natural dunes and in the areas with 1 year of re-vegetation, occupying intermediate positions between those along axis 1 (Fig. 2).
Changes in the structure of the soil microbial community of a native dune and re-vegetated sand dunes in response to different vegetation ages according to NMS ordination. The symbols represent the centroid of the samples under each condition of re-vegetation and sampling time (n = 4). The horizontal and vertical error bars indicate the standard deviation of the samples in relation to their centroids along axes 1 and 2, respectively. NSD: natural sand dune; a DP dry period; b RP rainy period; year(s): time of re-vegetation
In the dry season, the distribution of samples along axis 1 showed a negative correlation with AMF (r = −0.87; P < 0.001) and a positive correlation with GNB (r = 0.65; P < 0.01) (Table 4). During the rainy season, variations of AMF and GNB along axis 1 were similar to those observed in the dry season, while gradients of concentrations of biomarkers for saprobe fungi and Actinomycetes along this axis were observed only in the rainy season (Table 4). In this case, increments of saprobe fungi were observed in the direction of the natural dune in relation to the area with 1 year of re-vegetation, while those of Actinomycetes showed the opposite behavior.
Microbial components associated with axis 2 were observed in the dry season, in areas of native sand dunes; those re-vegetated for 1 year were located at the bottom of the chart, and presented a higher similarity between themselves than with the areas of older re-vegetation located at the top of the chart. In the dry season, the distribution of the samples in the upward direction of this axis was positively correlated with biomarkers of GPB, GNB, and AMF, and negatively with the saprobic fungi. Even though with the re-vegetation time the general similarity among mined and natural dunes had been incremented, the areas re-vegetated the longest (nine and 21 years) presented, during the dry season, more favorable conditions for the establishment of GPB, and were less favorable for the saprobic fungi in relation to natural dunes and the most recently re-vegetated areas (Table 4).
In the rainy season, GPB and saprobic fungi showed the same patterns of variation observed in the dry season regarding to the axis 2. However, unlike in the dry season, the distribution of samples along axis 2 in the rainy season was not linked to concentrations of a biomarker of AMF (Table 4).
Stands out that in the rainy season, saprobic fungi and AMF were negatively correlated with both axes, indicating that the establishment of these microbial groups was especially favored in the native dune areas, as opposed to the degraded areas, regardless of their time of re-vegetation.
The soil biological properties were correlated with changes in microbial community structure in the areas of mined and re-vegetated sand dunes. The negative correlations between the biological properties and axis 1 scores indicated an increment in the activity of the enzyme acid phosphatase (P < 0.001) and arylsulfatase (P < 0.05) toward the reference area during the dry season (Fig. 2; Table 5). The increase in the stress indicator 19:0cy/18:1ω7 ratio during this season occurred toward the younger re-vegetated area, as indicated by the positive correlation between this indicator and the scores of axis 1 (Fig. 2; Table 5). During the rainy season, the correlations among axis 1 and bacterial stress, PHOS, and ARYL were similar to those observed in the dry season. However, in the rainy season, the MBC and BETA were also associated with microbial community structure, with increments of these variables being observed toward the communities of native dune areas, along axis 1 (Table 5).
With respect to the components of community structure associated with axis 2, positive correlations were observed with PHOS and BETA in the dry season, indicating an increased gradient in the potential activity of these enzymes toward samples located in the upper part of the chart. However, correlations were not found among variables of microbiological properties and axis 2 scores of the structure of microbial communities in the rainy season (Fig. 2; Table 5).
Influence of Chronosequence on Soil Microbiological Properties
To characterize the seasonal effect on soil microbiological properties (SMP) during the dry and rainy seasons, both seasons were analyzed together. Figure 3 represents the differences between the SMP of the re-vegetated dune areas in both seasons. Most of the variation in the data (86 %) was associated with axis 1, while axis 2 represented only 8 % of the variability of the data (Fig. 3).
Differences among the soil microbiological properties of a native dune and re-vegetated sand dunes in response to temporal variations and different times of re-vegetation, in accordance with NMS ordination. The symbols represent the centroid of the samples under each condition of re-vegetation and sampling time (n = 4). The horizontal and vertical bars indicate standard deviation of the samples in relation to their centroids along axes 1 and 2, respectively. ND natural dune, DP dry period, RP rainy period; year(s): time of re-vegetation
The SMP did not differ (P = 0.061) between the seasons, dry and rainy, regardless of the vegetation age assessed, according to the MRPP technique (Table 6). However, when areas with the same vegetational conditions were compared with each other, in the two seasons, a difference in SMP among these locations was observed.
Like the changes in MCS in the re-vegetated sand dunes, SMP were also compared separately among the vegetational states during the dry and rainy seasons. The SMP of the area re-vegetated for 1 year were different from the other areas in the two seasons. Differences in the SMP of the natural dune and older re-vegetated areas were seen only in the dry season, with the SMP of the area re-vegetated 21 years ago similar to those of the area re-vegetated 9 years ago and natural dune. However, the SMP of the natural dune and re-vegetated for 9 years were different in this season.
The SMP of the re-vegetated area for 1 year differed from those of the other areas in both seasons. In the dry season, the SMP of the re-vegetated area 21 years ago were similar to the values registered in the dunes with 1 year of re-vegetation and in the natural dune. However, the SMP differed between these two last areas. During the rainy season, there were no differences in the SMP of the natural dune when compared to areas re-vegetated for nine and 21 years.
The negative correlations among the biological properties and the axis 1 scores indicated an increase in FDA hydrolysis (P < 0.001) and in the activity of the enzymes PHOS and ARYL (P < 0.001), BETA (P < 0.01) and URE (P < 0.05) toward the reference area during the dry season. In the rainy season, all the biological properties evaluated, with the exception of stress indicator 19:0cy/18:1ω7, were negatively correlated with axis 1, which is indicative of an increase in these properties toward the natural dune and areas re-vegetated for nine and 21 years. The increase of the stress indicator 19:0cy/18:1ω7, during the rainy season, occurred toward the younger re-vegetated area, as indicated by the positive correlation among scores of axes 1 and the 19:0cy/18:1ω7 ratio (r = 0.91; P < 0.001) (Fig. 4; Table 7).
Changes in soil microbiological properties of native dune and re-vegetated sand dunes in response to different vegetational ages according to NMS ordination. The symbols represent the centroid of the samples under each condition of re-vegetation and sampling time (n = 4). The horizontal and vertical bars indicate standard deviation of the samples in relation to their centroids along axes 1 and 2, respectively. NSD natural sand dune. a dry period. b rainy period; year(s): time of re-vegetation
Regarding to soil biological properties associated with axis 2 in the dry season, an increase in the stress indicator occurred toward the re-vegetated areas, located at the top of the chart, as indicated by the positive correlation between axis 2 scores and the 19:0cy/18:1ω7 ratio (r = 0.73; P < 0.001). The axis 2 correlated negatively with RES (r = −0.64; P < 0.01) and positively with the enzymes DEHYDR (r = .75; P < 0.001) and URE (r = .53; P < 0.05), indicating that potential activity of these enzymes increased toward areas located at the top of the chart (Fig. 4; Table 7). During the rainy season, there were no correlations among the biological properties of the soil and axis 2 (Table 7).
Discussion
Seasonal Effect on Soil Microbial Community Structure
During the dry season, there was an increase in the population of GPB compared to the number of GNB in the sand dune areas. This result can be related to the fact that gram-positive bacteria present thicker cell walls and are able to form more spores; together, these characteristics give them the capacity to tolerate this stressful condition (Bérard et al. 2011; Kavamura et al. 2013; Uhlířová et al. 2005). This assumption is supported by Harrison and Bardgett (2010) and Štursová and Baldrian (2011) who reported that GPB populations tend to increase in comparison with those of GNB when soil localities suffer periods of low soil humidity. However, the proportions of GNB tend to increase when stress situations have ceased (Bardgett et al. 2001; Romaniuk et al. 2011). Other studies also have shown that soil moisture regimes can have great influence on the whole microbial communities (Bardgett et al. 1999; Claassens et al. 2012; Swallow et al. 2009).
The microbial community structure in the natural dunes was less affected by season than in the re-vegetated areas, and this suggests a greater stability of the microbial communities in the natural areas, probably as a result of the greater maturity of the plant community in these areas. For example, soil microbial communities sampled from natural forests are more resistant to environmental changes (pH, temperature, and soil moisture) than those found in soils in the process of recovery (Hahn and Quideau 2013). According to these authors, these differences are due to a complex and indirect system of interactions that occur between soil and plants.
Effect of Re-vegetation on Soil Microbial Community Structure
Even though re-vegetation contributed to recovery of the microbial community, the results of this study showed that the microbial composition of the re-vegetated areas, regardless of the time of re-vegetation and sampling period, still differed from the natural dune area. However, with increasing age, the composition of the microbial community became more similar to that found in natural areas.
Studies comparing microbial fatty acid profiles between soils of native grasslands and restored grasslands have shown that there was an increase in the quantities of phospholipid fatty acid (PLFA) in the restored areas depending on their age. However, even 24 years after the beginning of restoration, the microbial profiles in these areas were still different when compared to the native areas (McKinley et al. 2005).
In our study, microbial community structure had recovered in the nine- and 21-year-old re-vegetation plots, but it was not yet identical to the MCS of natural dunes. In fact, some microbial components (associated with axis 2) are even more different in older areas from natural dunes when these are compared to the area with 1 year of re-vegetation. Some studies report that depending on the land use, the microbial community structure of restored soil though stabilized will never be the same as the microbial community found in native soils (Huang et al. 2011; Jangid et al. 2010).
In addition to showing positive effects on the microbial communities in soils of mined dunes, re-vegetation promoted the recovery of biological activity. The recovery of microbial activity does not happen immediately, as observed in the area with 1 year of re-vegetation, which was clearly different compared with the other three areas studied (Fig. 4). The exact time required for full recovery is difficult to predict and depends on the environmental conditions as much as the ecosystem in the process of recovery (Hahn and Quideau 2013).
With the exception of the Actinomycetes, which population increased in the younger area only during the rainy season, it was possible to observe an increase of specific microbial groups toward the same areas in the two seasons evaluated. As for Actinomycetes, assessments made in places with different plant formations have shown that the PLFA indicator of this group was found mainly in the area of deciduous conifers, moister, and richer in organic matter (Swallow et al. 2009). The topsoil left over from natural forest areas, used in the re-vegetation process of dunes, is rich in plant debris (trunks, leaves, and roots), which may have favored the increase in the population of Actinomycetes in the rainy season due to moisture conditions in this season. The growth of these microorganisms, possibly, was benefited. These results suggest that in addition to the temporal changes, other factors such as age of the locations and possible differences in the functioning of plant development appear to be related to the changes in the composition of the soil microbial community.
The increase in the quantities of AMF toward the natural dune indicates that mycorrhizal symbiosis may have been gradually restored in re-vegetated areas (Zhang et al. 2012). The community of AMF is described as having an important role in the activity of plants, as well as in the channeling of root-deposited C for the soil microbial community (Balasooriya et al. 2014). This result may be related to the fact that these microorganisms are directly involved in the C flow from plants to soil. The AMF participate in the transfer of soluble compounds of low molecular weight from the root exudates, while saprophytic fungi provide C through decomposition of litterfall, process that requires the synthesis of various hydrolytic and oxidative enzymes (Criquet et al. 2000; Baldrian 2009).
Taking into consideration that the GPB were most apparent in the season with little rainfall, where moisture conditions were limiting, the increase of GPB occurred in the areas in the older restoration process, in the two seasons, and not in the younger areas. This result shows that the analysis of the seasons together explains that the variations of MCS occur due to soil moisture as limiting factor, while the separate analysis of the seasons shows the changes in MCS and their relationships with soil properties as a function of the age of the areas.
The increase of GNB in the younger area, in the two seasons, indicates the capacity of these organisms to utilize simple C sources and to adapt quickly to different conditions of adversity, being more tolerant to soil disturbances than fungi (Bardgett et al. 2001; Carrasco et al. 2010; Zhang et al. 2012). Changes in bacterial community composition are described as resulting from significant changes in the plant community (Jangid et al. 2011).
The fungi/bacteria ratio (F/B) increased toward the natural dune area and the area with 21 years of re-vegetation, what could be related to better soil conditions in these areas. In re-vegetated areas, the F/B ratio increased consistently with the re-vegetation time and was correlated with the increase in organic C, total P, total N, available P, porosity, and other properties that indicate improvements in soil quality of the re-vegetated areas (Zhang et al. 2012), as observed in the present study. Besides, the increase in quantities of fungi due to decreased levels of disruption was reported by Allison et al. (2005) in prairie areas undergoing recovery in the United States. Studies of managed pasture areas showed that the proportion of F/B varies gradually with the intensity of management, being higher in the unfertilized areas and lower in fertilized areas; in this case, the decrease in the amount of fungi occurs with greater intensity of management and was responsible for variation in the F/B ratio (Bardgett et al. 1999).
The increase in the stress indicator 19:0cy/18:1ω7 occurred toward the younger area, as indicated by the positive correlation among the scores of axes 1 and this indicator, during the rainy season (Fig. 2; Table 4). In mined areas, 19:0cy/18:1ω7 ratio significantly decreased with time after re-vegetation and with the improvement of soil nutrients (Zhang et al. 2012). According to Claassens et al. (2012), the increase of 19:0cy/18:1ω7 ratio indicates higher levels of stress in coal and asbestos discard areas.
Effect of Re-vegetation on Biological Properties of the Soil and Its Relationship with the Soil Microbial Community Structure
The negative correlations among the biological properties and axis 1 scores indicate that there was an increase in enzyme activity and MBC toward the reference area during the seasons studied. The increase of these activities corresponded to the relative increase in the quantities of saprophytic and AMF symbiotic fungi. The highest phosphatase activity observed on the reference area indicate the important role of fungal populations in the P cycling, which can be confirmed by the significant correlation between β-glucosidase activity and FAME biomarkers of saprophytic fungi (decomposing of organic matter) and AMF (increase P availability for plants) (Vallejo et al. 2012).
According to Baldrian et al. (2010), there is a high correlation between the increase in the activity of hydrolase enzymes and the F/B ratio, even when there is no correlation with the increase in fungal community. The principal factor that affects the production of enzymes in the soil is the presence of microorganisms. The substrates and inducers of the expression of enzymes (Baldrian et al. 2010), soil quality, leaf litter, and the effects of the rhizosphere are also important factors that can regulate the enzyme activity in soils. The increase in microbial activity accompanying the plant succession reflects a likely increase in the complexity and diversity of the microbial community in soils of areas in the recovery process (Fioretto et al. 2009). Together with the successional changes, these microbial communities and their activities are affected by temporal fluctuations throughout the year (Baldrian et al. 2008).
The recovery of SMP was observed in the mined areas with increased re-vegetation time, with the oldest area appearing more similar to the natural dune area in the two seasons. Microorganisms play fundamental roles that determine productivity and nutrient availability in forest ecosystems, being important in the establishment and maintenance of soils in sustainable ecosystems (Badiane et al. 2001; Brockett et al. 2012). This makes it necessary to know how microbial communities, their structure, and function change or may indicate changes in functioning of environmental factors (Brockett et al. 2012), stress, and/or the restoration process (Claassens et al. 2012).
Soil microbial processes can be influenced by changes in the MCS resulting from seasonal variations (Bardgett et al. 1999). The correlations between enzyme activities (e.g., phosphatase activity) and microbial groups (e.g., saprophytic fungi and AMF) indicate how changes in MCS could affect soil functions, and consequently, important ecosystem services such as nutrient cycling (Vallejo et al. 2012). It is believed that redundancy of functions happens frequently in soils and, due to these functional redundancies, a reduction in any microbial group has little effect on the soil specific functions, since other microorganisms can continue performing the functions (Brockett et al. 2012; Claassens et al. 2012).
Conclusions
Although increased re-vegetation time results in a community structure generally closer to that of the original dune, this new community seems to stabilize (no difference between 9 and 21 years) without fully re-establishing the structure found in the natural dune. Our results demonstrate that changes in the MCS with re-vegetation time are related to changes in the SMP. Some microbial groups (saprophytic fungi and AMF) and enzymatic activities (acid phosphatase and β-glucosidase) being sensible indicators of the re-vegetation progress.
The seasonal variations affect MCS and the SMP in both natural and re-vegetated areas, and in the dry period, the effect of the re-vegetation time on SMP is more pronounced. These results highlight the importance of seasonal samplings in studies that aim to determine changes in soil microbial communities in field conditions. However, monitoring these areas for a longer period of time is required to confirm the trends observed and deepen knowledge about the factors that control the community composition in re-vegetated areas.
References
Alef K, Nannipieri P (1995) Methods in applied soil microbiology and biochemistry. Academic Press, San Diego
Allison VJ, Miller RM, Jastrow JD, Matamala R, Zak DR (2005) Changes in soil microbial community structure in a tallgrass prairie chronosequence. Soil Sci Soc Am J 69:1412–1421
Babuija LC, Hungria M, Franchini JC, Brookes PC (2010) Microbial biomass and activity at various soil depths in a Brazilian oxisol after two decades of no-tillage and conventional tillage. Soil Biol Biochem 42:2174–2181
Badiane NNY, Chotte JL, Pate E, Masse D, Rouland C (2001) Use of soil enzymes to monitor soil quality in natural and improved fallows in semi-arid tropical regions. Appl Soil Ecol 18:229–238
Balasooriya WK, Denef K, Huygens D, Pascal P (2014) Translocation and turnover of rhizodeposit carbon within soil microbial communities of an extensive grassland ecosystem. Plant Soil 376(1–2):61–73
Baldrian P (2009) Microbial enzyme-catalyzed processes in soils and their analysis. Plant Soil Environ 55(9):370–378
Baldrian P, Trogl J, Frouz J, Šnajdr J, Valášková V, Merhautová V, Cajthaml T, Herinková J (2008) Enzyme activities and microbial biomass in topsoil layer during spontaneous succession in spoil heaps after brown coal mining. Soil Biol Biochem 40:2107–2115
Baldrian P, Merhautová V, Cajthaml T, Petránková M, Šnajdr J (2010) Small-scale distribution of extracellular enzymes, fungal, and bacterial biomass in Quercus petraea forest topsoil. Biol Fert Soils 46:717–726
Bardgett RD, Cook R, Yeates GW, Denton CS (1999) The influence of nematodes on below-ground processes in grassland ecosystems. Plant Soil 212:23–33
Bardgett RD, Jones AC, Jones DL, Kemmitt SJ, Cook R, Hobbsq PJ (2001) Soil microbial community patterns related to the history and intensity of grazing in sub montane ecosystems. Soil Biol Biochem 33:1653–1664
Brockett BFT, Prescott CE, Grayston SJ (2012) Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western Canada. Soil Biol Biochem 44:9–20
Carrasco L, Gattinger A, Fließbach A, Roldán A, Schloter M, Caravaca F (2010) Estimation by PLFA of microbial community structure associated with the rhizosphere of Lygeum spartum and Piptatherum miliaceum growing in semiarid mine tailings. Microb Ecol 60:265–271
Casida LE, Klein DA, Santoro T (1964) Soil dehydrogenase activity. Soil Sci 98:371–376
Chaer G, Fernandes M, Myrold D, Bottomley P (2009) Comparative resistance and resilience of soil microbial communities and enzyme activities in adjacent native forest and agricultural soils. Microb Ecol 58:414–424
Chaer GM, Tótola MR (2007) Impacto do manejo de resíduos orgânicos durante a reforma de plantios de eucalipto sobre indicadores de qualidade do solo. Rev Bras Cienc Solo 31:1381–1396
Chodak M, Pietrzykowski M, Niklinska M (2009) Development of microbial properties in a chronosequence of sandy mine soils. Appl Soil Ecol 41:259–268
Claassens S, Riedel KJ, van Rensburg L, Morgenthal TL, van Rensburg PJ (2005) Soil microbial properties in coal mine tailings under rehabilitation. Appl Ecol Env Res 4:75–83
Claassens S, van Rensburg PJ, Maboeta MS, van Rensburg L (2008) Soil microbial community function and structure in a post-mining chronosequence. Water Air Soil Poll 194:315–329
Claassens S, van Rensburg PJ, Liebenberg D, van Rensburg L (2012) A Comparison of microbial community function and structure in rehabilitated asbestos and coal discard sites. Water Air Soil Poll 223:1091–1100
Criquet S, Joner E, Leglize P, Leyval C (2000) Anthracene and mycorrhiza affect the activity of oxidoreductases in the roots and the rhizosphere of lucerne (Medicago sativa L.). Biotechnol Lett 22:1733–1737
De-Polli H, Guerra JGM (1997) Determinação do carbono da biomassa microbiana do solo: Método da fumigação-extração. Embrapa-CNPAB, Seropédica
Fernandes MF, Barreto AC, Mendes IC, Dick RP (2011) Short-term response of physical and chemical aspects of soil quality of a kaolinitic Kandiudalfs to agricultural practices and its association with microbiological variables. Agr Ecosyst Environ 142:419–427
Fioretto A, Papa S, Pellegrino A, Ferrigno A (2009) Microbial activities in soils of a Mediterranean ecosystem in different successional stages. Soil Biol Biochem 41:2061–2068
Frostegård A, Bååth E (1996) The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol Fert Soils 22:59–65
Guckert JB, Hood MA, White DC (1986) Phospholipid ester-linked fatty-acid profile changes during nutrient deprivation of vibrio-cholerae - increases in the trans cis ratio and proportions of cyclopropyl fatty-acids. Appl Environ Microb 52:794–801
Hahn AS, Quideau SA (2013) Long-term effects of organic amendments on the recovery of plant and soil microbial communities following disturbance in the Canadian boreal forest. Plant Soil 363:331–344
Harrison KA, Bardgett RD (2010) Influence of plant species and soil conditions on plant–soil feedback in mixed grassland communities. J Ecol 98:84–395
Hinojosa MB, Carreira JA, García-Ruíz R, Dick RP (2004) Soil moisture pre-treatment effects on enzyme activities as indicators of heavy metal contaminated and reclaimed soils. Soil Biol Biochem 36:1559–1568
Izquierdo I, Caravaca F, Alguacil MM, Hernández G, Roldán A (2005) Use of microbiological indicators for evaluating success in soil restoration after re-vegetation of a mining area under subtropical conditions. Appl Soil Ecol 30:3–10
Jangid K, Williams MA, Franzluebbers AJ, Blair JM, Coleman DC, Whitman WB (2010) Development of soil microbial communities during tallgrass prairie restoration. Soil Biol Biochem 42:302–312
Jangid K, Williams MA, Franzluebbers AJ, Schmidt TM, Coleman DC, Whitman WB (2011) Land-use history has a stronger impact on soil microbial community composition than aboveground vegetation and soil properties. Soil Biol Biochem 43:2184–2193
Kaiser C, Frank A, Wild B, Koranda M, Richter A (2010) Negligible contribution from roots to soil-borne phospholipid fatty acid fungal biomarkers 18:2u6,9 and 18:1u9. Soil Biol Biochem 42:1650–1652
Kandeler E, Gerber H (1988) Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol Fert Soils 6:68–72
Kavamura VN, Taketani RG, Lancôni MD, Andreote FD, Mendes R, Melo IS (2013) Water regime influences bulk soil and rhizosphere of Cereus jamacaru bacteria communities in the brazilian Caatinga Biome. PLoS One 8(9):e73606
McCune B, Grace JB (2002) Analysis of ecological communities. MjM Software Design, Gleneden Beach, Oregon
McCune B, Mefford MJ (2011) PC-ORD. Multivariate analysis of ecological data. Version 6. MjM Software, Gleneden Beach, Oregon
McKinley VL, Peacock AD, White DC (2005) Microbial community PLFA and PHB responses to ecosystem restoration in tallgrass prairie soils. Soil Biol Biochem 37:1946–1958
Mendes IC, Fernandes MF, Chaer GM, Reis Junior FB (2012) Biological functioning of Brazilian Cerrado soils under different vegetation types. Plant Soil 359(1–2):183–195
Mummey DL, Stahl PD, Jeffrey SB (2002) Soil microbiological properties 20 years after surface mine reclamation: spatial analysis of reclaimed and undisturbed sites. Soil Biol Biochem 34:1717–1725
Ramsey PW, Gibbons SM, Rice P, Mummey DL, Feris KP, Moore JN, Rillig MC, Gannon JE (2012) Relative strengths of relationships between plant, microbial, and environmental parameters in heavy-metal contaminated floodplain soil. Pedobiologia 55:15–23
Romaniuk R, Giuffré L, Costantinia A, Nannipieri P (2011) Assessment of soil microbial diversity measurements as indicators of soil functioning in organic and conventional horticulture systems. Ecol Indic 11:1345–1353
Schutter ME, Dick RP (2000) Comparison of fatty acid methyl ester (FAME) methods for characterizing microbial communities. Soil Sci Soc Am J 64:1659–1668
Silva DKA, Freitas NO, Souza RG, Silva FSB, Araujo ASF, Maia LC (2012) Soil microbial biomass and activity under natural and regenerated forests and conventional sugarcane plantations in Brazil. Geoderma 189–190:257–261
Sokal RR (1979) Testing statistical significance of geographic-variation patterns. Syst Zool 28:227–232
Souza RG, Goto BT, Silva DKA, Silva FSB, Sampaio EVSB, Maia LC (2010) The role of arbuscular mycorrhizal fungi and cattle manure in the establishment of Tocoyena selloana Schum. in mined dune areas. Eur J Soil Biol 46:237–242
Štursová M, Baldrian P (2011) Effects of soil properties and management on the activity of soil organic matter transforming enzymes and the quantification of soil-bound and free activity. Plant Soil 338:99–110
Swallow M, Quideau SA, MacKenzie MD, Kishchuk BE (2009) Microbial community structure and function: The effect of silvicultural burning and topographic variability in northern Alberta. Soil Biol Biochem 41:770–777
Swisher R, Carrol GC (1980) Fluorescein diacetate hydrolysis as an estimator of microbial biomass on coniferous needle surfaces. Microb Ecol 6:217–226
Tabatabai MA, Bremner JM (1970) Arylsulfatase activity of soils. Soil Sci Soc Am Pro 34:225–229
Tabatabai MA, Bremner JM (1969) Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem 1:301–307
Vallejo VE, Arbeli Z, Teran W, Lorenz N, Dick RP, Roldan F (2012) Effect of land management and Prosopis juliflora (Sw.) DC trees on soil microbial community and enzymatic activities in intensive silvopastoral systems of Colombia. Agr Ecosyst Environ 150:139–148
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Huang Y-M, Michel K, Shao-Shan An, Zechmeister-Boltenstern S (2011) Changes in microbial-community structure with depth and time in a chronosequence of restored grassland soils on the Loess Plateau in northwest China. J Plant Nutr Soil Sc 174:765–774
Zhang B, He H, Ding X, Zhang X, Zhang X, Yang X, Filley TR (2012) Soil microbial community dynamics over a maize (Zea mays L.) growing season under conventional- and no-tillage practices in a rainfed agroecosystem. Soil Till Res 124:153–160
Acknowledgments
Thanks are due to the Millennium Inorganic Chemicals—a Cristal Company and ‘Embrapa Tabuleiros Costeiros’ for logistical support; FACEPE for a PhD scholarship to V.M. Santos; CNPq for a PhD scholarship to D.K.A. Silva and a research fellowship and grant support provided to L.C. Maia; and CAPES for a doctoral scholarship granted to I.E.C. Escobar and financing of the project: Microbial dynamics and viability of land-use coverage in the natural regeneration of dunes impacted by mining on the coast of Paraíba, Brazil (PNPD-CAPES/CNPq). We are indebted with Érika Anjos, Daniele Assis, and Daniela Tenório for helping with the analyses; David Bousfield for his useful suggestions; and Scott Heald for English’s review of the manuscript. We also thank the reviewers for their valuable comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Escobar, I.E.C., Santos, V.M., da Silva, D.K.A. et al. Changes in Microbial Community Structure and Soil Biological Properties in Mined Dune Areas During Re-vegetation. Environmental Management 55, 1433–1445 (2015). https://doi.org/10.1007/s00267-015-0470-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00267-015-0470-8