Abstract
Background
In Asia, the demand for cosmetic facial treatments has surged due to technological advancements, increased social acceptability, and affordability. Poly-l-lactic acid (PLLA) fillers, known for their biocompatibility and biodegradability, have emerged as a popular choice for facial contouring, yet studies specifically addressing their use in Asian populations are scarce.
Methods
This retrospective study examined 30 Chinese patients who underwent facial contouring with PLLA fillers, focusing on product composition, injection techniques, and safety measures. A comprehensive clinical evaluation was performed, including the Global Aesthetic Improvement Scale (GAIS) and Global Impression of Change Scale (GICS) for effectiveness and patient satisfaction, respectively.
Results
No significant difference in GAIS scores was observed between injectors and blinded evaluators over a 12-month period, indicating consistent effectiveness. Patient satisfaction remained high, with GICS scores reflecting positive outcomes. The safety profile was favorable, with no serious adverse events reported. The study highlighted the importance of anatomical knowledge to avoid complications, particularly in areas prone to blindness.
Conclusions
PLLA fillers offer a safe, effective option for facial contour correction in the Asian population, achieving high patient satisfaction and maintaining results over time. The study underscores the need for tailored approaches in cosmetic procedures for Asians, considering their unique facial structures and aesthetic goals. Further research with larger, multicenter cohorts is recommended to validate these findings and explore long-term effects.
Level of Evidence III
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In Asia, the number of people requesting and receiving cosmetic facial treatments has increased dramatically in the past decade. The reasons include technological progress and the improved effects of injection treatments such as botulinum toxin and soft tissue fillers, the increasing social acceptability of changing one’s appearance, and the enhanced affordability and availability of injection treatments. Most Asian aesthetic patients, whether young or old, prefer to avoid surgery whenever possible and turn to injection treatments to achieve natural results. To date, most studies and published recommendations on the use of facial injections refer to the standards for Western populations [1,2,3,4,5,6,7]. However, Asians differ in facial appearance and facial anatomy [8,9,10,11,12].
The charming and beautiful faces of people of different ethnic groups have unique ethnic characteristics that reflect harmony, symmetry and balance. However, when comparing the facial aesthetics of different races, they exhibit significant similarities in facial shape [13, 14]. Facial shape is a basic parameter of facial attractiveness, and there is a consensus among people of all ethnic backgrounds that an oval face is considered attractive (and youthful) [13, 15, 16].
An oval face in this context refers to a face with a smooth egg-shaped curve profile, with a smooth transition from the forehead through the temporal region, the surrounding cheeks, the preauricular region, the mandibular angle, the mandibular line, and to a chin with good protrusion. The average Asian face can present specific aesthetic challenges, including a square face shape, a lack of vertical height, a lack of anterior convexity, a flat wide nose, infraorbital depressions with prominent bags under the eyes, maxillary and chin retrusions, deep nasolabial folds, and a blunt nose. It is rarely possible to limit treatment to a specific region. Therefore, doctors need to treat Asians according to their contour characteristics to optimize facial aesthetics [13, 15, 16].
Poly-l-lactic acid (PLLA) is a widely used biodegradable and biocompatible polymer. It is a long-chain polymer formed by the polymerization of l-lactic acid molecules. PLLA is particularly popular in the medical and aesthetic fields because it can be gradually degraded into lactic acid in the body and eventually metabolized as carbon dioxide and water, making it a safe long-term implant material [17,18,19,20].
Currently, injectable PLLA has been approved for use in many countries around the world, including the Asia–Pacific region. The aesthetic medical market in this region is reported to be growing faster than that in the rest of the world, owing to the increasing affluence of the population and the rising popularity of aesthetic treatments [2]. Although Western recommendations for PLLA injection are feasible [18, 21, 22], guidance for the treatment of Asian patients is still needed because Asians have differences in facial structure (e.g., wider faces; shorter vertical heights; flatter or more concave features on the inside of the maxilla; flatter eyebrows, noses, and chins) [23].
However, studies on PLLA injection in Asians are relatively rare, and the safety and efficacy of PLLA injection for facial contouring need further investigation. CureWhite, a PLLA-PEG microsphere suspension in cross-linked HA hydrogels approved by NMPA in 2021, is indicated for the correction of moderate to severe nasolabial folds and wrinkles. Therefore, in this study, we retrospectively analyzed the cases of 30 Asian patients who received CureWhite injections for facial contouring and performed simulated injections on fresh cadaveric head specimens to verify its safety.
Methods
PLLA Products
The product (CureWhite) was produced by IMEIK Technology Development Co., Ltd., and was approved for marketing by the State Drug Administration in 2021. The gel mainly is composed of cross-linked sodium hyaluronate, l-lactic acid–ethylene glycol copolymer microspheres, lidocaine hydrochloride, and a phosphate buffer system, with a declared content of l-lactic acid–ethylene glycol copolymer microspheres of 18%. The specific product information is listed in Table 1.
Clinical Case Analysis
Subjects
Thirty Chinese subjects seeking injection for facial contouring were included in this study, including 2 males and 28 females; the average age was 34.5 ±6.4 years. The study follows the guiding principles of the Declaration of Helsinki. Patients were educated about the risks and benefits of treatment and signed informed consent forms before receiving injections. Patients with bleeding tendencies, coagulation disorders, severe diabetes, hypertension, hypertrophic scars or keloids, allergies to any components of the injection of PLLA, or other systemic diseases were excluded from the present study. Similarly, patients who had received facial laser treatments, chemical peelings, botulinum toxin injections, thread implantations, soft tissue fillers or surgery within 12 months were also excluded.
Injection Technique
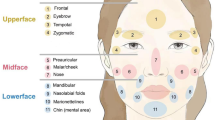
Before injection, the sample was withdrawn to reduce the risk of inadvertent intravascular injection. The injections were performed usin5g a slow, low-pressure, retrograde injection method. Injections were performed via the top-down approach, from the top (forehead) to the bottom (submental region). The injection sites, methods, and expected results are shown in Fig. 1 and Table 2.
Objective and Subjective Clinical Evaluation
Photographs were taken at baseline (before injection) and at 1, 3, 6, 9, and 12 months after treatment. Each subject was filmed under standard conditions, including the same photographer, consistent camera settings, standing posture, and uniform lighting.
Global Aesthetic Improvement Scale (GAIS) [24, 25]
After the injection, the operator and another plastic surgeon who was not involved in the study procedure evaluated the overall improvement in the patient’s facial contour. In particular, they analyzed the photographs of the subjects before and after treatment and evaluated the results according to the GAIS (1, very much improved; 2, much improved; 3, improved; 4, no change; 5, worse).
Patient Satisfaction [26]
The satisfaction of the subjects was evaluated using the Global Impression of Change Scale (GICS) at 1, 3, 6, 9, and 12 months after the injection. Each subject was asked: What is your overall impression of the change in facial appearance after treatment compared with that before the last injection? The answers were given on a 7-point scale from − 3 (very poor) to + 3 (very good).
Safety Assessment
Throughout the study, subjects were asked to report any adverse symptoms they experienced, and the duration of these events was recorded. The safety assessment included all abnormal reactions, including local reactions in the facial area that occurred during the clinical trials. Safety was evaluated by a physical examination performed during the clinical trial. All abnormal reactions were carefully recorded. Patient complaints included the presence and persistence of edema, bruising, palpable nodular lesions and even vision-related changes.
Cadaver Filler Injection and Fluoroscopic Imaging [27]
PLLA injections were performed using 2 cadavers to simulate injections for facial contouring. Visual analysis of the relationship between the filler and the artery was conducted. The injected radioactive PLLA was a mixture of commercially available PLLA product and the contrast agent iotroxic acid (350 mg/mL, Shanghai Pharma, China). After each filler injection, clear images of the artery and the filler were collected using the same 64-detector spiral CT scanner under the same conditions.
Data Analysis
SPSS version 22.0 (IBM Corporation) was used for the data analysis. Quantitative variables, such as baseline information, were expressed as the mean ± standard deviation. The GAIS scores of the two doctors and the GICS scores of the subjects were compared using a t test, and p<0.05 was considered statistically significant.
Results
Effectiveness
The patients' baseline characteristics are presented in Table 3. The GAIS was used to objectively evaluate the clinical outcomes. At 12 months after treatment, the median GAIS evaluated by injectors was 2.13±0.43; while, the median GAIS evaluated by blinded evaluators was 2.03±0.41 (Fig. 2). At 1, 3, 6, 9, and 12 months, there was no significant difference in GAIS scores between the injectors and the blind evaluators (p > 0.05) (Fig. 2).
Patient Satisfaction
The GICS was used to objectively evaluate patient satisfaction. Twelve months after treatment, patient satisfaction was 2.03±0.41. The GICSs of the subjects at 1, 3, 6, 9, and 12 months after injection were not significantly different (p > 0.05) (Fig. 3).
Safety
No serious adverse events, such as nodules, skin necrosis or vision-related damage, occurred in any of the patients. Injection-related reactions, such as swelling, were reported in 7 patients, and bruising, which disappeared within 1 week, was reported by 5 patients. No adverse events occurred during the follow-up period, indicating that PLLA is a safe and well-tolerated treatment for facial contour defects.
Discussion
CureWhite is composed of cross-linked sodium hyaluronate, l-lactic acid–ethylene glycol copolymer microspheres, lidocaine hydrochloride and a phosphate buffer system; the labeled content of the l-lactic acid–ethylene glycol copolymer microspheres was 18%. PLLA-polyethylene glycol (PEG) copolymer is a biodegradable and biocompatible polymer material. This copolymer combines the mechanical strength of PLLA with the water solubility and biocompatibility of PEG so that PLLA-PEG has unique physical, chemical, and biological properties [28]. PLLA-PEG is widely used in biomedical fields, including as a drug delivery system [29], for tissue engineering scaffolds [30], and in other medical devices, due to its ability to enhance material cytocompatibility and degradability and promote cell growth and tissue regeneration.
PLLA-PEG microspheres can be gradually degraded in the body through hydrolysis to release L-lactic acid and ethylene glycol, which are eventually cleared through normal metabolic pathways in the body. This degradation process allows the PLLA-PEG microspheres to be gradually absorbed without the need for surgical removal while simultaneously avoiding complications that may be caused by long-term indwelling foreign bodies [31].
The cross-linked sodium hyaluronate gel and PLLA-PEG microspheres injected into the face physically fill the soft tissues at the early stage and immediately improve facial contours. Over time, the microspheres are gradually degraded and absorbed, but they stimulate the regeneration of surrounding tissues, including collagen production, thereby achieving a long-term increase in volume. Moreover, the degradation process of the PLLA-PEG microspheres stimulates the cellular responses of the surrounding tissues, especially the stimulation of fibroblast proliferation and collagen synthesis. Collagen is a main component of the skin structure, and an increase in collagen can improve the elasticity and firmness of the skin, further optimizing the facial contours [32, 33].
In this study, the efficacy, patient satisfaction and safety of PLLA injections for the treatment of facial contour defects were investigated using the GAIS and the GICS. According to the results, after 12 months of treatment, the median GAIS scores given by the injectors and blinded evaluators were 2.13±0.43 and 2.03±0.41, respectively, indicating the effectiveness of the long-term effect of this material (Figs. 4, 5, 6). The difference in the GAIS score at different time points after treatment was not statistically significant, indicating the stability and durability of the PLLA treatment.
The evaluation results of patient satisfaction also showed that patient satisfaction after 12 months of treatment was 2.03 ± 0.41, which was not significantly different from the GICS at different time points during treatment, reflecting the sustained satisfaction of patients with the treatment outcomes. This is particularly important because patient satisfaction is a key indicator for evaluating the success of cosmetic plastic surgery.
In terms of safety, none of the patients in this study experienced serious adverse events, such as nodules, skin necrosis, or vision-related damage; 7 patients experienced injection-related reactions, such as swelling, and 5 patients experienced bruising, but these minor adverse events disappeared spontaneously within one week.
Skin necrosis and blindness are the most serious complications of facial soft tissue filler injections; hence, a detailed understanding of facial arteries is key to avoiding such complications. In this study, we added contrast agents to the fillers and simulated injections on specimens, followed by CT scans to show the relationship between the fillers and the blood vessels (Fig. 7). For forehead and superciliary arch injection, injector should avoid damage supratrochlear artery, supraorbital artery and superficial superior orbital arcade, cause normally both of them originated from the ophthalmic artery [34, 35]. For temple injection, superficial temporal artery and the deep temporal artery should be care when inject in between the superficial and deep temporal fascia and supraperiosteal layer [36, 37]. The nose and nasolabial fold are the area of the face where injections are most likely to lead to blindness. Therefore, injectors should maintain the injection level above the periosteum, as most nasal blood vessels are located superficially [38]. These findings indicate that this injection material is a safe and well-tolerated method for the treatment of facial contour defects.
Three-dimensional CT scan showing that different region arteries and its relationship with the filler. 1, superciliary arch; 2, zygoma; 3, nasolabial folds; 4, chin; 5, nose; 6, temple; 7, forehead; StA, supratrochlear artery; STA, superficial temporal artery; FA, facial artery; AA, angular artery; TFA, transverse facial artery; SOA, supraorbital artery; SSOA, superficial superior orbital arcade
Nevertheless, the present study has several limitations, such as the relatively small sample size and the single-center nature of the study, which may limit the generalizability of the results. Future studies should consider larger multicenter studies to further verify the efficacy and safety of PLLA treatment and to explore its comparative efficacy with other cosmetic treatments.
Conclusions
In summary, cross-linked sodium hyaluronate gel with PLLA-b-PEG microsphere injection therapy showed good efficacy, patient satisfaction, and safety in facial contour correction. These results support the use of PLLA as an effective choice for cosmetic facial plastic surgery. However, more studies are needed to better understand its long-term effects and optimize treatment regimens.
References
Rzany B, Fratila AA, Fischer TC et al (2013) Recommendations for the best possible use of botulinum neurotoxin type a (Speywood units) for aesthetic applications. J Drugs Dermatol 12(1):80–84
Dessy LA, Fallico N, Mazzocchi M, Scuderi N (2012) Aesthetic indications of botulinum toxin: a review of the efficacy and safety of currently available products
Raspaldo H, Gassia V, Niforos FR, Michaud T (2012) Global, 3-dimensional approach to natural rejuvenation: part 1—recommendations for volume restoration and the periocular area. J Cosmet Dermatol 11(4):279–289. https://doi.org/10.1111/jocd.12003
Fagien S, Cassuto D (2012) Reconstituted injectable hyaluronic acid: expanded applications in facial aesthetics and additional thoughts on the mechanism of action in cosmetic medicine. Plast Reconstr Surg 130(1):208–217. https://doi.org/10.1097/prs.0b013e318254b3f6
Raspaldo H, Baspeyras M, Bellity P et al (2011) Upper- and mid-face anti-aging treatment and prevention using onabotulinumtoxin A: the 2010 multidisciplinary French consensus—part 1. J Cosmet Dermatol 10(1):36–50. https://doi.org/10.1111/j.1473-2165.2010.00544.x
Raspaldo H, Niforos F-R, Gassia V et al (2011) Lower-face and neck antiaging treatment and prevention using onabotulinumtoxin A: the 2010 multidisciplinary French consensus—part 2. J Cosmet Dermatol 10(2):131–149. https://doi.org/10.1111/j.1473-2165.2011.00560.x
Carruthers JDA, Glogau RG, Blitzer A (2008) Advances in facial rejuvenation: botulinum toxin type A, hyaluronic acid dermal fillers, and combination therapies???-Consensus recommendations. Plast Reconstr Surg 121(Supplement):31S-33S. https://doi.org/10.1097/01.prs.0000313401.57532.60
Fang F, Clapham PJ, Chung KC (2011) A systematic review of interethnic variability in facial dimensions. Plast Reconstr Surg 127(2):874–881. https://doi.org/10.1097/PRS.0b013e318200afdb
Farkas LG, Katic MJ, Forrest CR (2005) International anthropometric study of facial morphology in various ethnic groups/races. J Craniofac Surg 16(4):615–646. https://doi.org/10.1097/01.scs.0000171847.58031.9e
Gu Y, McNamara JA, Sigler LM, Baccetti T (2010) Comparison of craniofacial characteristics of typical Chinese and Caucasian young adults. Eur J Orthod 33(2):205–211. https://doi.org/10.1093/ejo/cjq054
Le TT, Farkas LG, Ngim RCK, Levin LS, Forrest CR (2002) Proportionality in Asian and North American caucasian faces using neoclassical facial canons as criteria. Aesthet Plast Surg 26(1):64–69. https://doi.org/10.1007/s00266-001-0033-7
Sim RS, Smith JD, Chan ASY (2000) Comparison of the aesthetic facial proportions of southern chinese and white women. Arch Facial Plast Surg 2(2):113–120. https://doi.org/10.1001/archfaci.2.2.113
Liew S, Dart A (2008) Nonsurgical reshaping of the lower face. Aesthet Surg J 28(3):251–257. https://doi.org/10.1016/j.asj.2008.03.003
Rhee SC, Lee SH (2010) Attractive composite faces of different races. Aesthet Plast Surg 34(6):800–801. https://doi.org/10.1007/s00266-010-9606-7
Swift A, Remington K (2011) BeautiPHIcation™: a global approach to facial beauty. Clin Plast Surg 38(3):347–377. https://doi.org/10.1016/j.cps.2011.03.012
Wu WTL (2006) Non surgical facial rejuvenation with the 4R principle: innovative uses of BOTOX and facelifting with the woffles lift, a barbed suture sling. Springer, Berlin, Heidelberg
Shridharani SM, Tisch GM, Ebersole TG, Moak TN, Edwartz C (2021) Clinical experience of poly-l-lactic acid injections for body contouring treatment. J Cosmet Dermatol 20(6):1655–1662. https://doi.org/10.1111/jocd.14141
Vleggaar D, Fitzgerald R, Lorenc ZP et al (2014) Consensus recommendations on the use of injectable poly-l-lactic acid for facial and nonfacial volumization. J Drugs Dermatol 13(4 Suppl):S44–S51
Schierle CF, Casas LA (2011) Nonsurgical rejuvenation of the aging face with injectable poly-l-lactic acid for restoration of soft tissue volume. Aesthet Surg J 31(1):95–109. https://doi.org/10.1177/1090820x10391213
Chen SY, Lin JY, Lin CY (2019) Compositions of injectable poly-d, l-lactic acid and injectable poly-l-lactic acid. Clin Exp Dermatol 45(3):347–348. https://doi.org/10.1111/ced.14085
Harper J, Avelar L, Haddad A et al (2022) Expert recommendations on the use of injectable poly-l-lactic acid for contour deficiencies of the buttocks. J Drugs Dermatol 21(1):21–26. https://doi.org/10.36849/jdd.6180
Lin MJ, Dubin DP, Goldberg DJ, Khorasani H (2019) Practices in the usage and reconstitution of poly-l-lactic acid. J Drugs Dermatol 18(9):880–886
Liew S, Wu WTL, Chan HH et al (2016) Consensus on changing trends, attitudes, and concepts of Asian beauty. Aesthet Plast Surg. 40(2):193–201. https://doi.org/10.1007/s00266-015-0562-0
Liao Z-F, Yang W, Li X, Wang S-W, Liu F-C, Luo S-K (2023) Infraorbital rejuvenation combined with thread-lifting and non-cross-linked hyaluronic acid injection: a retrospective, case-series study. Aesthet Plast Surg. https://doi.org/10.1007/s00266-023-03740-1
Liao ZF, Yang W, Lin FC, Wang SW, Hong WJ, Luo SK (2023) A case study: comprehensive approach for treating horizontal neck wrinkles using hyaluronic acid injections and thread-lifting. Aesthet Plast Surg 47(2):765–771. https://doi.org/10.1007/s00266-022-03071-7
Muti GF (2019) Open-label, post-marketing study to evaluate the performance and safety of calcium hydroxylapatite with integral lidocaine to correct facial volume loss. J Drugs Dermatol 18(1):86–91
Liao Z-F, Cong L-Y, Luo C-E, Zhan W-F, Luo S-K (2023) New insight into glabellar arteries: a three-dimensional computed tomography and dissection study. Plast Reconstr Surg 151(5):979–987. https://doi.org/10.1097/prs.0000000000010075
Nagahama K, Ouchi T, Ohya Y (2008) Temperature-induced hydrogels through self-assembly of cholesterol-substituted star PEG-b-PLLA copolymers: an injectable scaffold for tissue engineering. Adv Funct Mater 18(8):1220–1231. https://doi.org/10.1002/adfm.200700587
Cho H, Gao J, Kwon GS (2016) PEG-b-PLA micelles and PLGA-b-PEG-b-PLGA sol-gels for drug delivery. J Control Release 240:191–201. https://doi.org/10.1016/j.jconrel.2015.12.015
Kerimoglu O, Alarcin E (2012) Poly(lactic-co-glycolic acid) based drug delivery devices for tissue engineering and regenerative medicine. ANKEM Derg 26(2):86–98. https://doi.org/10.5222/ankem.2012.086
Xie M, Ge J, Lei B, Zhang Q, Chen X, Ma PX (2015) Star-shaped, biodegradable, and elastomeric PLLA-PEG-POSS hybrid membrane with biomineralization activity for guiding bone tissue regeneration. Macromol Biosci 15(12):1656–1662. https://doi.org/10.1002/mabi.201500237
Fan M, Liao J, Guo G et al (2014) Dexamethasone-loaded poly(d, l-lactic acid) microspheres/poly(ethylene glycol)-poly(epsilon-caprolactone)-poly(ethylene glycol) micelles composite for Skin augmentation. J Biomed Nanotechnol 10(4):592–602. https://doi.org/10.1166/jbn.2014.1832
Ehashi T, Kakinoki S, Yamaoka T (2014) Water absorbing and quick degradable PLLA/PEG multiblock copolymers reduce the encapsulation and inflammatory cytokine production. J Artif Organs 17(4):321–328. https://doi.org/10.1007/s10047-014-0791-z
Liao ZF, Cong LY, Hong WJ, Luo CE, Luo SK (2022) Three-dimensional computed tomographic study of the supratrochlear artery and supraorbital artery to determine arterial variations and their relationship. Dermatol Surg 48(2):225–231. https://doi.org/10.1097/DSS.0000000000003347
Liao ZF, Hong WJ, Cong LY, Luo CE, Zhan WF, Ke JQ, Luo SK (2021) A case series: 3-dimensional computed tomographic study of the superior orbital vessels: superior orbital arcades and their relationships with the supratrochlear artery and supraorbital artery. J Am Acad Dermatol 84(5):1364–1370. https://doi.org/10.1016/j.jaad.2020.06.082
Zhou YH, Chen CL, Luo CE, Wang HB, Luo SK (2023) Deep temporal artery anatomy: implications for improving the safety of deep temporal injections. Aesthet Plast Surg 47(5):2045–2050. https://doi.org/10.1007/s00266-023-03341-y. (Epub 2023 Apr 19)
Chen CL, Cong LY, Kong XX, Zhao WR, Hong WJ, Luo CE, Luo SK (2021) Three-dimensional computed tomography scanning of temporal vessels to assess the safety of filler injections. Aesthet Surg J 41(11):1306–1313. https://doi.org/10.1093/asj/sjaa371
Cong LY, Liao ZF, Zhang YS, Li DN, Luo SK (2022) Three-dimensional arterial distribution over the midline of the nasal bone. Aesthet Surg J 42(7):784–790. https://doi.org/10.1093/asj/sjab432
Acknowledgments
None
Funding
None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript. The study is not supported by any funding source.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest to disclose
Ethical Approval
The study protocol was approved by the Institutional Review Board of the Hospital
Informed Consent
All patients provided written informed consent
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
The video of injection technique (MP4 13147 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liao, ZF., She, YH., Huang, JM. et al. Cross-linked Sodium Hyaluronate Gel with PLLA-b-PEG Microsphere for Facial Contouring in Chinese: A Retrospective Study. Aesth Plast Surg (2024). https://doi.org/10.1007/s00266-024-04195-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00266-024-04195-8