Abstract
Background
Autologous fat grafting is an effective form of soft tissue regeneration. However, the optimal fat particle size and graft retention pattern need more research.
Methods
The mouse inguinal fat pad was harvested and cut into fat particles of different diameters: ≥ 5 mm, 3–4 mm, 2–3 mm, 1–2 mm and 1 mm (micro-fat). A volume of 0.2 ml fat was transplanted into another mouse dorsum. Volume and retention rate were measured at 1, 4, 8 and 12 weeks. Histology analysis was performed. Immunofluorescence staining was used to evaluate M1 and M2 macrophage infiltration and graft angiogenesis.
Results
Fat retention was highest in the “> 5 mm” group and lowest in the “micro-fat” group. Large vacuoles were more common in larger-diametered fat particles. There was less collagen accumulation in the well-vascularized connective tissue in the “1–2 mm” group. The infiltrated nucleated cells peak at week 4 in groups of fat particles under 3 mm and at week 8 in in groups with fat particles above 3 mm. The number of M1 macrophages peaked at week 1 and then declined in all groups except for the “5 mm” group. The number of M2 macrophages peaked at week 4 and gradually decreased through 12 weeks in the groups below 3 mm, but increased through 12 weeks in the groups above 3 mm. Vascular intensity was similar to M2 macrophage prevalence.
Conclusions
Fat particles of different sizes may posses different retention patterns. Larger grafts have higher retention rate but worse quality. Meanwhile, smaller grafts have better quality with lower retention rate.
No Level Assigned
This journal requires that authors assign a level of evidence to each submission to which Evidence-Based Medicine rankings are applicable. This excludes Review Articles, Book Reviews, and manuscripts that concern Basic Science, Animal Studies, Cadaver Studies, and Experimental Studies. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autologous fat grafting has become a popular method for soft tissue regeneration, but there are still issues with the procedure. Despite improvements in processing techniques, the transplanted fat can still undergo necrosis, calcification, or develop oil cysts [1]. In addition, the retention rate of transferred fat remains unpredictable, and the mechanisms behind retention are still not well understood. The most popular fat grafting technique in clinical practice is harvesting through cannula with low negative pressure [2]. Previous studies reported that fat harvested with the same cannula could vary in particle size, with the largest particle matching the diameter of the cannula [3]. Therefore, clinical experts have sought to discover the optimal particle size for fat grafting and many laboratory investigations have focused on this area.
Clinical scientists believe that the best particle size for fat grafting ranges from 1 to 2 mm [4]. However, results of animal experiments remain controversial. While most of the studies agree that higher graft retention is observed in groups with larger harvesting cannulas, the quality of the grafted fat with larger diameters varies significantly in the long term [5, 6]. Poor-quality fat grafts may exhibit oil cysts and fibrosis, which represent the final status of the repair of grafted fat [7]. When assessing the retention results, both the ratio and quality of the grafted fat should be taken into consideration.
Fragile fat tissue may sustain some mechanical damage during harvesting and transplantation [8]. As the initial stage of repair, the damaged adipocytes and extracellular matrix (ECM) would induce immediate inflammation and attract macrophages [9]. The timing and degree of the infiltration of inflammatory cells are highly related to the particle size of the grafted fat [10]. After polarization, M1 macrophages remove debris and necrotic tissue by secreting pro-inflammatory factors [11], while M2 macrophages promote angiogenesis, stem cell migration and ECM synthesis by releasing anti-inflammatory cytokines [12]. Poor ECM remolding (both excessive and insufficient) can result in oil cysts of the grafted fat.
In this study, we aimed to investigate whether fat with different particle sizes undergoes different repair process, starting with inflammation and ending with ECM remolding. To test our hypothesis, we harvested the inguinal fat pad from mice, cut them into different sizes and transferred them onto the dorsum of other mice. The retention rate of the grafted fat was measured using the drainage method. Immunofluorescence staining was then performed to evaluate inflammation and angiogenesis within the grafted fat.
Materials and Methods
Animals
These experimental protocols were approved by the Ethics Review Board of the Fourth Military Medical University and were conducted according to the guidelines of the International Council for Laboratory Animal Science (ICLAS). A total of 140 C57BL/6 mice (embryo at − 28 day) were housed under a 12-h day/night cycle under conventional conditions with food and water ad libitum.
Fat Grafting
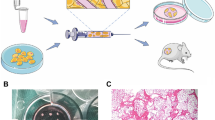
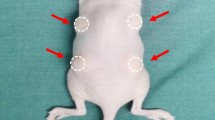
Our fat grafting protocol is shown in Fig. 1. Seven hundred mice were killed, and both sides of the inguinal fat pad were excised and trimmed into fat particles. Then fat particles were washed with PBS, immersed in PBS and photographed for size analysis according to an existing protocol. Briefly, a minimum of 50 particles was dispersed in PBS with a ruler included in each photograph and images were then automated measured with software ImageJ [10] (Figure S1). Then the fat particles were classified into five groups at 1-mm intervals (≥ 5 mm, 3–4 mm, 2–3 mm, 1–2 mm and 1 mm (micro-fat)) (n = 7) (Figure S2). A volume of 0.2 ml was used for fat grafting. Each mouse received five fat grafts on its dorsum, and each group contained six mouse. A total of 140 mice were grafted. Mice were euthanized 1, 4, 8 and 12 weeks after grafting. Final fat volume was measured using the drainage method: the harvested tissue was suspended on a fine cotton suture thread and immersed entirely in a beaker of saline that had been placed on a balance. Fat volume was calculated by measuring the displaced saline. Grafted fat was then fixed in 4% paraformaldehyde for 24 h and stored in 70% ethanol at 4 °C for histology and immunohistochemistry.
Illustration of the allogeneic fat grafting model. Both sides of the inguinal fat pad were harvested from C57BL/6 mice. Fat was cut into fat particles of the following diameters: ≥ 5 mm, 3–4 mm, 2–3 mm, 1–2 mm and 1 mm (micro-fat). A volume of 0.2 ml fat was transplanted into the mouse dorsum. Each mouse received five dorsal fat grafts
Histology and Immunofluorescence
Tissue samples were cut into 3-mm sections and stained with hematoxylin and eosin (H&E) according to standard protocols. Masson’s trichrome staining was also performed following an existing protocol. For immunofluorescence, sections were stained with primary antibodies against CD31 (ab28364), iNOS (ab178945), CD68 (ab201340), CD206 (ab64693) (1:200; Abcam, Cambridge) and then incubated with secondary antibodies. Nuclei were stained with 4′,6-diamidino-2-phenylindole (Thermo Fisher, Waltham, MA, USA). Images were obtained using a confocal microscope system (Zeiss, Jena, Germany). The number of inflammatory and vascular cells was measured by two of the authors, who counted the number of cells in at least five fields per sample in a double-blinded fashion.
Statistical Analysis
Continuous variables were expressed as mean±SD. A two-way analysis of variance (ANOVA) was used to analyze the time and study group effect on volume, cell number and vessel density at all time points, and a Tukey post hoc test was used to compare the any two groups at a single time point. One-way analysis of variance was performed to analyze the difference of oil cysts and collagen deposition area at week 12. A two-tailed p value < 0.05 was considered statistically significant.
Results
Gross Imaging and Retention
Fat graft retention rate decreased in all five groups over time, ranging from 19.00 ± 4.20 to 64.00 ± 3.80% (Fig. 2 right). The highest retention rate at week 12 was in the group with the largest fat particles. As the size of the fat particles grew, the retention rate increased. A two-way ANOVA revealed that there was a statistically significant interaction between the effect of time and study group (F = 7.022, p < 0.001). Because the interaction between was significant, we chose to ignore the two main effects and instead examined the study group simple main effect. The differences among the five groups were varied for four time points. Except for week 1, there was a significant difference of fat volume among the five groups (p < 0.001). Follow-up tests were conducted to evaluate the five groups’ pairwise differences at week 12. We were surprised to find that the volume between groups was not significantly different only in three pairs (micro-fat vs. 1–2 mm; 1–2 m vs. 2–3 mm; 3–4 mm vs. ≥ 5 mm). Same as other fat grafting murine model, there was a slim capsule surrounding the fat grafts which enabled us to separate them easily from the tissue around [13]. Additionally, a high degree of vascularization in the grafted fat of all groups was observed at week 1 (Fig. 2 left). Immunofluorescence staining was therefore performed to detect neo-angiogenesis at week 1.
Histological Analysis
H&E staining of the grafted fat at week 12 showed different outcomes in samples with different diameters (Fig. 3). A discernible number of inflammatory cells appeared in the center of the adipocytes in the “micro-fat” group, but were absent in all other groups. Large vacuoles (indicating oil cysts) were observed in every group. Generally, the larger the diameter of the fat sample, the more vacuoles that were present. Masson’s trichrome staining demonstrated that there was significant collagen accumulation around the large vacuoles in the “micro-fat” group. In contrast, collagen was seen around the well-vascularized connective tissue of the grafted fat in the “1–2 mm” group (Figure S3).
High-resolution images were obtained at each time points, and the nucleated cells were calculated to detect the infiltration of cells (Fig. 4). In groups of fat particles under 3 mm, the number of nucleated cells increased since week 1 and peaked at week 4. Meanwhile, there was a four-week delay of climax of nucleated cells in groups with fat particles above 3 mm. The amount of infiltrated cells was highest in micro-fat group at week 4 and was highest in “3–4 mm” group at week 8.
Angiogenesis
We used a CD31 antibody to stain vascular structures. Quantitative analysis showed that vessel intensity was greatest in the “micro-fat” group at week 1. For the “micro-fat” and “1–2 mm” groups, the intensity of the vascular peaked at week 4 and gradually decreased until week 12. A similar tendency was observed in fat grafts that were larger than 2 mm except that peak vascular intensity was detected at week 8 (Fig. 5).
Infiltration of the Macrophages in Grafted Fat
Angiogenesis was closely related to inflammation. M1 and M2 macrophages appeared in chronological order [14]. We stained with antibodies to F4/80 and iNOS to label M1 macrophages and F4/80 and CD206 to mark M2 macrophages. Quantitatively, the number of M1 macrophages in the “micro-fat,” “1–2 mm” and “2–3 mm” groups decreased over time after 1 week. In contrast, the M1 macrophages in the “3–4 mm” and “5 mm” groups peaked at week 12 (Fig. 6). The M2 macrophages in “≤ 3 mm” groups gradually increased after 1 week, peaked at week 4 and declined thereafter. On the contrary, the number of M2 macrophages in “≥ 3 mm” groups gradually increased until week 12 (Fig. 7).
Immunofluorescence staining for M2 macrophages in the fat grafts. The number of M2 macrophages rose after 1 week, peaked at week 4 and then gradually decreased through 12 weeks in the “micro-fat,” “1–2 mm” and “2–3 mm” groups. However, the number of M2 macrophages increased after 1 week in the “3–4 mm” and “5 mm” groups (below). The “micro-fat” group had the most M2 macrophages at week 4 (above). Scale bar = 50 μm
Discussion
Although several works have evaluated the effect of harvesting cannula diameter and aperture size on adipocyte survival, there is still no consensus on the optimal cannula size [15, 16]. De Arruda et al. [17] directly compared fat biopsies with diameters of 3 mm or 5 mm and found no significant differences in angiogenesis and adipogenesis between groups, but the rough diameters of the biopsies limited its clinical applicability. In this study, we first divided the inguinal fat pads of mice into fat particles with diameters at 1-mm intervals and found the retention pattern may be different among fat grafts with different-sized particles. Moreover, the initial volume of fat grafts was the same in each group, so the effects of interstitial fluid diffusion and perfusion were controlled [2, 18]. Histological analysis showed that while the graft retention rates of groups with larger fat particles were higher at week 12, the structure of the “1–2 mm” group most closely resembled mature adipose tissue.
The primary index used in most studies to evaluate the efficacy of fat grafting is the retention rate, defined as the percentage of mean volume gain out of the mean volume injected [19]. In our study, the “> 5 mm” group showed the highest retention rate at 64.00 ± 3.80%. This was consistent with a study conducted by Nguyen et al. [20], who injected 0.7 ml fat harvested using Coleman cannula or a multi-perforated 1-mm cannula into the flanks of nude mice. The retention rate was higher in groups that contained larger fat particles. However, retention rate alone does not fully represent the success of fat grafting, as fibrosis and structural integrity are also crucial factors. Histological analysis in the present work revealed that “1–2 mm” group obtained optimal adipose structure, with fewer large vacuoles (oil cysts) and least severe fibrosis compared to other groups. Interestingly, Kirkham et al. [6] injected 1g of fat harvested with either 3-mm or 5-mm cannula through a 14G micro-cannula into the back of nude mice and found that there was improved graft retention and quality when a larger aspiration cannula was used. One reasonable explanation for this difference was that the authors only provided short-term analysis of 6 weeks, when fat regeneration was still in process.
During harvesting, the shear force of multi-hole cannula and the negative pressure of the syringe may cause adipocytes rupture [21]. Aspirated fat containing dying adipocytes and a “broken” extracellular matrix would secret cytokines, damage-related molecular pattern molecules and some proteases [7]. These factors recruited neutrophils, which phagocytose cell fragments secrete chemokines and induce monocytes to migrate to the recipient sites [22]. Inflammation is also highly related to immune response. To minimize the influence, we used allogeneic C57BL/6 mice model for fat grafting, which is an inbred strain sharing nearly identical genotype. Compared with other inbred strain, Th1 immune response and cell-mediated immunity play a dominant role in C57BL/6 mice [23]. Allogeneic fat grafting in Th1 directed C57BL/6 mice may result in intenser activation of M1 macrophages and further destruction of fat structure [24]. Therefore, this situation must be considered when interpreting the study results. The infiltration of these nucleated inflammatory cells varied a lot among the groups. In summary, the peak time of the number of nucleated inflammatory cells was four week earlier in groups of fat particles under 3 mm than in groups with fat particles above 3 mm. Additionally, the number of infiltrated cells was also much higher in groups with smaller fat particles. The reason may be that the inflammatory cells always infiltrated from margin to center and they would travel sooner in smaller fat particles. What’s more, smaller fat particles usually sustain more damage, resulting in a more intense inflammatory response.
Those monocytes then differentiate into M1 macrophages. In our study, the number of M1 macrophages was highest in the “micro-fat” group, which had least structural integrity at week 1. We also found that the number of M1 macrophages decreased as the size of fat particles increased. After differentiation, M1 macrophages engage in phagocytic activity, produce pro-inflammatory mediators and promote local tissue fibrosis [25]. Because M1 macrophages could engulf cellular debris and digest damaged ECM components, excessive M1 macrophages would cause further destruction of fat tissue. Meanwhile, inadequate infiltration of M1 macrophages would lead to the development of large oil cysts and poor adipose retention [26]. Data from our study showed that the smallest fat particles accumulated significant amounts of collagen, while the largest size particles had more large vacuoles. The poor results seen in groups with larger fat particles could be partially explained by the reduced infiltration of M1 macrophages in these group and correspondingly fewer M2 macrophages, which would significantly impact angiogenesis and adipogenesis.
The grafted fat entered the “regeneration phase” from week 2 to week 12. M1 macrophages gradually differentiate to M2 macrophages during this phase. Ultimately, M2 macrophages become the dominant macrophage population and produce high levels of TGF-β, secrete angiogenic factors and induce endothelial cell migration [27]. Circulating stem cells migrate to the grafted site through neo-vessels and participate in adipose regeneration. Our quantitative results showed that the infiltration time and number of M2 macrophages varied among groups. Poor M1 macrophage infiltration led to few number of M2 macrophages which, in turn, caused poor angiogenesis. Therefore, the appearance time of M1 and M2 macrophages was crucial for successful regeneration of the transplanted fat. M1 macrophages were responsible for phagocytosing cell fragments, while M2 macrophages played an important role in angiogenesis and stem cell recruitment.
In this study, we discussed the effect of different-sized fat particles on inflammation and retention pattern of allografted inguinal fat with same initial volume. The larger the fat particles, the fewer vascular and more oil cysts shown in the central zone at late phases. Interestingly, the “micro-fat” group which was made up of the smallest particles exhibited well-vascularized tissue with moderate amount of oil cysts. Proper chronological order of macrophages in different-sized fat particles would result in varied retention patterns (Fig. 8). The retention pattern of fat grafts, which may be survival and regenerative at different situations, has become a hot but disputed issue. Eto et al. proposed the classical “three zone” theory in a en bloc (150 to 200 mg) autologous fat grafting mouse model which consisted of “surviving zone,” “regenerating zone” and “necrotic zone” [28]. We also observed a small number of oil droplets in the central zone of graft in all the groups since week one. Creatively, we explain this phenomenon by differences in angiogenesis and inflammation among groups. Delayed and mild inflammation followed by greater oil cysts was found in groups with larger fat particles. The variations in mechanical damage of fat may cause different onsets of inflammation and repair of adipose tissue, resulting in diverse retention patterns. Therefore, we presumed from the results that fat particles may undergo a regenerative phase up to a certain size, while experiencing a survival pattern beyond that size. Accordingly, the term “retention” was not perfectly suitable to describe the procedure after fat grafting anymore. Scholars have proposed a new term—“integration” to depict the process fat grafts interacting with the surrounding tissue [29]. We agreed with their opinion and would adopt the new concept in the following research. The study also highlighted the need for further research on integration patterns of clinically used fat grafts which range from nano- to micro-scale [30] and detection of an optimal ratio of varied-sized particles for clinical practice.
Possible mechanism for the retention patterns of grafted fat with same initial volume but composed of different-sized particles. The “micro-fat” and “1–2 mm” groups were well vascularized at week 1 and maintained this till week 12. In contrast, vascularity gradually increased in groups with fat particles larger than 5 mm. Proper chronological order of M1 and M2 macrophages would result in fewer oil cysts in the “1–2 mm” group.
Animal studies were essential for detection of influence of fat particle size on retention pattern. In order to establish the fine range of fat particles, we opted for “cut” instead of negative pressure-assisted liposuction. Previous studies showed that dissection by scissors would only destroy the vascular network and the connective tissue among cells [31]. The nuances among the particle size during separation and division by naked eyes may lead to results bias. To mitigate this, automated measurement with software ImageJ was applied. Moreover, manual “cutting” is an labor-intensive work and would have restricted clinical applications.
Conclusions
Fat particles of different sizes may posses different retention patterns. Larger grafts have higher retention rate but worse quality. Meanwhile, smaller grafts have better quality with lower retention rate. These results may be practically explained by the variation in timing and degree of infiltration of inflammatory cells in fat particle of different sizes.
References
Coleman SR, Saboeiro AP (2007) Fat grafting to the breast revisited: safety and efficacy. Plast Reconstr Surg 119(3):775–785; discussion 786–787. https://doi.org/10.1097/01.prs.0000252001.59162.c9
Khouri RK, Rigotti G, Cardoso E, Khouri RK Jr, Biggs TM (2014) Megavolume autologous fat transfer: part II. Pract Tech Plast Reconstr Surg 133(6):1369–1377. https://doi.org/10.1097/PRS.0000000000000179,Jun2014
Vazquez OA, Markowitz MI, Becker H (2020) Fat graft size: relationship between cannula and needle diameters. Cureus 12(4):7598. https://doi.org/10.7759/cureus.7598
Yun-Nan L, Shu-Hung H, Tsung-Ying L et al (2018) Micro-autologous fat transplantation for rejuvenation of the dorsal surface of the aging hand. J Plast Reconstr Aesthet Surg 71(4):573–584. https://doi.org/10.1016/j.bjps.2017.09.012,Apr2018
Tong Y, Liu P, Wang Y et al (2018) The effect of liposuction cannula diameter on fat retention-based on a rheological simulation. Plast Reconstr Surg Glob Open 6(11):e2021. https://doi.org/10.1097/GOX.0000000000002021
Kirkham JC, Lee JH, Medina MA 3rd, McCormack MC, Randolph MA, Austen WG Jr (2012) The impact of liposuction cannula size on adipocyte viability. Ann Plast Surg 69(4):479–481. https://doi.org/10.1097/SAP.0b013e31824a459f,Oct2012
Bi X, Li Y, Dong Z et al (2021) Recent developments in extracellular matrix remodeling for fat grafting. Front Cell Dev Biol 9:767362. https://doi.org/10.3389/fcell.2021.767362
Gause TM 2nd, Kling RE, Sivak WN, Marra KG, Rubin JP, Kokai LE (2014) Particle size in fat graft retention: a review on the impact of harvesting technique in lipofilling surgical outcomes. Adipocyte 3(4):273–279. https://doi.org/10.4161/21623945.2014.957987,Oct-Dec,2014
Mashiko T, Yoshimura K (2015) How does fat survive and remodel after grafting? Clin Plast Surg 42(2):181–190. https://doi.org/10.1016/j.cps.2014.12.008,Apr2015
Yang X, Egro FM, Jones T et al (2020) Comparison of adipose particle size on autologous fat graft retention in a rodent model. Plast Aesthet Res. https://doi.org/10.20517/2347-9264.2019.63
Chen Q, Liu S, Cao L, Yu M, Wang H (2021) Effects of macrophage regulation on fat grafting survival: improvement, mechanisms, and potential application—a review. J Cosmet Dermatol. https://doi.org/10.1111/jocd.14295,Jun15,2021
Dang J, Yang J, Yu Z et al (2022) Bone marrow mesenchymal stem cells enhance angiogenesis and promote fat retention in fat grafting via polarized macrophages. Stem Cell Res Ther 13(1):52. https://doi.org/10.1186/s13287-022-02709-2
Ye Y, Liao Y, Lu F, Gao J (2017) Daily suction provided by external volume expansion inducing regeneration of grafted fat in a murine model. Plast Reconstr Surg 139(2):392e–402e. https://doi.org/10.1097/PRS.0000000000003012,Feb2017
Cai J, Feng J, Liu K, Zhou S, Lu F (2018) Early macrophage infiltration improves fat graft survival by inducing angiogenesis and hematopoietic stem cell recruitment. Plast Reconstr Surg 141(2):376–386. https://doi.org/10.1097/PRS.0000000000004028,Feb2018
Fontes T, Brandao I, Negrao R, Martins MJ, Monteiro R (2018) Autologous fat grafting: harvesting techniques. Ann Med Surg (Lond) 36:212–218. https://doi.org/10.1016/j.amsu.2018.11.005,Dec2018
Strong AL, Cederna PS, Rubin JP, Coleman SR, Levi B (2015) The current state of fat grafting: a review of harvesting, processing, and injection techniques. Plast Reconstr Surg 136(4):897–912. https://doi.org/10.1097/PRS.0000000000001590,Oct2015
de Arruda EGP, Munhoz AM, Matsumoto W et al (2021) Impact of fat graft thickness and harvesting technique on adipocyte viability in a new porcine experimental model: an immunohistochemical analysis. Aesthet Surg J 41(6):NP616–NP630. https://doi.org/10.1093/asj/sjaa256
Khouri RK, Rigotti G, Cardoso E, Khouri RK Jr, Biggs TM (2014) Megavolume autologous fat transfer: part I. Theory and principles. Plast Reconstr Surg 133(3):550–557. https://doi.org/10.1097/01.prs.0000438044.06387.2a,Mar2014
Qin Z, Yu Z, Song B (2022) Efficacy and safety of external volume expansion (EVE) on fat grafting: a systematic review and single-arm meta-analysis. J Plast Reconstr Aesthet Surg 75(3):1073–1082. https://doi.org/10.1016/j.bjps.2021.11.032,Mar2022
Nguyen PS, Desouches C, Gay AM, Hautier A, Magalon G (2012) Development of micro-injection as an innovative autologous fat graft technique: The use of adipose tissue as dermal filler. J Plast Reconstr Aesthet Surg 65(12):1692–1699. https://doi.org/10.1016/j.bjps.2012.06.014,Dec2012
Egro FM, Coleman SR (2020) Facial fat grafting: the past, present, and future. Clin Plast Surg 47(1):1–6. https://doi.org/10.1016/j.cps.2019.08.004,Jan2020
Liu K, Cai J, Li H, Feng J, Feng C, Lu F (2018) The disturbed function of neutrophils at the early stage of fat grafting impairs long-term fat graft retention. Plast Reconstr Surg 142(5):1229–1238. https://doi.org/10.1097/PRS.0000000000004882,Nov2018
Song HK, Hwang DY (2017) Use of C57BL/6N mice on the variety of immunological researches. Lab Anim Res 33(2):119–123. https://doi.org/10.5625/lar.2017.33.2.119,Jun2017
Chen X, Chen Y, Wang Z et al (2022) Adipose-derived stem cells regulate CD4+ T-cell-mediated macrophage polarization and fibrosis in fat grafting in a mouse model. Heliyon 8(11):e11538. https://doi.org/10.1016/j.heliyon.2022.e11538
Cheng H, Luan J, Mu D et al (2019) M1/M2 macrophages play different roles in adipogenic differentiation of PDGFRalpha(+) preadipocytes in vitro. Aesthet Plast Surg 43(2):514–520. https://doi.org/10.1007/s00266-018-1294-8,Apr2019
Kato H, Mineda K, Eto H et al (2014) Degeneration, regeneration, and cicatrization after fat grafting: dynamic total tissue remodeling during the first 3 months. Plast Reconstr Surg 133(3):303e–313e. https://doi.org/10.1097/PRS.0000000000000066,Mar2014
Cai J, Li B, Liu K, Li G, Lu F (2017) Macrophage infiltration regulates the adipose ECM reconstruction and the fibrosis process after fat grafting. Biochem Biophys Res Commun 490(2):560–566. https://doi.org/10.1016/j.bbrc.2017.06.078,Aug19,2017
Eto H, Kato H, Suga H et al (2012) The fate of adipocytes after nonvascularized fat grafting: evidence of early death and replacement of adipocytes. Plast Reconstr Surg 129(5):1081–1092. https://doi.org/10.1097/PRS.0b013e31824a2b19,May2012
Guimaraes P, de Oliveira FBM, Lage FC et al (2022) Retropectoral fat graft survival in mammoplasty: evaluation by magnetic resonance imaging. Aesthetic Plast Surg 46(6):2712–2722. https://doi.org/10.1007/s00266-022-02999-0,Dec2022
Yao Y, Cai J, Zhang P et al (2018) Adipose stromal vascular fraction gel grafting: a new method for tissue volumization and rejuvenation. Dermatol Surg 44(10):1278–1286. https://doi.org/10.1097/DSS.0000000000001556,Oct2018
Dong Z, Peng Z, Chang Q et al (2015) The angiogenic and adipogenic modes of adipose tissue after free fat grafting. Plast Reconstr Surg 135(3):556e–567e. https://doi.org/10.1097/PRS.0000000000000965,Mar2015
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China under Grant 82102355, 82202479.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Approval
All procedures performed in studies involving animals were approved by the Ethics Review Board of the Fourth Military Medical University and were conducted according to the guidelines of the International Council for Laboratory Animal Science (ICLAS).
Informed Consent
For this type of study informed consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
266_2023_3368_MOESM1_ESM.jpg
Supplementary figure 1: Images of fat particles prepared from whole adipose tissue using surgical scissors dispersed in PBS. (JPG 590 KB)
266_2023_3368_MOESM3_ESM.tif
Supplementary figure 3: Fat grafts collagen deposition at twelve weeks. Less collagen presented around the well-vascularized connective tissue in “1-2mm” group, while significant collagen accumulation around the large vacuoles in the “micro-fat” group was observed. Scale bar = 200 μm (TIF 8266 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Z., Qin, Z., Tang, J. et al. Particles of Different Sizes Affect the Retention Pattern of the Fat Grafts in a Mouse Model. Aesth Plast Surg 47, 2106–2116 (2023). https://doi.org/10.1007/s00266-023-03368-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-023-03368-1