Abstract
Objective
The present study aimed to explore the efficacy and safety profile of liquid phase concentrated growth factor (LPCGF) in promoting autologous fat graft survival.
Methods
LPCGF/PRP was mixed with human fat tissues at different proportions and transplanted into nude mice. Three months after transplantation, the implanted fat tissues were retrieved for analysis. H&E staining was used to quantify the neovascularization. Immunohistochemical staining was applied to quantify the CD34-positive stem cells and the fluorescence intensity of VEGF and TGF-β.
Results
Addition of LPCGF to autologous fat reduced the fat absorption by 5–15%, especially at the early stage, and no complications were observed. In addition, the effect was improved with increased CGF. Liquid phase concentrated growth factor improves autologous fat graft survival, and the most suitable ratio of LPCGF/fat is 1:8.
Conclusion
LPCGF is rich in VEGF, TGF-β and CD34-positive stem cells, which can improve the fat transplantation effect, but the specific influence of a single component requires future evaluation.
No Level Assigned
This journal requires that authors assign a level of evidence to each submission to which Evidence-Based Medicine rankings are applicable. This excludes Review Articles, Book Reviews, and manuscripts that concern Basic Science, Animal Studies, Cadaver Studies, and Experimental Studies. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fat transplantation is an important treatment strategy for maxillofacial soft tissue deficiency, considering its excellence of biocompatibility and compliance in soft-tissue augmentation [1]. However, despite the ideal therapeutic proposal, several practical issues currently serve as roadblocks in the path. Unpredictable absorption rate is a major challenge for the application of fat transplantation procedure nowadays. The reported incidence varies, ranging from 30 to 70% among different cohorts [2]. Furthermore, transplant calcification, cyst and necrosis due to excessive single fat injection and poor angiogenesis may also contribute to poor prognosis of fat transplantation [3, 4]. It has been demonstrated that adipose tissue survival mainly depends on the neovascularization at the early stage of in vivo transplantation, as controlled by diverse pro-angiogenic factors. In recent years, concentrated platelet products, such as PRP and CGF, have been used as autologous materials to improve the fat survival rate [5,6,7,8,9,10] and have attracted increased attention [11,12,13,14,15,16,17]. In this study, we explored the effectiveness and safety of liquid phase concentrated growth factor (LPCGF) in promoting autologous fat graft survival and compared the effect of the main components to that of platelet-rich plasma (PRP).
Materials and Methods
Preparation of Free Fats
A volunteer was recruited from the Department of Oral and Craniomaxillofacial Surgery, Shanghai Ninth People’s Hospital, School of Medicine, Shanghai Jiaotong University. After receiving a conventional physical and laboratory tests to exclude underlying diseases, the volunteer signed the informed consent form and donated 30 ml of fat and 60 ml of peripheral venous blood for three separate times.
Animals
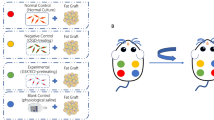
Twenty-four 4-week-old experimental-level NU/NU male nude mice (Shanghai SLAC Experimental Animals Co., Ltd., China) with body weights ranging from 18 to 24 g were selected. All nude mice had freely available food and water. The experiment started one week after all mice were housed in the SPF-level animal laboratory. Twenty-four nude mice were numbered 1–24 and then divided into eight groups. The mixture of LPCGF and fat and the mixture of PRP and fat were prepared for injection into the PRP group. LPCGF and PRP were prepared using the donated blood from the volunteer as described before [18, 19].
Fat Transplantation
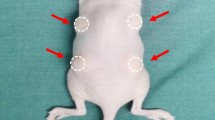
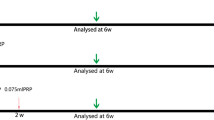
The collected fat was divided into eight portions with 0.8 ml in each portion, and each portion was placed into a 2 ml syringe. The groups were labeled as control, CGF1, CGF2, CGF3, control, PRP1, PRP2 and PRP3. Fat was mixed with LPCGF or PRP at various proportions (blank, 1:8, 1:4 and 1:2; volume/volume). The transplant mixture (0.2 ml fat and LPCGF or fat and PRP at the corresponding proportions) was subcutaneously injected into the upper left, lower left, lower right and upper right areas of the backs of the nude mice with the corresponding numbers: The upper left, lower left, lower right and upper right areas on the backs of the No. 1, 2 and 3 nude mice were injected with the control, CGF1, CGF2 and CGF3 samples, respectively; the upper left, lower left, lower right and upper right areas of the backs of the nude mice marked No. 1 were injected with the control, PRP1, PRP2 and PRP3 samples, respectively, and the remaining experiments were performed in the same manner. After injection, the masses of the empty syringes were weighed, and the transplant masses were recorded (Table 1). Six nude mice were killed at each time point (15 d, 30 d, 45 d and 60 d), and all four transplanted fat samples were removed from subcutaneous tissue on the back of each nude mouse for further analysis. Tissues were harvested post-implantation to calculate the residual rate.
Histological Evaluation
Nude mice were killed through carbon dioxide suffocation at different time points and transplantation material harvested. After weighing and general observation, the obtained materials were fixed in 4% paraformaldehyde, dehydrated and embedded in paraffin. Serial sections of the tissue blocks were processed, stained and photographed with digital camera.
After H&E staining, the central necrosis degree, inflammatory reaction and cytolysis of the transplant were evaluated under a 10× microscope, and the scores were recorded by levels. Scoring criteria were as follows: 5 scores, unseen; 4 scores, mild manifestation; 3 scores, mild and moderate manifestation; 2 scores, moderate manifestation; 1 score, moderate and severe manifestation; 0 score, severe manifestation. The fibrosis degree of transplant tissues was evaluated under a 10× microscope. Scoring criteria are contrary to those specified above.
Immunohistochemistry and Immunofluorescence Staining
The following primary and secondary antibodies were used for cell immunohistochemistry and immunofluorescence staining: CD34+ monoclonal antibody, VEGF monoclonal antibody, TGF-β monoclonal antibody, biotin-labeled IgG antibody, Alexa Fluor® 568 goat anti-rabbit IgG, Fluor® 488 goat anti-mouse IgG antibody, 4,6-diamidino-2-phenylindole dihydrochloride. The staining protocols were described elsewhere [20, 21].
Images were acquired. The staining intensities and fluorescence intensities were quantified and analyzed with ImageJ software.
Results
Comparison of Fat Transplant Survival Rates
The initial mass (g) and residual mass of the fat transplants in three groups at different time points were recorded, and the residual ratios were calculated (Table 1). As no differences were observed among different PRP groups and the control group (data not shown) in fat survival and other indexes, PRP1 group, PRP2 group and PRP3 group were merged for data analysis to avoid redundancy and distraction and labeled as PRP group only in all results. Through analysis of variance of independent samples, differences in the fat transplant survival rates between the LPCGF group and PRP group with different proportions during different time periods were compared, and the following results were obtained (Fig. 1a, b).
(1) After 30 days, the average absorption rate in the LPCGF group was the lowest, and the PRP group showed no obvious difference from the blank control group; the absorption rates of the CGF2 and CGF3 groups were significantly lower than those of the PRP group (p < 0.05). At 60 days, the absorption rates of the CGF1, CGF2 and CGF3 groups were significantly lower than those of the PRP group or blank control group (p < 0.05).
Observation and Scoring Results Based on H&E Staining of the Fat Transplant
No obvious differences existed in the CGF groups in the quantity of new vessels in the fat transplant tissues (Fig. 2a, b). Since the early phase, significant differences existed between the LPCGF groups and the PRP group. The LPCGF group with the lowest concentration of vessels still had more than those in the PRP group, and thus, LPCGF could obviously promote the formation of new vessels and improve the survival of the fat transplant. The quantity of new vessels in the same fat transplant showed few changes at different time points, and the promoting effects of LPCGF and PRP were mainly exerted within 15 days.
Through a quantitative comparison of the histological manifestations of the fat transplant, fat liquefaction ranges in the LPCGF and PRP groups were smaller than those in the blank control group in the early phase, and obvious fibrotic septa appeared between cells that appeared earlier with more new vessel formation and were more obvious in the LPCGF groups than the other groups. However, no obvious differences existed in the inflammatory reactions between the adipose tissues and cellular morphological changes. The higher the proportion of LPCGF to fat, the better the transplantation performed, but when these transplants were compared with the transplant with a low concentration of LPCGF (1:8), there was no significant difference (Fig. 3).
Immunohistochemical Staining of CD34
After immunohistochemical staining, DAB-labeled CD34-positive stem cells were observed under a microscope (Fig. 4a). Most staining sites were located in new vessels, and some were inside the fibrous septum between fats. The staining area was calculated. Based on one-way analysis of variance, the staining areas of all LPCGF groups were significantly larger than those of the PRP group and blank control group at four time points, and no significant difference existed between the PRP group and blank control group (Fig. 4b).
For the intragroup comparison (LPCGF groups), there were no significant differences in CD34-positive stem cells in the different groups (different proportions of LPCGF). No obvious changes occurred as time passed. Correlation analysis between the staining areas in the groups and the quantities of new vessels was conducted. The correlation coefficient reached 0.71 (p < 0.01); thus, CD34-positive stem cells presented a significant correlation with the quantity of new vessels.
Results of VEGF and TGF-β Immunofluorescent Intensities
After immunofluorescence staining, TGF-β showed red fluorescence and VEGF showed green fluorescence, and the cell nucleus showed blue fluorescence. Two cytokines were mostly located in intercellular regions (Fig. 4a).
Compared with the PRP and blank control groups at the same time point, the LPCGF group had higher levels of VEFG and TGF-β. The higher the proportions of LPCGF, the higher the fluorescent intensities of the two cytokines. At different time points, the contents of both cytokines in the LPCGF groups, PRP group and blank control group slightly declined with time, but the differences were not significant (Fig. 4b). A correlation analysis of the two cytokines and histological scores in part two was conducted. The correlation coefficient between the VEGF fluorescence intensity and the score reached 0.55 (p < 0.01), while that between the TGF-β fluorescence intensity and the score was 0.57 (p < 0.01).
Discussion
Analysis of the Mass Change in Fat Transplants
One highlight of the current study is that we took a step forward to analyze the auxiliary effects of CGF in fat graft survival at different doses, on the basis that a wide variety of studies have already revealed the role of PRP. According to our experiment, the absorption degree of adipose tissues with time was an intuitive index. The results showed that CGF reduced the absorption of fat transplants, and the fat absorption rate in the blank control group was similar to clinically reported data, which further supported this conclusion. However, we could not ensure that only transplants without adipose tissues were obtained from nude mice and that there was no transplant loss. Thus, certain errors existed in the experimental results. Furthermore, in terms of experimental design, if a large animal experiment was adopted and materials were taken from the same body continuously, the experimental results would show an increased reliability.
Relationship Between New Vessels in the Fat Transplant and Survival Rate
There are two viewpoints regarding the outcome of fat transplantation: (1) transplanted fat cannot survive, and adipose tissues experience fibrosis [22, 23]; (2) transplanted fat can survive and is present for a long period of time due to sufficient blood supply and nutrition. In this study, the results of the blank control group indicated that subcutaneous blood supply on the back of the nude mice was not abundant, which inhibited the survival of adipocytes; however, new vessels increased in the tissues following LPCGF addition, and they became basically stable from 45 to 60 days. Existing animal experiments have reported that PRP also has a similar effect, and the fat transplant stably grows in the nude mouse body until 90 days. What’s more, we failed to observe the onset of fibrosis in this study, maybe because of the relatively short experimental duration and the low injection dose.
In this study, the quantities of new vessels in all CGF groups were significantly higher than those in the PRP and blank control groups, and the quantity was directly proportional to the results in the first experimental part and the other experiments in this part. Thus, the effect exerted by the generation of new vessels on the survival of the fat transplant was confirmed. A previous biological study on autologous fat transplantation-induced vascularization indicated [24] that some cytokines play significant roles in improving the survival rate of ischemic tissues, and VEGF is a powerful angiogenic factor that can obviously promote initial angiogenesis with high conservatism, stimulate the migration of endothelial cells and maintain the normal state of blood vessels. Thus, the content and effect of VEGF in LPCGF will be further investigated.
PRP has been speculated to improve the fat retention rate in autologous fat transplantation by a wide variety of research [25]. Although PRP was proved able to release growth factors and subsequently facilitate angiogenesis in vivo, the duration period of this biological action was documented only lasting for 8 days after implantation [2]. In the current study, the PRP group seems a little bit superior to control in terms of average residual mass of transplanted fat at 60 d, but no significant differences were observed. Here, we offer several explanations for this phenomenon. First, the biological action of PRP may be too short after implantation to influence the long-term (60 d) fat survival. In a sense, this is the reason why we aim to explore the application value of LPCGF and compare it with PRP. Second, we collected blood samples from one single volunteer, which may lead to selection bias. In future studies, we would expand the sample size and recruit volunteers with different demographic features from different centers. Last but not least, we chose a classical isolation method, which may potentially influence the biological capacities of PRP.
Relationship Between CD34-Positive Stem Cells and Fat Transplantation
CD34 is mainly expressed on the surface of hematopoietic stem cells (HSCs) in blood and primarily maintains the stability of blood compositions through differentiation of mature hemocytes; this molecule also plays a role in regulating and maintaining the physiological equilibrium of cell components in the tissue under stress states such as injury and inflammation. A major advantage of LPCGF is that it contains CD34-positive stem cells, which are not contained in PRP.
In this study, CD34 was expressed in the fat transplant, and LPCGF contained high levels of CD34-positive cells. The promoting effect of HSCs on the survival rate of fat transplantation has seldom been discussed so far, and most studies have positive attitudes toward it [26]. However, CD34 will also be expressed in endothelial cells in new vessels; thus, its positive expression could also be observed in the PRP groups. VEGF in LPCGF can obviously promote angiogenesis; thus, this study cannot prove that the LPCGF-mediated improvement in the survival rate of fat transplantation has a direct bearing on CD34-positive stem cells, and further experimental exploration is needed.
Conclusion
Complete fat transplantation operations provide a safer and more natural operation mode for soft tissue restoration, but the limitations of high fat absorption rates and the low levels of transplanted material remain a challenge. LPCGF, which is rich in various growth factors, has gained increased attention. In this study, the results demonstrated the following conclusions:
-
(1)
LPCGF is safe and effective when applied to autologous fat transplantation.
-
(2)
When compared to PRP, LPCGF has a greater promoting effect on fat transplantation, and its effect is obvious when the proportion of LPCGF/fat tissues is 1:8. Increasing the proportion of LPCGF does not significantly improve the fat transplantation effect. In consideration of the clinically collected blood quantity and the quantity needed for fat transplantation, a proportion of 1:8 is recommended.
-
(3)
LPCGF is rich in VEGF, TGF-β and CD34-positive stem cells, which can improve the fat transplantation effect, but the specific influence of a single component requires future evaluation.
Abbreviations
- LPCGF:
-
Liquid phase concentrated growth factor
- PRP:
-
Platelet-rich plasma
- HSCs:
-
Hematopoietic stem cells
References
Denadai R, Raposoamaral CA, Buzzo CL et al (2018) Autologous free fat grafting for management of the facial contour asymmetry. J Craniofac Surg 29(4):878–886
Jin R, Zhang L, Zhang YG (2013) Does platelet-rich plasma enhance the survival of grafted fat? An update review. Int J Clin Exp Med 6(4):252–258
Pinski KS Jr, RH. (1992) Autologous fat transplantation. J Dermatol Surg Oncol 18(3):179–184
Kaufman MR, Bradley JP, Dickinson B et al (2007) Autologous fat transfer national consensus survey: trends in techniques for harvest, preparation, and application, and perception of short- and long-term results. Plast Reconstr Surg 119(1):323–331
DohanDM ChoukrounJ, DissA, et al (2006) Platelet-rich fibrin (PRF): a second generation platelet concentrate PartII: platelet relatedbiologicfeatures. Oral Surg Oral Med Oral Pathol Oral RadiolEndod 101(3):45–50
Landesberg R, Roy M, G lickman RS. (2000) Quantification of growth factor levels using a simplified method of platelet rich plasma gel preparation. J Oral MaxillofacSurg 58:297–300
Zhang CQ, Yuan T, Zeng BF (2004) Experimental study of the effect of Platelet-rich plasma on osteogenesis in rabbit. Chin Med J 117(12):1853–1855
Marx RE, Carlson ER, Eichstaedt RM et al (1998) Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radio-Endod 85:638–646
Sacco L (2006) Lecture, International academy of implant prosthesis and osteoconnection. 12:4
Rodella LF, Favero G, Boninsegna R et al (2011) Growth factors, CD34 positive cells, and fibrin network analysis in concentrated growth factors fraction. Microsc Res Tech 74(8):772–777
Yoshimura K, Sato K, Aoi N et al (2007) Cell-assisted lipotransfer for cosmetic breast augmentation supportive use of adipose derived stem/stromal cells. Aesthetic PlastSurg 32(1):48–55
Yoshimura K, Sato K, Aoi N et al (2008) Cell-assisted lipotransfer for facial lipoatrophy: efficacy of clinical use of adipose-derived stem cells. DermatolSurg 34(9):1178–1185
Chung CW, Marra KG, Li H et al (2012) VEGF microsphere technology to enhance vascularization in fat grafting. Ann PlastSurg 69(2):213–219
Lu F, Li J, Gao J et al (2009) Improvement of the survival of human autologous fat transplantation by using VEGF-transfected adipose-derived stem cells. PlastReconstrSurg 124(5):1447–1449
Yuksel E, Weinfeld AB, Cleek R et al (2000) Increased free fat-graft survival with the long-term, local delivery of insulin, insulin-like growth factor-I, and basic fibroblast growth factor by PLGA/PEG microspheres. PlastReconstrSurg 105(5):1712–1720
Hamed S, Egozi D, Kruchevsky D et al (2010) Erythropoietin improves the survival of fat tissue after its transplantation in nude mice. PLoS ONE 5(11):419–453
Yun H, Yichen J, Muyao W et al (2018) Concentrated growth factor enhanced fat graft survival. Dermatol Surg 44(7):976–984
Rodella LF, Favero G, Boninsegna R et al (2011) Growth factors, CD34 positive cells, and fibrin network analysis in concentrated growth factors fraction. Microsc Res Tech 74(8):772–777
Lei X, Liu H, Pang M et al (2019) Effects of platelet-rich plasma on fat and nanofat survival: an experimental study on mice. Aesthetic Plast Surg 43(4):1085–1094
Laukka M, Hoppela E, Salo J et al (2020) Preperitoneal fat grafting inhibits the formation of intra-abdominal adhesions in mice. J Gastrointest Surg 24(12):2838–2848
Li S, Liu W, Kang D et al (2019) Locally increased level of inorganic phosphate induced nodules or calcification after bolus fat grafting. Aesthet Plast Surg 43(6):1646–1656
Qi HX (2014) Analysis of component and content of bioactive factors of autologous concentrated growth factors fibrin gel(liquid). Heibei Medical University
Scarano A, Valbonetti L, Marchetti M et al (2016) Soft tissue augmentation of the face with autologous platelet-derived growth factors and tricalcium phosphate microtomography evaluation of mice. J.Craniofac Surg 27(5):1212–1214
Isenberg JS, Romeo MJ, Abuasab M et al (2007) Increasing survival of ischemic tissue by targeting CD47. Circ Res 100(5):712
Li Y, Mou S, Xiao P et al (2020) Delayed two steps PRP injection strategy for the improvement of fat graft survival with superior angiogenesis. Sci Rep 10(1):5231
Roh DS, Orgill DP (2018) Discussion: early macrophage infiltration improves fat graft survival by inducing angiogenesis and hematopoietic stem cell recruitment. Plast Reconstr Surg 141(2):387
Acknowledgements
We would like to acknowledge the reviewers for their helpful comments on this paper.
Funding
None.
Author information
Authors and Affiliations
Contributions
XW finished study design, TZ, YX finished experimental studies, JD, LY finished data analysis, TZ, JD finished manuscript editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and Animal Rights
This study was approved and supervised by the animal ethics committee of National Clinical Research Center for Oral Diseases, Shanghai Key Laboratory of Stomatology & Shanghai Research Institute of Stomatology. The treatment of animals in all experiments conforms to the ethical standards of experimental animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tianjia Zhang and Jiewen Dai are co-first authors.
Rights and permissions
About this article
Cite this article
Zhang, T., Dai, J., Xu, Y. et al. Liquid Phase Concentrated Growth Factor Improves Autologous Fat Graft Survival In Vivo in Nude Mice. Aesth Plast Surg 45, 2417–2422 (2021). https://doi.org/10.1007/s00266-021-02336-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-021-02336-x