Abstract
Background
Suction-assisted lipoplasty (SAL; liposuction) is an established aesthetic procedure in plastic surgery. The main parameters differentiating one method of lipoplasty from another are safety, consistency of results, and other more technical parameters. Due to the recent popularity of lipotransfer, the quality of extracted fat has become a relevant parameter. We compare the viability of extracted adipocytes after dry SAL, hyper-tumescent PAL (power-assisted lipoplasty), and water-assisted lipoplasty (WAL).
Methods
We used fluorescent microscopy to differentiate viable from necrotic/apoptotic cells after liposuction using each of the mentioned methods.
Results
The ratio of living cells between the three methods was significantly different with dry liposuction yielding inferior ratios (p = 0.011). When omitting extreme results, we found that the body-jet technique (WAL) yielded higher ratios of living cells than the hyper-tumescent technique (p < 0.001). The total number of cells was highest in the hyper-tumescent method (p = 0.013).
Conclusions
Our results indicate that the hyper-tumescent technique yields the highest number of cells, whereas the body-jet technique yields the highest living cells ratio. The dry technique is clearly inferior to both.
No Level Assigned
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Suction-assisted lipoplasty (SAL; liposuction) is an established aesthetic procedure in plastic surgery. First attempted by surgeons in the 1920s–1960s and abandoned, it was re-introduced by Illouz in the late 1970s and early 1980s [1, 2]. At first, the procedure was marked by blood loss, electrolyte imbalance, and early disappointing results [3]. However, later refinements of the technique and development of new techniques [i.e., power-assisted lipoplasty (PAL), water-assisted lipoplasty (WAL)] [3–9] have mostly rendered these concerns obsolete [10, 11].
Traditionally, the main parameters differentiating one method of lipoplasty from another are safety (i.e., blood loss, fluid and electrolyte imbalance, etc.), consistency of results, and other more technical parameters such as ease of performance, learning curve, duration of hospitalization, and recovery time. The quality or viability of the extracted fat has never been a relevant parameter. However, in the previous decade, lipotransfer (the use of the extracted fat as a graft in other receiving areas) has been introduced to both reconstructive [12, 13] and aesthetic [14, 15] plastic surgery. This development has made the viability of extracted fat a major determining factor [16] in the take of the grafted fat. However, to date, this issue has not been resolved.
Objective
To compare the viability rates of extracted adipocytes after dry SAL, hyper-tumescent PAL, and water-assisted lipoplasty.
Patients and Methods
Patients
Sixteen female patients (age range 35–76 years) undergoing routine SAL as part of a reconstructive procedure were included. Patient medical history and personal demographic and characteristics were collected. All patients signed an informed consent form as required by the medical center’s internal review board.
Lipoplasty
Before commencement of the procedure each patient underwent dry SAL, hyper-tumescent PAL, and body-jet WAL. Twenty cubic centimeters of lipoaspirate were extracted in each method, all from the flanks, and transferred for further processing.
Dry SAL: As previously described [17, 18] after general anesthesia, fat was extracted using a blunt tip, 26-cm-long cannula, outer diameter of 4 mm (Byron™, MENTOR, Santa Barbara, California, MER426L) and a 20-cc luer lock syringe.
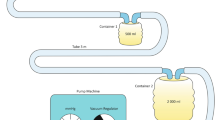
Body-Jet WAL: Following dry SAL patients underwent WAL using the body-jet system (Body-Jet; Human Med, Eclipse Ltd., Dallas, TX, USA) with the LipoCollector™ 3 and a standard 3.8 mm, 25 cm long cannula (REF 500092). The procedure has been previously described [19]. In the first phase, minimal volumes of standard tumescent solution are infiltrated in a pulsatile fashion to the treated area. In the second phase, fat is extracted simultaneously with the continued infiltration, collected, and separated from the fluids in a LipoCollector™. Adipocytes were sampled into a 20 cc lure lock syringe. In the final stage, suction is performed without infiltration to dry the treated area. It has to be emphasized that the 20 cc of lipoaspirate that were used to evaluate the viability of the cells were extracted from a different area than the dry SAL.
Hyper-Tumescent PAL: After fat extraction using the dry and body-jet methods, the patients underwent hyper-tumescent PAL as previously described [20]. In short, the treated area was infiltrated with high volumes (three times or more of the expected aspiration volume. Actual infiltration volume ranged 1–8 L per patient depending on the amount of expected fat harvest) of tumescent solution (prilocaine 2 %, 30 mL/3 L, lidocaine 2 %, 30 mL/3 L, NaHCO3 8.4 %, 20 mL/3 L), followed by fat extraction using a MicroAire PAL®-600E (Power Assisted Liposuction) LipoSculptor™, with reciprocating 4 mm cannulas 22 and 30 cm in length (4000 oscillations/min) into a negative pressure sterile collector, and adipocytes were sampled into a 20-cc Lure Lock syringe. As with WAL, hyper-tumescent PAL was performed in a previously unsuctioned site.
Lipoaspirate Processing
As previously described by Son [21], adipocytes were separated from blood cells, debris, and supernatant by centrifugation at 3000 rpm for 10 min (Eppendorf 5810R, 1800G) at room temperature (Fig. 1). One milliliter of the adipocyte layer was digested with 5 mL of 0.1 % collagenase 1A (Crude Type IA C2674-100MG from Clostridium histolyticum) in phosphate-buffered saline at 37 °C for 40 min, the collagen degradation reaction was stopped with 6 mL of DMEM (DMEM With 4.5 g/L d-Glucose Without l-Glutamine, Biological Industries Kibbutz BeitHaemek). Mature adipocytes were separated from the pellets by centrifugation at 1000 rpm for 10 min (Eppendorf 5810R, 200G). Specimens were divided into four layers: oil, mature adipocytes, blood, and pre-adipocytes. Then 400 µL was taken from the middle of the mature adipocyte layer. Twelve micromolar of fluorescein diacetate (Fluorescein Diacetate, Sigma 100 µL of 0.02 mg/µL) and 3.75 µM of propidium iodide (propidium iodide, Sigma 50 µL, 1 mg/µL) were added together with 250 µL of PBS, mixed, and inverted. After 5 min, 10 µL of the adipocytes was placed on a disposable hemocytometer (C-Chip Disposable Hemocytometer, Digital Bio).
Viability Evaluation
Mature adipocytes were extracted and purified from each lipoaspirate specimen harvested by one of the three different aspiration methods. Two sets of hemocytometers were prepared from each sample. The hemocytometers were examined under a fluorescence microscope (Olympus IX81) and ultraviolet illumination. Viable adipocytes fluoresced bright green (particularly around the edges; Fig. 2), whereas the nuclei of dead (necrotic/apoptotic) cells fluoresced red (propidium iodide; Fig. 3). Five high power fields (magnification ×20) were evaluated in every hemocytometer, cells were counted, and the number of stained cells alive or dead was recorded (Fig. 4).
Statistical Analysis
Adipocyte viability is presented as a live to total adipocyte (dead and alive) ratio. Viability ratios were compared between the different harvesting methods and analyzed using the ANOVA test repeated measures. The mean value was calculated and reported with standard deviation. Comparison of the viability ratios and total cell counts (dead + alive) within the same harvesting method between patients with normal BMI and normal medical history, and patients with elevated BMI and systemic illnesses was performed using the unpaired t test. Comparison of the total cell counts between the different harvesting methods was performed using the ANOVA test in repeated measures.
All of the statistical analyses were performed using InStat, Version 3.1a; GraphPad Software Inc and http://vassarstats.net.
Results
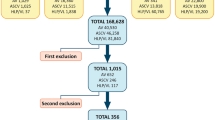
Sixteen female patients, 35–76 years old, underwent routine SAL. The patient BMIs ranged from 21.6 to 34.8. Ten patients had a normal BMI whereas the rest were either overweight or obese. None of the patients were smokers, and all of the patients had a past medical history involving Ca of Breast. Other medical conditions were asthma, dyslipidemia, and hypothyroidism and hypertension. Table 1 summarizes patient characteristics.
Lipoaspirate volume ranged between 100 and 1500 cc, with a mean volume of 450 cc (not including fat harvested in the various methods for the current study). In all patients, fat was harvested from the lower abdomen and flanks. No adverse events or complications were noted in any of the patients. Table 2 summarizes the volume of fat harvested in each patient.
Table 3 summarizes the cell count for each patient in each harvest method. In all three methods, the lowest total number of cells per high power field was obtained from patient number 1. This finding is coupled with the lowest ratio of living to dead cells in all three methods. In fact, patient 1 is the only patient displaying a lower than 1 ratio (more dead than living cells), and does so in all three methods of fat harvest. In contrast, the highest ratio of living cells was observed in specimens harvested from patient 6. In the dry SAL specimens, patients with normal BMI and normal medical history the ratios of living cells were similar to those found in the elevated BMI group (1.96 ± 0.65 vs. 1.4 ± 0.5; p = 0.13) as well as their total cell count (145.1 ± 23.5 vs. 134.2 ± 56.6; p = 0.67). In the WAL specimens, patients with normal BMI and normal medical history had higher ratios of living cells (3.4 ± 0.8 vs. 2.1 ± 1; p = 0.03), but their total cell count was not higher than that of the elevated BMI patients (182.8 ± 59.3 vs. 155 ± 122.9; p = 0.62). In the hyper-tumescent PAL specimens, patients with normal BMI and normal medical history had higher ratios of living cells, but this difference did not reach statistical significance (3.2 ± 2.1 vs. 1.75 ± 1.1; p = 0.18). Their total cell count was not higher than that of the elevated BMI patients (208 ± 65 vs. 186 ± 105; p = 0.67).
The total number of cells was highest in the hyper-tumescent PAL method (Table 3; p = 0.013). When comparing the average ratio of living cells between the three harvesting methods, we found a statistically significant difference in which dry SAL yielded ratios that were inferior to those of the two other methods (Table 4; p = 0.011). We did not find a statistically significant difference between the hyper-tumescent PAL technique and the body-jet WAL technique (p = 0.49). However, when omitting extremely high ratios (patient 6) and extremely low ratios (patient 1), the body-jet PAL technique had a statistically significant advantage (2.84 vs. 2.18; p < 0.001).
Discussion
The popularity of fat transfer has been rising in recent years. The techniques of harvesting and grafting vary widely, with equally varying results. However, standardization is lacking and an agreed upon set of guidelines is not available. This study aims to clarify whether or not the method of adipose tissue harvest plays a role in the final result.
Free fat graft viability evaluation presents a challenging target. Current methods include [22] glucose uptake, trypan blue staining, GPDH uptake assays, FDA-PI staining, and lately MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrasodium bromide] colorimetric assay. Most of the above methods require in vivo isolation of the adipocyte from the surrounding stromal components. The use of FDA-PI [23] staining to determine cell viability is well described, commonly used, and reliable, and we found this method easy to implement.
In a recent study by Agostini et al. [24], the viability of fat cells was compared following the dry and wet techniques. In that study, no difference was found between the methods.
Our results, in contrast, show clear and significant differences between the methods. We found lower cell counts and viability ratios in the dry technique. The hyper-tumescent PAL technique yielded higher total cell counts, whereas the body-jet WAL technique yielded higher living cells ratios. Because adipocytes and pre-adipocytes are considered more vulnerable to mechanical trauma, as well as to changes in the chemical environment (i.e., the infiltration solution), the authors carefully raise the hypothesis that other, more resilient cells, namely mesenchymal stem and progenitor cells, may follow the same pattern [25, 26], thus rendering the harvest method critical to the final result of more complex procedures such as mesenchymal cell extraction and enrichment of fat grafts. However, this study did not aim to examine this question, and the design of the study does not allow the authors to draw a conclusion on this subject. Further research is needed to validate this hypothesis.
Although different cannula diameters were used in each lipoaspirate method and may influence the adipocyte viability, the overall change in cannula diameter was not prominent enough to solely justify our findings.
Another notable finding was that the ratio of living cells was higher in patients with normal BMI and unremarkable medical history only in the body-jet WAL specimens compared to patients with elevated BMI and other illnesses, but not in other harvest methods. The finding of a higher ratio of living cells in the body-jet WAL specimens in patients with normal BMI was to be expected; however, this is the first study to prove it. More surprising is the lack of statistically significant difference (between normal and elevated BMI patients) in other methods. Whether this is a result of the small group or not is a matter for further research.
Implications and Future Research
As mentioned before, our results indicate that the harvesting methods influence the quantities of cells per extraction volume, and their viability ratios. Thus, the authors recommend using either the body-jet WAL or hyper-tumescent PAL techniques for fat harvesting procedures. The dry technique should be avoided in this setup.
When the body-jet technique WAL is used, normal BMI/medical history patients have a better prognosis for a successful procedure.
References
Hetter GP, Herhahn F (1983) Experience with “lipolysis”: the Illouz technique of blunt suction lipectomy in North America. Aesthet Plast Surg 7(2):69–76
Illouz YG (1983) Body contouring by lipolysis: a 5-year experience with over 3000 cases. Plast Reconstr Surg 72(5):591–597
Klein JA (1990) Tumescent technique for regional anesthesia permits lidocaine doses of 35 mg/kg for liposuction. J Dermatol Surg Oncol 16(3):248–263
Klein JA (1990) The tumescent technique. Anesthesia and modified liposuction technique. Dermatol Clin 8(3):425–437
Lauber JS, Abrams HL, Coleman WP 3rd (1990) Application of the tumescent technique to hand augmentation. J Dermatol Surg Oncol 16(4):369–373
Apfelberg D (1992) Laser-assisted liposuction may benefit surgeons, patients. Clin Laser Mon 10(12):193–194
Zocchi M (1992) Ultrasonic liposculpturing. Aesthet Plast Surg 16(4):287–298
Dillerud E, Heden P (1993) Circulation of blood and viability after blunt suction lipectomy in pig buttock flaps. Scand J Plast Reconstr Surg Hand Surg 27(1):9–14
Baker TM et al (1996) What’s new in aesthetic surgery. Clin Plast Surg 23(1):3–16
Dolsky RL (1990) Blood loss during liposuction. Dermatol Clin 8(3):463–468
Apfelberg DB et al (1994) Progress report on multicenter study of laser-assisted liposuction. Aesthet Plast Surg 18(3):259–264
Ducic Y, Pontius AT, Smith JE (2003) Lipotransfer as an adjunct in head and neck reconstruction. Laryngoscope 113(9):1600–1604
Ducic Y (2008) Fat grafting in trauma and reconstructive surgery. Facial Plast Surg Clin North Am 16(4):409–416 v–vi
Williams EF 3rd, Smith SP Jr (2007) Minimally invasive midfacial rejuvenation: combining thread-lift and lipotransfer. Facial Plast Surg Clin North Am 15(2):209–219 vii
DeFatta RJ, Williams EF 3rd (2008) Fat transfer in conjunction with facial rejuvenation procedures. Facial Plast Surg Clin North Am 16(4):383–390 v
Smith P et al (2006) Autologous human fat grafting: effect of harvesting and preparation techniques on adipocyte graft survival. Plast Reconstr Surg 117(6):1836–1844
Fournier PF, Otteni FM (1983) Lipodissection in body sculpturing: the dry procedure. Plast Reconstr Surg 72(5):598–609
Dolsky RL (1984) Body sculpturing by lipo-suction extraction. Aesthet Plast Surg 8(2):75–83
Sasaki GH (2011) Water-assisted liposuction for body contouring and lipoharvesting: safety and efficacy in 41 consecutive patients. Aesthet Surg J 31(1):76–88
Sattler G et al (1999) Tumescent liposuction in Germany: history and new trends and techniques. Dermatol Surg 25(3):221–223
Son D et al (2010) Viability of fat cells over time after syringe suction lipectomy: the effects of cryopreservation. Ann Plast Surg 65(3):354–360
Macrae JW et al (2003) Human adipocyte viability testing: a new assay. Aesthet Surg J 23(4):265–269
Jones KH, Senft JA (1985) An improved method to determine cell viability by simultaneous staining with fluorescein diacetate–propidium iodide. J Histochem Cytochem 33(1):77–79
Agostini T et al (2012) Wet and dry techniques for structural fat graft harvesting: histomorphometric and cell viability assessments of lipoaspirated samples. Plast Reconstr Surg 130(2):331e–339e
Matsumoto D et al (2006) Cell-assisted lipotransfer: supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng 12(12):3375–3382
Yoshimura K et al (2008) Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthet Plast Surg 32(1):48–55 discussion 56–7
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest to disclose.
Additional information
This study was presented in the “Oncoplastic & Reconstructive Surgery of the Breast” meeting in Milan, Italy/December 2011.
Rights and permissions
About this article
Cite this article
Harats, M., Millet, E., Jaeger, M. et al. Adipocytes Viability After Suction-Assisted Lipoplasty: Does the Technique Matter?. Aesth Plast Surg 40, 578–583 (2016). https://doi.org/10.1007/s00266-016-0645-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-016-0645-6