Abstract
Objective
Anesthetic agents are often combined to enhance their therapeutic effects while minimizing adverse events. The aim of this study was to evaluate the effects of two different sedation regimens of ketamine and propofol combination via infusion on perioperative variables in patients who underwent plastic and reconstructive surgery.
Methods
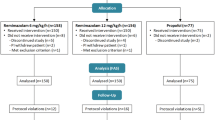
This randomized double-blind clinical trial was done on 80 patients who were randomized to two groups; group 1 (n = 40) received a 2:1 mixture of 9 mg/ml propofol and 4.5 mg/ml ketamine, and group 2 (n = 40) received a 4:1 mixture of 9 mg/ml propofol and 2.25 mg/ml ketamine. After premedication and before local anesthetic injection, the infusion of mixtures was adjusted to attain the Ramsay sedation scores of 5 in both groups. We recorded induction time, sedation efficacy, cardiovascular and respiratory events, recovery time, and incidence of adverse events during and after the procedure.
Results
The mean of volume infusion of mixtures in the beginning of the procedure was higher in group 2 (3.2 ± 1. 2 ml) than in group 1 (2.4 ± 0.8 ml) (p < 0.001). The induction time for sedation was 2.8 ± 0.8 min and 2.6 ± 0.4 min in group 1 and group 2, respectively (p = 0. 92). The number of oversedated patients was greater in group 2 compared to group 1 but not statistically significant (p = 0. 80). The sedation efficacy was similar between the two groups. The hemodynamic changes during the procedure were greater in group 2 compared to group 1 (p = 0. 001). The recovery time was not significantly different between the two groups (p = 0.43). The mean pain score in the recovery room was lower in group 1 than group 2 (1.2 ± 0.8 vs 2.8 ± 1.8, p = 0. 01). Moreover, 4 (10 %) patients in group 1 and 10 (25 %) patients in group 2 needed opioid administration (p = 0. 02). Other postoperative adverse events were similar between the two groups.
Conclusion
We recommend the use of a 2:1 combination of propofol–ketamine, because it reduced the rescue propofol requirement and consequently produced lower cardiovascular and respiratory depression effects and also less postoperative pain.
Level of Evidence I
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many plastic and reconstructive surgeries such as basal cell carcinoma, squamous cell carcinoma, and melanoma can be performed with monitored anesthesia care, especially in elderly patients because many of these patients have low cardiovascular reserves and general anesthesia in these subjects might be difficult. This strategy needs efficacious and safe sedation with analgesia. The goal of appropriate sedation includes a sufficient level of sedation and amnesia, minimizing pain, anxiety, and adverse drug-related complications, and maintaining stable hemodynamics and ventilation during the procedure. The reasonable agents for sedation are better to reach these goals. Also, these agents would be safe, especially in elderly patients and have a short recovery time and are inexpensive. Unfortunately, we don’t know a single agent that has all of these characteristics, so anesthesiologists for achieving efficacious sedation administer combinations of different agents to obtain many of these desired goals. The major problems in the administration of propofol for sedation are dose-dependant hypotension and respiratory depression [1]. Also, ketamine use can produce psychotomimetic effects and increase the incidence of postoperative nausea and vomiting [2]. Previous studies showed that a small dose of ketamine possesses analgesic properties [3, 4]. This property of low-dose ketamine can complement the sedation provided by propofol [5]. It is clear that the use of a mixture of these two agents may preserve sedation efficacy and decrease their adverse events, because many potential adverse effects are dose dependent [6]. However, if we administer a combination of these agents, the dose of each agent can be reduced [7]. Also, when we use a propofol and ketamine combination the hemodynamics remain stable because the cardiovascular effects of the two agents oppose each other. This study was aimed to evaluate the effects of two different sedation regimens that include a combination of ketamine and propofol via infusion on hemodynamic variables, sedation efficacy, analgesia, time of recovery, and adverse events during and after plastic and reconstructive surgery.
Methods
Our randomized, double-blind clinical trial was done from January to September 2013 on 80 consenting ASA physical status I–III patients who underwent plastic and reconstructive surgery, including basal cell carcinoma (BCC), squamous cell carcinoma (SCC), and melanoma on the scalp or head and neck. Two surgeons performed all procedures with patients under sedation. Our study was approved by the ethical committee in our hospital. Exclusion criteria included patients with clinically significant cardiovascular, respiratory, and neurological disease, a history of psychological problems, substance abuse or chronic pain. Number of subjects in each group provided a 90 % power for detecting a 40 % difference in opioid administration postoperative with an alpha level of 0.05. Patients were randomized to two groups according to a computer-generated randomization schedule. Group 1 received a propofol–ketamine combination from syringes prepared as a 2:1 mixture of 9 mg/ml propofol and 4.5 mg/ml ketamine (combination of 50 ml propofol 1 % with 5 ml of ketamine). The infusion rate in this group was 1.5 mg/kg/hr of propofol and 0.8 mg/kg/hr of ketamine during surgery. Group 2 received mixtures prepared as a 4:1 ratio of 9 mg/ml propofol and 2.25 mg/ml ketamine (combination of 50 ml propofol 1 % with 2.5 ml of ketamine). The infusion rate in group 2 was 1.5 mg/kg/hr of propofol and 0.4 mg/kg/hr of ketamine during surgery. Propofol–ketamine mixture syringes were prepared by an anesthesia nurse who was not directly involved in this study. Midazolam (15 µg/kg) and fentanyl (1 µg/kg) IV were given to all our patients as premedication. Non-invasive blood pressures, heart rate, oxygen saturation via pulse oximetry were recorded at the beginning of the operation and then every 5 min until the end of the operation. Pain evaluation was determined by the visual analog scale (zero = no pain—10 = worst pain which was experienced) in two groups during and after the procedure. Ventilation was assessed by end-expiratory carbon dioxide and recording of respiratory rate. End-expiratory carbon dioxide was monitored via a plastic catheter through a nasal cannula. The sedation level of the patients was assessed by Ramsay sedation scores [7]. After administration of premedication and before injection of local anesthesia, the infusion of the propofol–ketamine combination was adjusted to attain the Ramsay sedation scores of 5 in both groups and then 10–15 ml lidocaine 2 % plus epinephrine 1/200,000 was injected in both groups. If the sedation score during the procedure was less than 5, an additional dose of the mixture was infused to obtain this score in both groups. We recorded the induction time of sedation that was defined as the interval from the beginning of infusion of propofol–ketamine mixture until the time that the Ramsay sedation score of 5 was achieved. Sedation efficacy was defined as the patients not having an unpleasant recall of the operation and no sedation-related adverse events during the procedure. Other secondary outcomes included a total infusion of the propofol–ketamine combination dose, operation time, recovery time, desaturation (SpO2 < 90 %), respiratory depression, and nausea and vomiting during the procedure. After completion of the procedure, based on the Aldrete recovery score patients the patients were transferred to the recovery room and vital signs and level of sedation were assessed every 15 min. Statistical analysis of our data was performed with SPSS 16.0. Parametric variables were analyzed with the t test and compared between the two groups. Blood pressure and heart rate were analyzed using repeated measurement analysis. Categorical variables were compared between the two groups by χ2 or Fisher’s exact tests. All data were presented as means with standard deviations (SD). The results were considered significant at a p value < 0.05.
Results
Demographic characteristics were similar between the two groups (Table 1). Also, types of procedures were not significantly different between the two groups (Table 2). The duration of surgical intervention was similar between two groups (88.2 ± 18.8 min in group 1 and 84.6 ± 22.2 min in group 2, respectively p = 0. 43). The mean volume of the infused propofol–ketamine combination in the beginning of the procedure until the level of sedation reached a Ramsay sedation score of 5 was higher in group 2 (3.2 ± 1. 2 ml) than in group 1 (2.4 ± 0.8 ml) (p = 0. 001). The induction times for sedation were 2.8 ± 0.8 min and 2.6 ± 0.4 min in group 1 and group 2, respectively (p = 0. 92). Eight (20 %) patients in group 1 and 14 (35 %) patients in group 2 needed an additional infusion of the propofol–ketamine mixture intraoperatively to maintain a Ramsay sedation score of 5 (p = 0. 01). The dose of propofol–ketamine mixture used in group 1 was 18.2 ± 6.4 ml compared to 24.4 ± 8.2 ml in the other group (p = 0. 03). The mean of the Ramsay sedation score during the operation was 4.6 ± 1.4 in group 1 and 4.8 ± 1.2 in group 2 (p = 0. 09). The number of oversedated patients (Ramsay sedation score >5) was greater in group 2 compared to group 1, 6 (15 %) patients versus 4 (10 %) patients, but was not statistically significant (p = 0. 80). The dose of lidocaine 2 % plus epinephrine that was injected for local anesthesia was 12.2 ± 4.2 ml in group 1 and 12.8 ± 6.4 ml in group 2 (p = 0. 82). The sedation efficacy was similar between the two groups. The surgeon’s satisfaction during the operation did not differ between the groups (95 % in group 1 versus 87 % in group 2, respectively p = 0. 09). The hemodynamic changes during the procedure were greater in group 2 compared to the other group (p < 0.001). (Table 3, 4, 5) (Fig. 1, 2, 3). Moreover, the intraoperative heart rate changes were statistically significantly greater in group 2 compared to group 1 (Table 6) (Fig. 4). No patient in either group became hypotensive (decrease of 30 % of systolic blood pressure from baseline measurement) that required treatment. Desaturation (SpO2 <90 %) was observed in 2 (5 %) patients in group1 and in 5 (12.5 %) patients in group 2 during the procedure (p = 0.64). All of these cases required simple reposition of the airway with the head tilt or chin lift and supplemental oxygen, and none needed mask ventilation or endotracheal intubation. None of the patients in either group showed apnea. The mean respiratory rates measured by capnography were higher in group 1 compared to group 2 during the procedure (14.8 ± 2.4 vs 12.4 ± 4.2, p = 0.03). No patients in either group developed agitation or hallucinations. Also, none of the patients experienced rash, bradycardia, and shivering through and after the procedure. The duration of phase 2 recovery was not significantly different between the two groups (group 1, 34.4 ± 10.2 min, group 2, 38.2 ± 14.4 min, p = 0.43). The mean VAS score in the recovery room was lower in group 1 than group 2 (1.2 ± 0.8 versus 2.8 ± 1.8, p = 0.01). However, 4 (10 %) patients in group 1 and 10 (25 %) patients in group 2 needed opioid administration postoperatively (p = 0.02). None of the patients in both groups experienced the psychotomimetic response postoperatively. None of the patients in group 1 and 2 (5 %) patients in group 2 experienced nausea and vomiting after the operation (p = 0.37).
Discussion
The present study showed that a 2:1 mixture of propofol–ketamine appears to be effective and safe with lower respiratory and cardiovascular depression effects and also less postoperative pain compared to a 4:1 mixture in patients undergoing plastic and reconstructive surgery. It is preferable that anesthetic agents are often combined to lead to favorable endpoints and less dose-dependent adverse events. In our study addition of ketamine to propofol not only provided analgesia, but also, counteracts the cardiovascular and respiratory depression of propofol. The combination in group 2 (4:1; 9 mg/ml propofol and 2.25 mg/ml ketamine) required more additional infusion of the mixture in the beginning and during the procedure and also more unwanted deep sedation compared to the other group. These observations are supported by previous studies that found the combination of propofol and ketamine results in deeper sedation than propofol alone, and leads to a decreased amount of propofol administration [8, 9]. As a result, fewer patients in the propofol–ketamine mixture need repeated doses of agents to maintain nickel sedation during their procedure. Erden who compared two different doses of ketamine for sedation during interventional radiology procedures showed that the higher dose of ketamine in combination with propofol resulted in lower administration of drugs and lower incidence of desaturation [6]. In our study, desaturation episodes were greater in group 2 with lower ketamine doses but not statistically significant. It is known that to achieve desirable sedation with adequate analgesia, use of ketamine against opioids and combining with propofol could provide fewer adverse airway events [10, 11]. Previous studies showed that respiratory adverse events of propofol are dependent on the rate of its administration [12, 13]. It was shown that ketamine preserved respiratory function [14] and combination of it with propofol counterbalances the respiratory depression associated with propofol alone [15]. This protective effect of ketamine enables us to obtain the desired sedation depth with minimum doses of propofol when the combination is used for sedation during procedures [16]. In our study, supplemental oxygen and airway repositioning such as head extension and chin lift were enough to correct respiratory depression and there was no need for mask ventilation or endotracheal intubation. One study reported that use of a propofol–ketamine combination provided desaturation and 2.6 % of patients required airway manipulation and 0.9 % needed bag ventilation [17]. Moreover, one study showed that the use of ketamine by reducing total doses of propofol can significantly improve ventilation and decrease end-expiratory CO2 [4]. The hemodynamic changes of group 2 in our study were greater compared to group 1. This finding can relate to administration of higher doses of propofol for group 2. Therefore, use of the mixture of group 1 is suitable for sedation especially in elderly subjects because of the low cardiovascular reserves. However, some previous studies identified that hemodynamic variables did not differ with different doses of ketamine [5, 6]. One of the major problems of ketamine is emergence delirium, but it was shown that this response was low when ketamine was combined with propofol [18]. Also, it is reported that the incidence of psychotomimetic responses to propofol–ketamine mixtures was low and often occurred in the mixture of large doses of ketamine [5]. This adverse event was not statistically different between the two groups in our study. The median recovery time for both groups in our study was similar. Previous studies showed that the median recovery time of sedation with propofol–ketamine combinations was small [6, 19] also, other studies observed recovery times of propofol–ketamine were shorter than fentanyl–midazolam combination [20], propofol alone [21], and ketamine alone [22]. The use of ketamine can lead to nausea and vomiting with incidences of 5 and 15 % [23]. However, when it is combined with propofol, this problem is compensated by the antiemetic activity of propofol [24]. There are no statistically significant differences between the two groups. In our study, the intraoperative and postoperative pain evaluated with VAS scores decreased more in group 1 compared to the other group. This result may be related to the preemptive analgesic effect of low-dose ketamine that was identified in previous studies [25–28]. Moreover, there was no difference in the amount of postoperative opioid administration in our groups. We think that with an increased sample size, this variable may be statistically significant. The main limitation of our protocol was the small sample size. In conclusion, use of a 2:1 mixture of 9 mg/ml propofol and 4.5 mg/ml ketamine for sedation during plastic and reconstructive surgery showed little need to use propofol and minimum respiratory depression and hemodynamic changes during procedures compared to the 4:1 (9 mg/ml propofol and 2.25 mg/ml ketamine) mixture. Both combinations appeared to have short recovery times and few postoperative complications. Therefore, infusion of propofol–ketamine combinations on the order of that used in group 1 appears to be safe and effective for sedation during surgery with less oversedation and also lower cardiovascular and respiratory depression effects.
References
Hug CC Jr, McLeskey CH, Nahrwold ML, Roizen MF, Stanley TH, Thisted RA, Walawander CA, White PF, Apfelbaum JL, Grasela TH et al (1993) Hemodynamic effects of propofol: data from over 25,000 patients. Anesth Analg 77(4 Suppl):S21–S29
Bowdle TA, Radant AD, Cowley DS, Kharasch ED, Strassman RJ (1998) Roy-Byrne PP. Psychedelic effects of ketamine in healthy volunteers: relationship to steady-state plasma concentrations. Anesthesiology 88(1):82–88
Suzuki M, Tsueda K, Lansing PS, Tolan MM, Fuhrman TM, Ignacio CI, Sheppard RA (1999) Small-dose ketamine enhances morphine-induced analgesia after outpatient surgery. Anesth Analg 89(1):98–103
Mortero RF, Clark LD, Tolan MM, Metz RJ, Tsueda K, Sheppard RA (2001) The effects of small-dose ketamine on propofol sedation: respiration, postoperative mood, perception, cognition, and pain. Anesth Analg 92(6):1465–1469
Badrinath S, Avramov MN, Shadrick M, Witt TR, Ivankovich AD (2000) The use of a ketamine-propofol combination during monitored anesthesia care. Anesth Analg 90(4):858–862
Erden IA, Pamuk AG, Akinci SB, Koseoglu A, Aypar U (2010) Comparison of two ketamine-propofol dosing regimens for sedation during interventional radiology procedures. Minerva Anestesiol 76(4):260–265
Ramsay MA, Savege TM, Simpson BR, Goodwin R (1974) Controlled sedation with alphaxalone-alphadolone. Br Med J 2(5920):656–659
Andolfatto G, Abu-Laban RB, Zed PJ, Staniforth SM, Stackhouse S, Moadebi S, Willman E (2012) Ketamine-propofol combination (ketofol) versus propofol alone for emergency department procedural sedation and analgesia: a randomized double-blind trial. Ann Emerg Med 59(6):504-12.e1-2
David H, Shipp J (2011) A randomized controlled trial of ketamine/propofol versus propofol alone for emergency department procedural sedation. Ann Emerg Med 57(5):435–441
Erden IA, Pamuk AG, Akinci SB, Koseoglu A, Aypar U (2009) Comparison of propofol-fentanyl with propofol-fentanyl-ketamine combination in pediatric patients undergoing interventional radiology procedures. Paediatr Anaesth 19(5):500–506
Chiaretti A, Ruggiero A, Barone G, Antonelli A, Lazzareschi I, Genovese O, Paiano S, Sammartino M, Maurizi P, Riccardi R (2010) Propofol/alfentanil and propofol/ketamine procedural sedation in children with acute lymphoblastic leukaemia: safety, efficacy and their correlation with pain neuromediator expression. Eur J Cancer Care (Engl) 19(2):212–220
Claeys MA, Gepts E, Camu F (1988) Hemodynamic changes during anaesthesia induced and maintained with propofol. Br J Anaesth 60(1):3–9
Frazee BW1, Park RS, Lowery D, Baire M (2005) Propofol for deep procedural sedation in the ED. Am J Emerg Med. 23(2):190-5
Kim G, Green SM, Denmark TK, Krauss B (2003) Ventilatory response during dissociative sedation in children-a pilot study. Acad Emerg Med 10(2):140–145
Green SM, Andolfatto G, Krauss B (2011) Ketofol for procedural sedation? Pro and con. Ann Emerg Med. 57(5):444–448
Aouad MT, Moussa AR, Dagher CM, Muwakkit SA, Jabbour-Khoury SI, Zbeidy RA, Abboud MR, Kanazi GE (2008) Addition of ketamine to propofol for initiation of procedural anesthesia in children reduces propofol consumption and preserves hemodynamic stability. Acta Anaesthesiol Scand 52(4):561–565
Willman EV, Andolfatto G (2007) A prospective evaluation of “ketofol” (ketamine/propofol combination) for procedural sedation and analgesia in the emergency department. Ann Emerg Med 49(1):23–30
Idvall J, Ahlgren I, Aronsen KR, Stenberg P (1979) Ketamine infusions: pharmacokinetics and clinical effects. Br J Anaesth 51(12):1167–1173
Ghadami Yazdi A, Ayatollahi V, Hashemi A, Behdad Sh, Ghadami Yazdi E (2013) Effect of two Different Concentrations of Propofol and Ketamine Combinations (Ketofol) in Pediatric Patients under Lumbar Puncture or Bone Marrow Aspiration. Iran J Ped Hematol Oncol. 3(1):187–192
Taylor DM, O’Brien D, Ritchie P, Pasco J, Cameron PA (2005) Propofol versus midazolam/fentanyl for reduction of anterior shoulder dislocation. Acad Emerg Med 12(1):13–19
Vardi A, Salem Y, Padeh S, Paret G, Barzilay Z (2002) Is propofol safe for procedural sedation in children? A prospective evaluation of propofol versus ketamine in pediatric critical care. Crit Care Med 30(6):1231–1236
Dachs RJ, Innes GM (1997) Intravenous ketamine sedation of pediatric patients in the emergency department. Ann Emerg Med 29(1):146–150
Strayer RJ, Nelson LS (2008) Adverse events associated with ketamine for procedural sedation in adults. Am J Emerg Med 26(9):985–1028
Langston WT, Wathen JE, Roback MG, Bajaj L (2008) Effect of ondansetron on the incidence of vomiting associated with ketamine sedation in children: a double-blind, randomized, placebo-controlled trial. Ann Emerg Med 52(1):30–34
Behdad A, Hosseinpour M, Khorasani P (2011) Preemptive use of ketamine on post operative pain of appendectomy. Korean J Pain. 24(3):137–140
Nesek-Adam V, Grizelj-Stojčić E, Mršić V, Rašić Z, Schwarz D (2012) Preemptive use of diclofenac in combination with ketamine in patients undergoing laparoscopic cholecystectomy: a randomized, double-blind, placebo-controlled study. Surg Laparosc Endosc Percutan Tech. 22(3):232–238
Singh H, Kundra S, Singh RM, Grewal A, Kaul TK, Sood D (2013) Preemptive analgesia with ketamine for laparoscopic cholecystectomy. J Anaesthesiol Clin Pharmacol 29(4):478–484
Naghibi K, Kashefi P, Abtahi AM (2013) The comparison of preemptive effects of propofol, remifentanil and ketamine on post-operative pain scores and analgesic requirements in elective lower abdominal surgery under general anesthesia: a randomized, double-blinded study. J Res Med Sci. 18(7):567–572
Acknowledgments
The authors thank Zahra Mokhtari, Nahid Jafarkhan, Fatemeh Daroughezadeh, Hooriyeh Naeimi, and Faezeh Keshavarz for their assistance in data collection and Zohreh Shahabi for her assistance in statistical analysis.
Conflicts of interest
The authors declare that they have no conflicts of interest to disclose
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sanatkar, M., Abianeh, S.H., Ghazizadeh, S. et al. The Comparison of Infusion of Two Different Sedation Regimens with Propofol and Ketamine Combination During Plastic and Reconstructive Surgery. Aesth Plast Surg 39, 141–146 (2015). https://doi.org/10.1007/s00266-014-0419-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-014-0419-y