Abstract
Animals compete for limited resources such as food, mating partners, and territory. The outcome of this intraspecific competition should be determined by individual variation in behavioral traits, such as aggressiveness and dominance status. Consistent among-individual differences in behavior likely contribute to competitiveness and predispose individuals to acquire specific dominance ranks during parts of their adult life. Nevertheless, how dominance rank is correlated with animal personality traits remains largely unclear. In a first step towards better understanding these functional links, we studied trait integration into behavioral syndromes, using 26 captive male bank voles (Myodes glareolus). We repeatedly assessed boldness in an emergence test, exploration in an open-field test, aggressiveness in staged dyadic encounters, and the among-individual correlations between these behaviors. We further related these personality traits to dominance rank, from quantifying urine marking value (UMV), as marking in bank voles is related to dominance rank. We found repeatable variations in boldness, exploration, aggressiveness, and UMV, which were correlated at the among-individual level. Aggressiveness tended to be negatively correlated with body condition, a proxy for fitness. Thus, key personality traits and social rank are functionally integrated into a behavioral syndrome of intraspecific competitiveness. By joining social and non-social aspects of personality, our findings contribute to the ecological validation of personality traits and suggest how they contribute to higher-order component traits, such as dominance which directly affect fitness components.
Significance statement
The outcome of intraspecific competition is a key determinant of fitness. Consistent among-individual variation in behavior is expected to contribute to competitiveness and predispose individuals to acquire dominance ranks in a given environmental and social context. Yet, studies assessing links between animal personality and dominance rank, are contradictory and often assess trait integration at the phenotypic level. By repeatedly assessing individual variation in behavior and urine marking patterns under controlled conditions in male bank voles, we find positive among-individual correlations between boldness, exploration, aggressiveness, and urine marking value, an indicator of dominance rank in voles. Thus, key personality traits are functionally integrated with social rank, forming a behavioral syndrome of competitiveness. This trait integration can help explain how personality traits affect fitness components and personality-dependent space use, and help better understand how and why behavioral syndromes evolve within and affect animal societies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animals compete with conspecifics for limited resources such as food, mating partners, and territory and the outcome of this competition has consequences for survival and reproductive success. Therefore, individual variation in competitiveness, i.e. the ability to access limited resources, will contribute to fitness (e.g. Nicholson 1954; Maynard Smith and Parker 1976; Łomnicki 2008). Behavioral traits contribute to an individual’s competitiveness and the importance of specific traits may differ among forms of competition. Under interference or contest competition, resource use is reduced as a result of antagonistic interactions among competitors, and traits that increase an individual’s potential to monopolize resources should be selected for. In contrast, under scramble competition, resources are reduced through exploitation, and traits that increase the ability to locate and access resources should be advantageous (Nicholson 1954).
Dominance ranks are typically described as long-lasting positions within groups (Kaufmann 1983) and are also assigned to short-term winners of dyadic contests (Drews 1993). They are associated with asymmetric aggression and priority access to resources or reproduction and are ubiquitous and important aspects of social structure mediating social interactions (Rowell 1974; Kaufmann 1983; see Tibbetts et al. 2022 for a review). Dominance rank is assessed by opponents and bystanders in repeated direct agonistic interactions, via signals of fighting ability, e.g. weapons, scent markings, and badges, or eavesdropping (Rohwer 1977; Clutton-Brock 1982; Kaufmann 1983; Rozenfeld et al. 1987; Rozenfeld and Rasmont 1991; Drews 1993; Hurd and Enquist 2001; Johnstone 2001; van Staaden et al. 2011; Rat et al. 2015). An individual’s dominance rank determines its access to safety, food, and reproduction, with higher-ranking individuals limiting the access of lower-ranking ones to good quality territories according to the ideal despotic distribution (Fretwell 1972; Rowell 1974). Dominance rank is associated with a wide range of physiological (Creel 2001) and behavioral traits and states (Colléter and Brown 2011; Majolo et al. 2012), and can affect fitness components such as reproductive success, longevity, and offspring survival (Kaufmann 1983; Majolo et al. 2012). Traits facilitating the acquisition of higher dominance ranks are often heritable (bank voles: Horne and Ylönen 1996; deer mice: Dewsbury 1990; house mice: Fang et al. 2016). Consistent among-individual differences in behavior, i.e. animal personality (Gosling 2001), likely contribute to competitiveness and predispose individuals to acquire specific dominance ranks in a given environmental and social context. Specifically, the personality trait aggressiveness, i.e. an individual’s agonistic reaction towards conspecifics often assessed in social or non-social contexts (Réale et al. 2007), ought to define an individual’s competitiveness under intraspecific interference competition and should be related to the dominance rank an individual can obtain in social hierarchies. Aggressiveness is usually assessed in mirror image stimulus tests (Réale et al. 2007; Wilson et al. 2011), playback experiments (Scales et al. 2011), via social network analysis (Panaccio et al. 2021), and via staged encounters with direct contact (Verbeek et al. 1996; Guerra and Pollack 2010; Wilson et al. 2011). Although dominance rank previously has been found to associate with various personality traits, these relationships, particularly between aggressiveness and dominance, remain contradicting and under-examined (Dingemanse and de Goede 2004; Colléter and Brown 2011; Wilson et al. 2013; Duckworth 2014; Seyfarth et al. 2014; Rudin et al. 2017). Whereas some studies show that dominance correlates with exploration, activity, risk-taking, neophilia and aggressiveness (Dingemanse and de Goede 2004; Colléter and Brown 2011; David et al. 2011; Riebli et al. 2011; Wilson et al. 2013; Favati et al. 2014), others find no relationships (Korpela et al. 2011; Devost et al. 2016) or that dominance can erode behavioral syndromes (Rudin et al. 2017). Additionally, despite the necessity of disentangling within- and between-individual correlation to evaluate behavioral syndromes at the correct level (Dingemanse et al. 2012; Brommer 2013; Brommer and Class 2017), some previous results are based on estimates of raw phenotypic correlations (e.g. Verbeek et al. 1996; Colléter and Brown 2011; Korpela et al. 2011).
Behavioral syndromes, i.e. suites of correlated behaviors consistent over time and context, (Sih et al. 2004) can be adaptive if ecological or social conditions favor combinations of traits (Wilson 1998; Bell 2004). Across taxa, boldness (i.e. an individual’s reaction to any risky non-novel situation; Réale et al. 2007) is often positively associated with aggressiveness at the among-individual level (Dingemanse et al. 2007; Sih and Bell 2008). An individual may only bear the cost of higher boldness (i.e. reduced survival) if it is more competitive (i.e. aggressive) at the same time. In three-spined stickelbacks (Gasterosteus aculeatus) aggressiveness and boldness have been found to positively align under predation risk, i.e. when there is a cost to boldness (Bell and Sih 2007). Boldness also commonly positively correlates with exploration and activity (Wilson and Godin 2009; Mazue et al. 2015; Dammhahn et al. 2022). If bolder individuals are more aggressive, active, and explorative, they might be more competitive and occupy larger and/or higher quality territories (e.g. Webster et al. 2009; Ward-Fear et al. 2018; Schirmer et al. 2019) and thus locate and access food faster (Dammhahn and Almeling 2012) and may have better access to mates (see Schuett et al. 2010 for an overview). Variations in spatiotemporal selection regimes, such as resource abundance, population densities, and predation pressure, could explain why these behavioral variations are maintained in populations (Mangel and Stamps 2001; Sih et al. 2004; Mouchet et al. 2021). Correlations between boldness and other personality traits are likely due to shared underlying mechanisms such as genetic correlations or physiological constraints (Sih et al. 2004). Such shared proximate mechanisms can constrain responses to selection and create trade-offs across different selection regimes, producing sub-optimal behavior (Sih et al. 2004). Therefore, knowing how behavioral traits are correlated becomes essential to explaining variations in behavior under different environmental and social contexts (e.g. resource abundance, predation risk, and population density) and determining broader effects on behavior, ecological interactions and fitness. We are now starting to better understand the ecological relevance of personality traits (Réale et al. 2007; e.g. mammals: Schirmer et al. 2020; birds: Dingemanse et al. 2003; fish: Webster et al. 2009; invertebrates: Santostefano et al. 2016), but whether and how they affect social interactions is still much less resolved (Gartland et al. 2022). In a first step towards better understanding these functional links, we aimed to study trait integration into behavioral syndromes related to among-individual differences in competitiveness. By examining correlations between personality traits and how they associate with social dominance, we aim to resolve the structure of behavioral syndromes across social and non-social traits. Better understanding such trait integration will help us illuminate higher-order composite traits, such as competitiveness, which directly affect fitness components (Réale et al. 2007). Embedding animal personality into social status may further help integrating the function and evolution of behavioral syndromes into our understanding of animal societies (Gartland et al. 2022).
As study species we used a small rodent, the bank vole (Myodes glareolus, family Cricetidae). In this solitary living species, females mate promiscuously and males compete for access to estrous females (Gipps 1985). Females defend exclusive territories against other females, whereas male home ranges are overlapping with several females and males (Bujalska 1985). Males form stable dominance hierarchies (Viitala 1977) and dominance-related traits are heritable (Horne and Ylönen 1996). Under experimental conditions, most offspring are sired by dominant males (Klemme et al. 2006) with offspring from dominant males being heavier than offspring from subordinates (Horne 1998). Bolder individuals of both sexes occupy larger and more exclusive home ranges under natural conditions (Schirmer et al. 2019). Under interspecific competition, breeding males reduce space use (Eccard et al. 2011). Competition in bank voles is not experienced equally among individuals in a population (e.g. Eccard and Ylönen 2003), with shy animals experiencing more intraspecific competition than bold conspecifics (Schirmer et al. 2020). Personality also predicts microhabitat use in this species, with bolder and more active individuals foraging in risky physical environments (Mazza et al. 2019; Dammhahn et al. 2022). The social context might, however, affect the strength and direction of this relationship (Schirmer et al. 2019) but this remains to be investigated.
In this study, we test the hypotheses that animals exhibit among-individual correlations (1) between boldness, activity/exploration, and aggressiveness, as well as (2) cross context of competitive behavior, aggressiveness and dominance-related behavior. We predict positive pairwise among-individual correlations between boldness, activity/exploration, aggressiveness, and dominance-related behavior. Additionally, we test the hypothesis that aggressiveness (a behavior for competitiveness) is positively correlated with body condition, a proxy for fitness (Jakob et al. 1996; Stevenson and Woods 2006; Peig and Green 2009).
Materials and methods
Study animals
We conducted this study on captive animals from July-February of 2021 and 2022 in the animal facilities of the Animal Ecology Group in Potsdam (Germany). Study animals were male bank voles (Myodes glareolus) captured from the wild in a small forest patch near Potsdam (Germany, 52°24’14.0"N 13°00’32.6"E) (n = 20) or born in captivity (n = 6) as F1 of wild-caught parents. Prior to the experiments, all animals spent 3 to 5 months in the laboratory and were sexually mature. Because males fight in direct encounters (Gipps 1985), they were kept individually. Voles were housed in standard polycarbonate cages (type III and type IV) provided with wood shavings and hay as bedding, cardboard rolls as shelter, and a running disk for enrichment. Water and food pellets (Ssniff, NM, V 1244-0) were available ad libitum. Bedding was changed at least every two to three weeks. Light duration and humidity largely reflected natural conditions and temperature was regulated between 14 and 25 °C.
Personality assessment
Emergence test and open-field test
We tested 26 males twice (inter-test interval: 3 to 10 days; median: 5 days, interquartile range: 4 to 6 days) in two established behavioral tests to quantify among-individual differences in activity, exploration, and boldness (Mazza et al. 2019; Schirmer et al. 2019). A circular arena (100 cm diameter surrounded by 35 cm high walls) with a white floor connected with an opaque tube (11 × 30 cm) with a sliding door was used in both tests. By drawing on the floor, the arena was visually divided into a circular middle area (70 cm in diameter) where the vole was fully exposed and a border area (15 cm wide) by the wall, representing a safer area. Each area was additionally visually divided into eight sections.
We quantified boldness-related behaviors via an emergence test. The individual was placed in the tube for one minute of habituation, after which the door to the arena was opened by pulling a string from the neighboring room. Based on direct observation (or via video analysis), one observer (FE) measured two behavioral variables: the time to emerge with the head including ears (latency head) and the latency to emerge with the full body excluding the tail (latency body).
We quantified activity- and exploration-related behaviors via open-field tests. In this test, individuals were placed in an inverted beaker at the edge of the arena and left to habituate for one minute. Thereafter the beaker was gently lifted with a rope from the neighboring room and the individual was observed for 10 min. We recorded the activity (running, jumping, scanning, grooming) instantaneously every 10 s, latency to enter the center part of the arena with the full body excluding the tail (latency center), number of crossings of the full body excluding the tail into the center part (crossings), and the number of different sections entered with its full body excluding the tail (sections).
Staged dyadic encounters (SDE)
To measure among-individual variation in behavior towards a conspecific, we ran staged dyadic encounters (SDE) between various dyads formed by the 26 male bank voles. Staged dyadic encounters are commonly used to assess aggressive behavior in bank voles where dominance hierarchies are established through fighting and chasing and maintained by olfactory signals (Rozenfeld et al. 1987). A total of 26 took part in three SDE (inter-test interval: 1 to 17 days; median: 7 days, interquartile range: 5 to 10 days) against three different opponents, except for one male for which only two encounters (with two different opponents) could be performed. To create dyads, we matched individuals of similar weight allowing a maximal weight difference of 6 g (corresponding to ≤ 25% of the body mass; median and inter-quartile ranges: 1.5 g (6%), 0.6 to 2.7 g (3 to 11%).
SDEs were performed in a rectangle box (56.5 × 36.5 × 59.5 cm) divided by a plastic wall into two similar-sized compartments (27 × 36.5 × 59.5 cm). The dividing wall had a wire grid and a sliding door at the bottom to allow visual, acoustic, and olfactory contact between the two compartments after the sliding door was pulled up (see Fig. SI1 in online resource 1). We did not allow the voles to physically interact to avoid injuries. To simplify the video analysis of the placement of voles in the arena, we divided the area into three equally big zones (with zone one closest to the divider, zone two in the middle, and zone three furthest away from the divider) with lines on a paper placed at the bottom of the arena. Animals were weighed immediately before testing. We placed one male in each compartment of the arena for one minute of habituation with the divider down (no direct visual and olfactory contact). The arena was then moved to the test room and left for another five minutes of habituation without visual contact. The observer then raised the sliding door from the neighboring room and the voles were left to have visual, acoustic, and olfactory contact for 30 min. We chose a 30-minute test duration because in pilot observations of previously established SDE for urine markings most voles moved during the first 30 min (FE personal observation).
Attack is the rapid approach of an opponent, resulting in a fight or a chase (Rozenfeld et al. 1987). Since a majority of direct interactions between mature male bank voles are agonistic (ca. 68% of close encounter SDEs end in fighting, Gipps 1984), and because we prohibit direct interactions, we interpret approaches to the barrier (i.e. the closest an individual can get to its opponent) as attacks. Contrastingly, departures and time spent in Zone 3 (far away from the opponent) likely represent fleeing behavior. From the videos one observer (FE) quantified movements of the focal vole between the three zones of the arena, where movements towards the divider and the opponent vole were defined as ‘approaches’ (Fig. SI1.A1-A4 in online resource 1) and movements away from the divider and the opponent as ‘departures’ (Fig. SI1.D1-D4 in online resource 1). We quantified approaches and departures by counting crossings of the focal vole between zones with the whole body excluding the tail. For each movement between the zones, we additionally specified if the vole moved to or from the side of the arena in which the opponent vole was located (left or right of the vertical middle), i.e. if the vole crossed from one side over the arena, over the midline, to the other side of the arena with its full body (excluding the tail). For each of these crossings, we also noted down the zone position of the opponent vole. Furthermore, we quantified the time spent with its whole body (excluding the tail) in the section closest to the divider (‘zone one seeker’) and time spent in this section while the opponent also occupies zone one in his compartment (‘opponent seeker’). If a vole spent the whole test immobile with the majority of its body in zone one, we also considered that as time spent in zone one. Because most of these crossing variables were zero-inflated we combined variables into fewer variables explaining departures and approaches to opponent. These variables include four variables of number of approaches, four variables of number of departures, and one variable for all crossings (‘totcross’). The four approach variables were: A1) number of approaches to zone one (closest to the divider) disregarding opponent position, A2) number of approaches to opponent (to zone one when the opponent is on the same side and approaches to the same side as the opponent within zone one), A3) number of approaches to zone one only when the opponent also is in zone one, A4) number of approaches to opponent (to zone one when the opponent is in the same side of zone one and approaches within zone one to the side the opponent vole is located in zone one). The four departure variables are the opposite movement (i.e. from zone one or opponent) to the above-described approach movements (from here on referred to as ‘D1’, ‘D2’, ‘D3’, ‘D4’). See Fig. SI2 in online resource 1 for a visual demonstration on how the variables were combined and online resource 2 for a video demonstration.
No observer was present in the room of the setup during the emergence test, open-field, and SDE. All tests were recorded using cameras (ABUS, Mini Dome Camera HDCC35560) and analyzed single-blinded to the vole identity after tests had been conducted.
Urine marking value
Dominance-subordinate relationships established through direct agonistic interactions (fighting and chasing) relate to urine-marking behavior in bank voles (Rozenfeld et al. 1987). Urine-marking behavior distinctly differs between dominant and subordinate male bank voles. Whereas subordinate males stop marking and only deposit large concentrated spots of urine in the presence of a dominant male, dominant males cover large areas with fine traces of squiggly-shaped markings (Rozenfeld et al. 1987; Rozenfeld and Rasmont 1991; Horne and Ylönen 1996). Assessing dominance status from these markings is a reliable method and has the advantage of avoiding injuries from direct encounters (Horne and Ylönen 1996). In addition to the SDE described above, we ran a series of repeated paired trials (inter-test interval: range 1 to 65 days; median: 15 days, interquartile range: 5 to 23 days) to assess urine marking behavior of 24 males. Males were tested in the same dyads as in the behavioral SDE test. This setup consisted of two boxes (27.5 × 40 × 19 cm) attached side by side without a floor. The joint walls were perforated with small holes (ca. 0.5 cm diameter) to allow olfactory but only limited visual contact between the voles in each box. Filter paper was placed under the boxes to capture urine markings. We placed dyads in the setup, and left them to interact for two hours. Using UV light, we evaluated the filter paper from each vole by dividing it into 5 cm x 5 cm squares (35 in total) and counted how many of these squares were marked with a few large blots of marking (typical for subordinate status) or with many squiggly lines (typical for dominant status); this classification is based on Klemme et al. (2006). An individual only marks typical for subordinate status or dominant status, not both types. We calculated a urine marking value (UMV) expressed as the proportion of the number of squares marked with urine markings of any style, subordinate or dominant urine marking patterns, divided by the total number of squares. Because individuals marking with lines typical for dominant status cover a larger surface area (i.e. more squares) than individuals marking with blots typical for subordinate status, we used the original quantitative variable of the proportion of squares marked. Consequently, we keep variation in the trait and avoid reducing the trait to a factor variable of dominant versus subordinate.
All tests were conducted in separate testing rooms outside of the animal housing rooms. The emergence, open-field, and SDE were conducted in similar environmental and light conditions as the housing rooms. The UMV assessment was conducted under dark light conditions. Because bank voles, like other microtine species, have a polyphasic activity rhythm (Halle and Stenseth 2000), animals were tested between the hours of 8:00 and 18:00 when found active. Due to difficulties in finding both individuals in a dyad active at the same time, animals were tested in the SDE and UMV whether or not they were found active in the cage before the time of the test. Animals were not tested on days when cages were cleaned. After each behavioral test (emergence, open-field, SDE, UMV assessment), the animal was transferred back to its home cage, and the test arenas were cleaned with ethanol. Animals were transferred to and from test setups in the opaque beakers used in the emergence and open-field test. Each vole was exposed to any of the setups only once per day. The emergence and open-field tests were conducted two to four months before the SDE and UMV assessment. The lower sample size of the UMV assessment and the lack of three repeats for one male in the SDE was caused by two males dying before these tests were conducted, probably of old age (exact age of wild-caught individuals was unknown).
Body condition
At the end of the SDE, one observer (FE) measured the head width (mm) of all animals and calculated body condition using the scaled body mass index suggested by Peig and Green (2009). This index was calculated as the mass (g) of an individual standardized to the mean head width (mm) as a measure of body size of all individuals in the population retaining the population-specific allometric relationship in grams.
Statistical analyses
To analyze among-individual correlations between behaviors expressed in the different tests we first estimated repeatability of all behavioral variables. Because the time spent in zone one in the SDE was bimodal, we first transformed it into a binomial variable using the function “cutoff” from the package “cutoff” (Choisy 2015). We log-transformed variables (all approaches, departures, and total crossings from the SDE as well as latency to emerge head and body from the emergence test) to allow running linear models (H. Schielzeth, personal communication) and arcsine square root transformed the proportion spent active in the open-field test and proportion of squares with urine marking (UMV). We estimated repeatability using the rpt function from the package rptR (Nakagawa and Schielzeth 2013; Stoffel et al. 2017), using 1000 simulations to estimate confidence intervals and 1000 permutations to estimate p-values. Because Schielzeth et al. (2020) found Gaussian repeatability models to be robust to non-gaussian data, we used Gaussian family distribution for variables from the SDE which were zero-inflated, despite not all variables meeting the requirements of a Gaussian distribution. To confirm the robustness, we additionally ran the repeatability with the distribution reflecting the data, see Table SI1, which yielded qualitatively similar results.
Second, we reduced repeatable variables in separate principal component analyses (PCA) for each test (emergence, open-field, and SDE) with oblimin rotation for the open-field test and SDE. PCA assumptions were checked by inspecting the correlation matrix, Bartlett test, and Kaiser-Mayer-Olkin (KMO) criterion (Field et al. 2012). We included two variables for the PCA of the emergence test, three variables in the PCA for the open-field test, and two variables in the PCA of the SDE (Table SI2). Because both behavioral variables had the same loading on the first component of the PCA on the emergence test data, we used the one original variable (latency body) in subsequent bivariate mixed models (BMMs). Similarly, two behavioral variables loaded strongly on the first component of the PCA on the open-field test data. Because one of these behavioral variables (crossings) fulfilled the assumption of normality of residuals, we used the original values for crossings in the subsequent bivariate mixed models. We only included components from the PCA with Eigenvalues > 1 in subsequent bivariate mixed models (BMMs) and therefore excluded a second component representing activity from the PCA on the open-field test. We included repeated measurements per individual in the PCA. This approach violates the PCA’s assumption of independent data (Budaev 2010) but is a common procedure in behavioral syndrome studies to retain repeated measures of composite variables for subsequent analyses. Due to small sample size limitations, we decided against structural equation modeling proposed by Araya-Ajoy and Dingemanse (2014). Since our samples are balanced, with two or three (depending on analysis) measures per individual, we assume that PCA procedures should be robust against some non-independence of data points.
Third, to estimate the fixed effects we ran univariate linear mixed effect models (for Gaussian distribution) using the lmer function from the lme4 package (Bates et al. 2015) or generalized mixed effect models (for Poisson and Binomial distribution) using the glmmTMB function from the glmmTMB package (Brooks et al. 2017) for each response variable with individual identity as a random effect. We evaluated the effect of controlling for test occasion (i.e. first, second or third time tested) on all response variables, and for body condition on the composite variable from the SDE. Due to the large percentage of animals (38%) that did not move in the SDE, we additionally ran a GLMM on a binomial variable of ‘aggressiveness’, and an LMM on a subset of data with only individuals that move as well as controlling for zero-inflation to further assess this relationship.
Lastly, to investigate among-individual correlations between pairs of variables we ran bivariate mixed models (BMMs), using the ‘mcmcglmm’ function from the MCMCglmm package (Hadfield 2010; following procedures described in Dingemanse and Dochtermann 2013 and Houslay and Wilson 2017). We ran six BMMs to evaluate pair-wise correlations between two composite variables obtained from the PCAs, crossings, and UMVs. All models included individual identity as a random effect and variable-specific fixed effects (test occasion for crossings and body condition and test occasion for component from SDE). We decided against running one single multivariate mixed effects model with all response variables because our sample size restricted power for such a complex model and because fitting the model with response variables of different error distributions often causes convergence issues. To retain all data in the SDE, we used the observations of both individuals in a dyad as independent observations. This approach allowed us to follow 3R principles of reduction of animals used for experimental research (Russell and Burch 1959), but the data of two individuals in one SDE is not independent. Therefore, we initially controlled for the opponent’s behavior in the SDE test by adding opponent identity as a random effect in the models to account for this non-independence. Simple univariate mixed models showed no effect of opponent identity on behavior in the SDE (models with and without opponent identity had dAIC < 2 for both aggressiveness and UMV, random effect had LRT = 1.204, df = 1, p-value = 0.272 for aggressiveness, and LRT = 0.123, df = 1, p-value = 0.726 for UMV). Therefore, and because each individual was tested against several opponents, we did accept some non-independence in our data set. Nevertheless, non-random assignments of partners and limited number of pairing will result in conflation of indirect and direct effect and inflate repeatability estimates. We fixed the within-individual variance to 0 because we did not estimate all variables at the same time. Since we did not have much information on the relationships between variables in the model, we ran all models with different priors as suggested by Hadfield 2010 (three informative, one non-informative priors). Model results were robust against different priors (results not shown). The reported model results are based on a weak prior (V = diag(2), nu = 1.002, i.e. inverse gamma). We used 250,000 iterations, a thinning interval of 100, and a burn-in of 50,000. Finally, using the posterior distributions from the bivariate mixed models, we calculated repeatability for each dependent variable, pairwise among-individual correlations, and their credibility intervals based on Houslay and Wilson (2017).
In line with the ‘language of evidence’ (Muff et al. 2022) and following Dingemanse et al. (2020) we interpreted an effect as “strongly supported” when zero was not included in the 95% CIs, “moderately supported” when the point estimate was skewed away from zero whilst the 95% CIs overlapped zero and strong support for the absence of an effect when estimates were centered at zero. All analyses were carried out in R, Version 4.3.1 (R Core Team 2023).
Results
A total of 94% of the animals emerged from the emergence test and all animals exhibited some activity in the open-field test. A total of 38% of the animals did not move in the SDE and 13% did not leave any urine markings in the UMV assessment. Animals ranged between 16.9 and 36.0 g in the scaled body mass index (i.e. body condition) with a mean of 24.4 g.
Among-individual variation
Both emergence test variables, most open-field variables, six variables from the SDE, and urine markings from UMV assignment were repeatable over time (all R > 0.3, Table SI1). Variables from the emergence test were reduced to one meaningful component (Eigenvalue 1.8, loadings = 0.95), interpreted as boldness, which explained 90% of the variance. Variables from the open-field tests were reduced to one meaningful component (Eigenvalue = 2.49, loadings: 0.89 for crossings, 1 for latency center), interpreted as exploration, explaining 58% of the variance. In the SDE, total crossings between all sections (‘totcross’) and crossings into zone one when the opponent is in zone one (‘A3’) were included in the PCA (Table SI2 in online resource 1) and one meaningful component was extracted, explaining 81% of the variance (Eigenvalue = 1.63, both loadings = 0.9). It represented approaches towards a conspecific and activity in the presence of a conspecific. Since a majority of direct interactions between mature male bank voles are agonistic (ca. 68% of close encounter SDEs end in fighting, Gipps 1984), we interpreted this component as “aggressiveness”. Boldness, exploration, aggressiveness, and UMV were repeatable over time calculated from the bivariate mixed model variance components (Fig. 1, Table SI3).
Repeatability of behavioral indices obtained from 24–26 captive male bank voles. Composite behavioral variables and urine marking value, obtained from repeated behavioral tests (boldness: emergence test; exploration: open-field test; aggressiveness: staged dyadic encounters, urine marking value (UMV): paired trial for assessing urine markings), were repeatable over time. Shown are the mean estimates of repeatability (dot) and their 95% credible intervals (lines). Inter-test interval: boldness and exploration: median: 5 days, interquartile range: 4 to 6 days; aggressiveness: median: 7 days, interquartile range: 5 to 10 days; UMV: median: 15 days, interquartile range: 5 to 23 days
Among-individual correlations
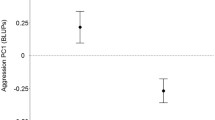
There was strong support for positive among-individual correlations between boldness and exploration and between boldness and aggressiveness, hence bolder individuals were more explorative and aggressive (Fig. 2, Table SI3). Further, there was strong support for positive among-individual correlations between exploration and aggressiveness and between exploration and UMV, hence more explorative individuals were also more aggressive and had higher UMV (Fig. 2, Table SI3). A positive correlation between boldness and UMV was moderately supported (Fig. 2, Table SI3). Lastly, we found strong support for positive among-individual correlation between aggressiveness and UMV, thus more aggressive individuals had higher UMV (Fig. 2, Table SI3).
Among-individual correlations between composite behavioral variables of 24–26 captive male bank voles, quantified in emergence test (boldness), open-field test (exploration), staged dyadic encounters (aggressiveness), and assessment for urine markings (urine marking value, i.e. UMV) estimated from bivariate Bayesian mixed effects models. Shown are mean estimates (dots), 95% credibility intervals (black lines) and 89% credibility intervals (grey lines). Abbreviations: w/ indicating ‘with’
Body condition
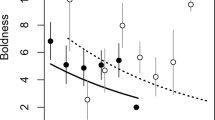
Animals with better body condition scored lower in aggressiveness (df = 26.71, t = -2.10, p = 0.045, marginal R2 = 0.14, conditional R2 = 0.46, y = 1.61–0.07x, Fig. 3). Body condition did not differ among non-aggressive versus aggressive animals (binomial model, intercept = 2.851, β = -0.104, z = -1.48, p = 0.139). Among the individuals that showed aggressiveness, more aggressive animals had lower body condition (model controlling for zero inflation, intercept = 1.614, β = -0.072, z = -2.19, p = 0.029; model on data without non-aggressive individuals, intercept = 2.527, β = -0.088, df = 21.01, t = -2.42, p = 0.025). If the individual with the extreme body condition (35 g) is removed, the relationship disappears for all models except the model without non-aggressive individuals (see Table SI5 in online resource 1).
Relationship between body condition (as scaled body mass index) and the composite behavioral variable ‘aggressiveness’ from linear mixed model without controlling for zero values. For illustration purposes, the mean aggressiveness and body condition per individual is shown. The transparent grey area represents the 95% confidence interval. The linear mixed model has the equation y = 1.61–0.07x and a marginal and conditional R2 of 0.136 and 0.463 respectively. Note that this relationship disappears if the individual with body condition > 35 g is excluded
Discussion
By repeatedly assessing individual behavior in four different assays under laboratory settings we found consistent among-individual variations in three out of five described personality traits, boldness, exploration, and aggressiveness (Réale et al. 2007), as well as in urine marking value as an indicator for dominance status, an important social phenotype in bank voles, henceforth referred to as dominance-related behavior (Rozenfeld et al. 1987; Horne and Ylönen 1996). In line with previous findings across many taxa, a majority of these traits were correlated at an among-individual level, forming behavioral syndromes (Dingemanse et al. 2012). Aggressiveness, exploration, and boldness were positively correlated with dominance-related behaviors, suggesting that these behaviors contribute to individual variation in competitiveness. Under ad libitum food availability in laboratory conditions, more aggressive individuals had lower body condition than less aggressive conspecifics suggesting that among-individual variation in behavior associated with behaviors for competitiveness might affect fitness components.
Personality traits form behavioral syndromes
As predicted, repeated behavioral tests of bank voles revealed consistent among-individual differences in boldness, exploration, and aggressiveness. As confirmed by ecological validation (e.g. risk-taking under perceived predation risk, microhabitat characteristics, home range size: Schirmer et al. 2019; Dammhahn et al. 2022), we interpret latencies to emerge as boldness. In line with previous studies on small mammals (e.g. Herde and Eccard 2013; Montiglio et al. 2014; Schirmer et al. 2019) and as suggested by associated variation in movement behavior and information gathering (MD, unpublished), we interpret behaviors expressed in the open-field as exploration. We interpret patterns of approach-depart behaviors expressed in SDE as indicative of aggressiveness in contrast to some other studies on different taxa (e.g. Freitas et al. 2008; Schoepf and Schradin 2012; Begall et al. 2022), which used similar test set-ups to quantify sociability, i.e. non-aggressive reactions to conspecifics (Réale et al. 2007). In bank voles, females are territorial and males directly compete for females (Gipps 1985). When placed in direct contact with an opponent male under laboratory conditions, males are known to approach each other and directly fight each other even in the absence of females (Gipps 1984). Therefore, we assume that approaches represent aggressiveness, rather than sociability. Since the chosen set-up prohibits direct contacts between opponents we could not verify whether approaches would lead to attacks (i.e. rapid approaches of opponent resulting in fights or chases (Rozenfeld et al. 1987). Future studies should cross-validate behaviors shown in SDE and direct encounters. Positive among-individual correlations between boldness, activity, exploration, and aggressiveness have previously been found in many species (Verbeek et al. 1996; Dingemanse et al. 2007; Sih and Bell 2008; Wilson and Godin 2009; Mazue et al. 2015; Koenig and Ousterhout 2018) and among-individual correlations between activity and boldness have already been reported in bank voles (Dammhahn et al. 2022). Here, in line with our predictions, we found that bolder bank voles are more explorative and aggressive than shyer individuals. This finding supports that personality traits are organized into behavioral syndromes, likely due to shared underlying proximate mechanisms in the form of genetic correlations, environmental experiences or neuroendocrine and other physiological mechanisms (Sih et al. 2004).
Noteworthy, because thigmotaxis (i.e. the avoidance of open spaces) is a common anxiety/fear reaction in small mammals (Treit and Fundytus 1988), proportion of time spent in the center section of the open-field test is sometimes interpreted as boldness rather than exploration (e.g. Treit and Fundytus 1988; Choleris et al. 2001; Gracceva et al. 2014). Consequently, latency to enter and number of crossings into the center section may also reflect boldness. The among-individual correlation we found between exploration and boldness may thus instead reflect an among-individual correlation between two aspects of boldness. However, the literature is ambiguous and a correct interpretation remains to be determined (Walsh and Cummins 1976; Perals et al. 2017). Future studies are needed to validate the interpretation of use of the center section.
A competitiveness syndrome?
Interestingly, we additionally found consistent individual differences in urine marking behavior across repeated interactions with different opponents. Voles with higher dominance related behavior were more aggressive, explorative, and tentatively also bolder (but see discussion on moderate correlations below) than conspecifics scoring lower on dominance related behavior. The positive among-individual correlation between dominance related behavior and aggressiveness suggests that SDE measures aggressiveness as an indicator of competitiveness rather than an indicator of defensive aggressiveness. Consequently, in line with our predictions, we found both among-individual correlations between classical personality traits, and traits indicative for competitiveness in social interactions. We interpret this trait integration as indicating a “competitiveness syndrome” (see also below). Joining social and non-social aspects of personality, this finding contributes to the ecological validation of personality traits (sensu Réale et al. 2007) and suggests how they contribute to higher-order component traits, such as dominance (Réale et al. 2007), which directly affect fitness components. Dominance rank is substantially related to an individual’s competitive ability and impacts individuals’ access to resources such as food, mating partners, and territory (Rowell 1974). Whether certain personality traits predispose individuals to acquire higher dominance ranks is often unclear, but commonly assumed to be affected by aggressiveness, an important trait for defending and usurping resources (Huntingford and Turner 1987). Some studies have found that bolder, more explorative, active, and aggressive individuals are dominant over shy, less explorative, active, and aggressive conspecific (Dingemanse and de Goede 2004; McGhee and Travis 2010; Colléter and Brown 2011; David et al. 2011; Riebli et al. 2011; but see Korpela et al. 2011; Devost et al. 2016). Our findings are in contrast to a previous study on bank voles, which could not detect associations between dominance-related traits, infanticide, novelty seeking, and extroversion at the raw phenotypic level (Korpela et al. 2011). Here we applied the statistical definition of Dingemanse et al. (2012) for behavioral syndromes and excluded other sources of within-individual variation (e.g., correlated errors or confounds). However, additional differences between this study and the one by Korpela et al. (2011), e.g. different test setups and statistical analyses, may also contribute to the different outcomes. Here, we find strong evidence for an integration of exploration, boldness, aggressiveness and dominance-related behavior, a “competitiveness syndrome”, at the among-individual level (i.e. the level selection can act upon). This apparent contrast between studies corroborates arguments presented elsewhere (e.g. in Dingemanse et al. 2012; Dingemanse and Dochtermann 2013) that we cannot always play the “individual gambit” and assume phenotypic correlations representing underlying between-individual correlations (Brommer 2013).
Recent work has pointed out that among-individual variation in spatial and social behavior are tightly linked with the spatial (e.g. landscape of fear) and social (e.g. population density and competition) environment (Wolf and Weissing 2012; Webber et al. 2023). Indeed, previous research points out the environmental and social context can have implications on personality-dependent spatial behavior such as space use and dispersal by affecting the strength and direction of correlations (Dingemanse et al. 2003; Boyer et al. 2010; Cote et al. 2010; Aguillon and Duckworth 2015; Schirmer et al. 2019; Wauters et al. 2021; Stiegler et al. 2022). How boldness relates to other aspects of ecology, such as risk-taking and space use, is already well established (Schirmer et al. 2019, 2020; Dammhahn et al. 2022). Our results on trait integration into a competitiveness syndrome additionally help explain previous findings and suggest that bolder animals occupy larger home ranges because they are also more explorative, and more exclusive home ranges because they are also more aggressive and dominant (Schirmer et al. 2019). Aggressive individuals could displace less aggressive conspecifics (Christian 1970; Schradin and Lamprecht 2002), similar to the idea of ideal despotic distribution where dominant individuals constrain the habitat selection of subordinate ones by displacing subordinates from high-quality patches (Fretwell 1972). Our results support this mechanism and additionally suggest that bolder and more explorative individuals could constrain the habitat use of shy and less explorative individuals due to their higher competitiveness. Testing whether and how this among-individual variation in the “competitiveness syndrome” also helps explaining individual variation in niche choice (Trappes et al. 2022) and realized individual niches (Takola and Schielzeth 2022) will be fascinating areas of future research.
This competitiveness syndrome indicates that both scramble (for resources and mates) and contest (for mates) competition is important for intraspecific competition in our study system. In collared flycatchers (Ficedula albicollis), for example, a syndrome of boldness and aggressiveness predicts fledgling success likely due to more competitive, bold, and aggressive individuals being able to secure higher quality resources and breeding territory (Tamin et al. 2023). Moreover, there seems to be no trade-off between scramble (traits that increase ability to locate and access resources; boldness and exploration) and contest (traits that increase resource monopolization; aggressiveness and dominance) competition, with bolder, more explorative aggressive, and dominant individuals having an advantage in both. Alternatively, the among-individual trait integration could reflect an overall stress in response to being subjected to a new situation in all test setups. Yet, how stress relates to dominance rank and animal personalities is less clear (Tamashiro et al. 2005; Łopuch and Matuła 2008; Koolhaas et al. 2010; Montiglio et al. 2012; Ferrari et al. 2013). Thus, properly evaluating stress response during these standardized behavioral assays needs further research. If, indeed, no trade-off exists, how and why is this syndrome maintained in the population? Variation in fitness peaks with spatiotemporal selection regimes and life-history trade-offs are commonly proposed to maintain behavioral variation (Mangel and Stamps 2001; Sih et al. 2004; Mouchet et al. 2021). In bank voles, fluctuating density-dependent selection might explain the maintenance of variation. Fluctuations in density is a key aspect of small mammal ecology associated with changes in reproductive rates, mortality, dispersal, gene frequency and behavior (Krebs and Myers 1974). When population density and, thus, competition is high, a competitive type (i.e. individuals that are more bold, explorative, aggressive, and dominant) should be favored. Indeed, recent empirical evidence suggests that when vole population densities are high, individuals with better stress-coping mechanisms have higher survival probability (Brehm and Mortelliti 2024). On the other hand, when population density and, thus, competition is low, predation pressure is often elevated because the predator peak density tends to lag behind prey density (Lotka 1925). Under such environmental conditions of low competition but elevated predation pressure, individuals that are shy, less explorative, less aggressive and less dominant might have an advantage (see results on common voles: Gracceva et al. 2014) or the behavioral syndrome might break down. Given that population density fluctuations accompanied by fluctuations in predation pressure are a key feature of small mammal natural history (Krebs and Myers 1974), small mammals provide an ideal system to test adaptive syndrome structure fluctuations under natural conditions in the future.
It is noteworthy that there was only moderate support for bolder individuals expressing higher dominance-related behavior than shy conspecifics. The lack of strong support, in concert with the strong support for correlations between boldness, exploration, and aggressiveness, might on the other hand suggest an indirect correlation between boldness and UMV (but see discussion on the interpretation of crossings as boldness instead of exploration) and that associations between behavioral traits might be more complex than previously thought. Previous research has shown that behavioral syndromes can break down under certain environmental contexts (Bell and Sih 2007; Scales et al. 2011). For example, in three-spined sticklebacks a behavioral syndrome between boldness and aggressiveness was only found under predation risk, when there was a cost to boldness (Bell and Sih 2007). This raises the question under which contexts this particular behavioral syndrome exists, and what proximate mechanism underlies these behavioral correlations. Yet, even under artificial lab conditions, we found strong or moderate support for positive correlations between all behavioral variables. Additional research should test for associations between boldness and UMV in the wild and under different social and environmental contexts.
Potential fitness consequences of among-individual variation in aggressiveness
Body size or condition can contribute to competitiveness. Counter to what we expected, aggressiveness did not positively correlate with body condition, a commonly used fitness proxy (Jakob et al. 1996; Stevenson and Woods 2006; Peig and Green 2009). As such, body condition does not seem to contribute to behavioral traits of competitiveness under lab conditions. Instead, under laboratory conditions, aggressive individuals appeared to have a lower body condition than less aggressive conspecifics. Individuals in better physical condition could be either more or less risk-taking, i.e. bold, according to two contrasting hypotheses (1) the ‘asset protection principle’ predicts that individuals in better condition have higher future reproductive potential and are thus more risk averse (Clark 1994), and (2) the ‘state-dependent safety’ hypothesis predicts that individuals in high conditions are more likely to survive and benefit from risky situation and, thus, make seemingly riskier choices (McElreath et al. 2007). Other components of the behavioral syndrome including boldness could similarly be correlated with physical condition. A meta-analysis of the association between risk-taking and physical condition demonstrates that lower condition individuals on average show about 26% greater tendency of risk-taking (Moran et al. 2021), i.e. supporting the asset protection principle. Because more aggressive individuals were more risk-taking in our study, the asset protection principle might explain why more aggressive bank vole had lower body condition. Nevertheless, when we exclude the individual above 35 g the relationship disappears. Therefore, the correlation between aggressiveness and body condition needs further investigation, preferably under field conditions. Additionally, the validation for body condition as a fitness proxy remains to be done.
Conclusions
Most personality traits and dominance-related behaviors are functionally integrated into a competitiveness syndrome in male bank voles. Individual differences in competitiveness likely result in differential access to resources and survival. Thus, trait integration might explain how variation in boldness, exploration and aggressiveness translate into fitness differences via intraspecific competitive interactions. Moreover, personality-dependent space use, shown in several studies (e.g. Dingemanse et al. 2003; Aguillon and Duckworth 2015; Schirmer et al. 2020; Dammhahn et al. 2022), might also result from individual variation in competitiveness affecting movement and habitat choices of other individuals. Further, integrating animal personality with social status may contribute to the ecological validation of personality traits (sensu Réale et al. 2007) and help to better understand how and why behavioral syndromes evolve within and affect animal societies (Gartland et al. 2022).
Data availability
Data are accessible on dryad DOI https://doi.org/10.5061/dryad.dncjsxm61.
References
Aguillon SM, Duckworth RA (2015) Kin aggression and resource availability influence phenotype-dependent dispersal in a passerine bird. Behav Ecol Sociobiol 69:625–633. https://doi.org/10.1007/s00265-015-1873-5
Araya-Ajoy YG, Dingemanse NJ (2014) Characterizing behavioural ‘characters’: an evolutionary framework. Proc R Soc B 281:20132645. https://doi.org/10.1098/rspb.2013.2645
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting Linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Begall S, Bottermann L, Caspar KR (2022) Self-domestication underground? Testing for social and morphological correlates of animal personality in cooperatively-breeding ansell’s mole-rats (Fukomys Anselli). Front Ecol Evol 10:862082. https://doi.org/10.3389/fevo.2022.862082
Bell AM (2004) Behavioural differences between individuals and two populations of stickleback (Gasterosteus aculeatus): behavioural syndromes. J Evol Biol 18:464–473. https://doi.org/10.1111/j.1420-9101.2004.00817.x
Bell AM, Sih A (2007) Exposure to predation generates personality in threespined sticklebacks (Gasterosteus aculeatus). Ecol Lett 10:828–834. https://doi.org/10.1111/j.1461-0248.2007.01081.x
Boyer N, Réale D, Marmet J et al (2010) Personality, space use and tick load in an introduced population of siberian chipmunks Tamias sibiricus. J Anim Ecol 79:538–547. https://doi.org/10.1111/j.1365-2656.2010.01659.x
Brehm AM, Mortelliti A (2024) Environmental heterogeneity modifies the link between personality and survival in fluctuating small mammal populations. J Anim Ecol 93:196–207. https://doi.org/10.1111/1365-2656.14037
Brommer JE (2013) On between-individual and residual (co)variances in the study of animal personality: are you willing to take the individual gambit? Behav Ecol Sociobiol 67:1027–1032. https://doi.org/10.1007/s00265-013-1527-4
Brommer JE, Class B (2017) Personality from the perspective of behavioral ecology. In: Vonk J, Weiss A, Kuczaj SA (eds) Personality in Nonhuman animals. Springer International Publishing, Cham, pp 73–107
Brooks ME, Kristensen K, van Benthem KJ et al (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9:378–400. https://doi.org/10.32614/RJ-2017-066
Budaev SV (2010) Using principal components and factor analysis in animal behaviour research: caveats and guidelines. Ethology 116:472–480. https://doi.org/10.1111/j.1439-0310.2010.01758.x
Bujalska G (1985) Regulation of female maturation in Clethrionomys species, with special reference to an island population of C. Glareolus. Ann Zool Fenn 22:331–342
Choisy M (2015) cutoff: Identify a cutoff value from bimodal data, https://github.com/choisy/cutoff
Choleris E, Thomas AW, Kavaliers M, Prato FS (2001) A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci Biobehav Rev 25:235–260. https://doi.org/10.1016/s0149-7634(01)00011-2
Christian JJ (1970) Social subordination, population density, and mammalian evolution. Science 168:84–90. https://doi.org/10.1126/science.168.3927.84
Clark CW (1994) Antipredator behavior and the asset-protection principle. Behav Ecol 5:159–170. https://doi.org/10.1093/beheco/5.2.159
Clutton-Brock TH (1982) The functions of antlers. Behaviour 79:108–124. https://doi.org/10.1163/156853982X00201
Colléter M, Brown C (2011) Personality traits predict hierarchy rank in male rainbowfish social groups. Anim Behav 81:1231–1237. https://doi.org/10.1016/j.anbehav.2011.03.011
Cote J, Clobert J, Brodin T et al (2010) Personality-dependent dispersal: characterization, ontogeny and consequences for spatially structured populations. Phil Trans R Soc B 365:4065–4076. https://doi.org/10.1098/rstb.2010.0176
Creel S (2001) Social dominance and stress hormones. Trends Ecol Evol 16:491–497. https://doi.org/10.1016/S0169-5347(01)02227-3
Dammhahn M, Almeling L (2012) Is risk taking during foraging a personality trait? A field test for cross-context consistency in boldness. Anim Behav 84:1131–1139. https://doi.org/10.1016/j.anbehav.2012.08.014
Dammhahn M, Lange P, Eccard JA (2022) The landscape of fear has individual layers: an experimental test of among-individual differences in perceived predation risk during foraging. Oikos 2022:e09124. https://doi.org/10.1111/oik.09124
David M, Auclair Y, Cézilly F (2011) Personality predicts social dominance in female zebra finches, Taeniopygia guttata, in a feeding context. Anim Behav 81:219–224. https://doi.org/10.1016/j.anbehav.2010.10.008
Devost I, Jones TB, Cauchoix M et al (2016) Personality does not predict social dominance in wild groups of black-capped chickadees. Anim Behav 122:67–76. https://doi.org/10.1016/j.anbehav.2016.10.001
Dewsbury DA (1990) Fathers and sons: genetic factors and social dominance in deer mice, Peromyscus maniculatus. Anim Behav 39:284–289. https://doi.org/10.1016/S0003-3472(05)80872-3
Dingemanse NJ, de Goede P (2004) The relation between dominance and exploratory behavior is context-dependent in wild great tits. Behav Ecol 15:1023–1030. https://doi.org/10.1093/beheco/arh115
Dingemanse NJ, Dochtermann NA (2013) Quantifying individual variation in behaviour: mixed-effect modelling approaches. J Anim Ecol 82:39–54. https://doi.org/10.1111/1365-2656.12013
Dingemanse NJ, Both C, van Noordwijk AJ et al (2003) Natal dispersal and personalities in great tits (Parus major). Proc R Soc Lond B 270:741–747. https://doi.org/10.1098/rspb.2002.2300
Dingemanse NJ, Wright J, Kazem AJN et al (2007) Behavioural syndromes differ predictably between 12 populations of three-spined stickleback. J Anim Ecol 76:1128–1138. https://doi.org/10.1111/j.1365-2656.2007.01284.x
Dingemanse NJ, Dochtermann NA, Nakagawa S (2012) Defining behavioural syndromes and the role of ‘syndrome deviation’ in understanding their evolution. Behav Ecol Sociobiol 66:1543–1548. https://doi.org/10.1007/s00265-012-1416-2
Dingemanse NJ, Moiron M, Araya-Ajoy YG et al (2020) Individual variation in age‐dependent reproduction: fast explorers live fast but senesce young? J Anim Ecol 89:601–613. https://doi.org/10.1111/1365-2656.13122
Drews C (1993) The concept and definition of dominance in animal behaviour. Behaviour 125:283–313. https://doi.org/10.1163/156853993X00290
Duckworth RA (2014) Ecological and evolutionary feedbacks in the evolution of aggression. In: Yasukawa K (ed) Function and evolution of animal behavior. Praeger, Santa Barbara, pp 295–326
Eccard JA, Ylönen H (2003) Who bears the costs of interspecific competition in an age-structured population? Ecology 84:3284–3293. https://doi.org/10.1890/02-0220
Eccard JA, Fey K, Caspers BA, Ylönen H (2011) Breeding state and season affect interspecific interaction types: indirect resource competition and direct interference. Oecologia 167:623–633. https://doi.org/10.1007/s00442-011-2008-y
Fang Q, Zhang Y, Shi Y et al (2016) Individuality and transgenerational inheritance of social dominance and sex pheromones in isogenic male mice. J Exp Zool Part B 326:225–236. https://doi.org/10.1002/jez.b.22681
Favati A, Leimar O, Løvlie H (2014) Personality predicts social dominance in male domestic fowl. PLoS ONE 9:e103535. https://doi.org/10.1371/journal.pone.0103535
Ferrari C, Pasquaretta C, Carere C et al (2013) Testing for the presence of coping styles in a wild mammal. Anim Behav 85:1385–1396. https://doi.org/10.1016/j.anbehav.2013.03.030
Field AP, Miles J, Field Z (2012) Discovering statistics using R. Sage, Thousand Oaks, CA
Freitas JNS, El-Hani CN, Da Rocha PLB (2008) Affiliation in four echimyid rodent species based on intrasexual dyadic encounters: evolutionary implications. Ethology 114:389–397. https://doi.org/10.1111/j.1439-0310.2008.01481.x
Fretwell SD (1972) Populations in a seasonal environment. Princeton University Press, Princeton, NJ
Gartland LA, Firth JA, Laskowski KL et al (2022) Sociability as a personality trait in animals: methods, causes and consequences. Biol Rev 97:802–816. https://doi.org/10.1111/brv.12823
Gipps JHW (1984) The behaviour of mature and immature male bank voles (Clethrionomys glareolus). Anim Behav 32:836–839. https://doi.org/10.1016/S0003-3472(84)80160-8
Gipps JHW (1985) Spacing behaviour and male reproductive ecology in voles of the genus Clethrionomys. Ann Zool Fenn 22:343–351
Gosling SD (2001) From mice to men: what can we learn about personality from animal research? Psychol Bull 127:45–86. https://doi.org/10.1037/0033-2909.127.1.45
Gracceva G, Herde A, Groothuis TGG et al (2014) Turning shy on a winter’s day: effects of season on personality and stress response in microtus arvalis. Ethology 120:753–767. https://doi.org/10.1111/eth.12246
Guerra PA, Pollack GS (2010) Colonists and desperadoes: different fighting strategies in wing-dimorphic male Texas field crickets. Anim Behav 79:1087–1093. https://doi.org/10.1016/j.anbehav.2010.02.002
Hadfield JD (2010) MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R Package. J Stat Softw 33:1–22. https://doi.org/10.18637/jss.v033.i02
Halle S, Stenseth NC (eds) (2000) Activity patterns in small mammals. Springer, Berlin
Herde A, Eccard JA (2013) Consistency in boldness, activity and exploration at different stages of life. BMC Ecol 13:49. https://doi.org/10.1186/1472-6785-13-49
Horne T (1998) Evolution of female choice in the bank vole. Ph.D. Thesis, University of Jyväskylä
Horne TJ, Ylönen H (1996) Female bank voles (Clethrionomys glareolus) prefer dominant males; but what if there is no choice? Behav Ecol Sociobiol 38:401–405. https://doi.org/10.1007/s002650050257
Houslay TM, Wilson AJ (2017) Avoiding the misuse of BLUP in behavioural ecology. Behav Ecol 28:948–952. https://doi.org/10.1093/beheco/arx023
Huntingford FA, Turner AK (1987) Animal conflict. Springer Netherlands, Dordrecht
Hurd PL, Enquist M (2001) Threat display in birds. Can J Zool 79:931–942. https://doi.org/10.1139/z01-062
Jakob EM, Marshall SD, Uetz GW (1996) Estimating fitness: a comparison of body condition indices. Oikos 77:61–67. https://doi.org/10.2307/3545585
Johnstone RA (2001) Eavesdropping and animal conflict. P Natl Acad Sci USA 98:9177–9180. https://doi.org/10.1073/pnas.161058798
Kaufmann JH (1983) On the definitions and functions of dominance and territoriality. Biol Rev 58:1–20. https://doi.org/10.1111/j.1469-185X.1983.tb00379.x
Klemme I, Eccard JA, Ylönen H (2006) Do female bank voles (Clethrionomys glareolus) mate multiply to improve on previous mates? Behav Ecol Sociobiol 60:415–421. https://doi.org/10.1007/s00265-006-0181-5
Koenig AM, Ousterhout BH (2018) Behavioral syndrome persists over metamorphosis in a pond-breeding amphibian. Behav Ecol Sociobiol 72:184. https://doi.org/10.1007/s00265-018-2595-2
Koolhaas JM, De Boer SF, Coppens CM, Buwalda B (2010) Neuroendocrinology of coping styles: towards understanding the biology of individual variation. Front Neuroendocrinol 31:307–321. https://doi.org/10.1016/j.yfrne.2010.04.001
Korpela K, Sundell J, Ylönen H (2011) Does personality in small rodents vary depending on population density? Oecologia 165:67–77. https://doi.org/10.1007/s00442-010-1810-2
Krebs CJ, Myers JH (1974) Population cycles in small mammals. Adv Ecol Res 8:267–399
Łomnicki A (2008) Competition and behavior. In: Jørgensen S, Fath D B (eds) Encyclopedia of Ecology. Elsevier, Amsterdam, pp 695–700. https://doi.org/10.1016/B978-008045405-4.00005-7
Łopuch S, Matuła B (2008) Is there a relationship between dominance rank and condition in captive male bank voles. Clethrionomys glareolus? Acta Ethol 11:1–5. https://doi.org/10.1007/s10211-007-0035-9
Lotka AJ (1925) Elements of physical biology. Williams & Wilkins Company, Baltimore, MA, USA
Majolo B, Lehmann J, de Bortoli Vizioli A, Schino G (2012) Fitness-related benefits of dominance in primates. Am J Phys Anthropol 147:652–660. https://doi.org/10.1002/ajpa.22031
Mangel M, Stamps J (2001) Trade-offs between growth and mortality and the maintenance of individual variation in growth. Evol Ecol Res 3:583–593
Maynard Smith J, Parker GA (1976) The logic of asymmetric contests. Anim Behav 24:159–175. https://doi.org/10.1016/S0003-3472(76)80110-8
Mazue GPF, Dechaume-Moncharmont F-X, Godin J-GJ (2015) Boldness-exploration behavioral syndrome: interfamily variability and repeatability of personality traits in the young of the convict cichlid (Amatitlania Siquia). Behav Ecol 26:900–908. https://doi.org/10.1093/beheco/arv030
Mazza V, Jacob J, Dammhahn M et al (2019) Individual variation in cognitive style reflects foraging and anti-predator strategies in a small mammal. Sci Rep 9:10157. https://doi.org/10.1038/s41598-019-46582-1
McElreath R, Luttbeg B, Fogarty SP et al (2007) Evolution of animal personalities. Nature 450:E5–E5. https://doi.org/10.1038/nature06326
McGhee KE, Travis J (2010) Repeatable behavioural type and stable dominance rank in the bluefin killifish. Anim Behav 79:497–507. https://doi.org/10.1016/j.anbehav.2009.11.037
Montiglio P-O, Garant D, Pelletier F, Réale D (2012) Personality differences are related to long-term stress reactivity in a population of wild eastern chipmunks, Tamias striatus. Anim Behav 84:1071–1079. https://doi.org/10.1016/j.anbehav.2012.08.010
Montiglio P, Garant D, Bergeron P et al (2014) Pulsed resources and the coupling between life-history strategies and exploration patterns in eastern chipmunks (Tamias striatus). J Anim Ecol 83:720–728. https://doi.org/10.1111/1365-2656.12174
Moran NP, Sánchez-Tójar A, Schielzeth H, Reinhold K (2021) Poor nutritional condition promotes high‐risk behaviours: a systematic review and meta‐analysis. Biol Rev 96:269–288. https://doi.org/10.1111/brv.12655
Mouchet A, Cole EF, Matthysen E et al (2021) Heterogeneous selection on exploration behavior within and among west European populations of a passerine bird. P Natl Acad Sci USA 118:e2024994118. https://doi.org/10.1073/pnas.2024994118
Muff S, Nilsen EB, O’Hara RB, Nater CR (2022) Rewriting results sections in the language of evidence. Trends Ecol Evol 37:203–210. https://doi.org/10.1016/j.tree.2021.10.009
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142. https://doi.org/10.1111/j.2041-210x.2012.00261.x
Nicholson A (1954) An outline of the dynamics of animal populations. Aust J Zool 2:9–65. https://doi.org/10.1071/ZO9540009
Panaccio M, Ferrari C, Bassano B et al (2021) Social network analysis of small social groups: application of a hurdle GLMM approach in the Alpine marmot (Marmota marmota). Ethology 127:453–464. https://doi.org/10.1111/eth.13151
Peig J, Green AJ (2009) New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118:1883–1891. https://doi.org/10.1111/j.1600-0706.2009.17643.x
Perals D, Griffin AS, Bartomeus I, Sol D (2017) Revisiting the open-field test: what does it really tell us about animal personality? Anim Behav 123:69–79. https://doi.org/10.1016/j.anbehav.2016.10.006
R Core Team (2023) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org
Rat M, van Dijk RE, Covas R, Doutrelant C (2015) Dominance hierarchies and associated signalling in a cooperative passerine. Behav Ecol Sociobiol 69:437–448. https://doi.org/10.1007/s00265-014-1856-y
Réale D, Reader SM, Sol D et al (2007) Integrating animal temperament within ecology and evolution. Biol Rev 82:291–318. https://doi.org/10.1111/j.1469-185X.2007.00010.x
Riebli T, Avgan B, Bottini A-M et al (2011) Behavioural type affects dominance and growth in staged encounters of cooperatively breeding cichlids. Anim Behav 81:313–323. https://doi.org/10.1016/j.anbehav.2010.11.001
Rohwer S (1977) Status signaling in Harris sparrows: some experiments in deception. Behaviour 61:107–129. https://doi.org/10.1163/156853977X00504
Rowell TE (1974) The concept of social dominance. Behav Biol 11:131–154. https://doi.org/10.1016/S0091-6773(74)90289-2
Rozenfeld FM, Rasmont R (1991) Odour cue recognition by dominant male bank voles, Clethrionomys glareolus. Anim Behav 41:839–850. https://doi.org/10.1016/S0003-3472(05)80351-3
Rozenfeld FM, Boulangé EL, Rasmont R (1987) Urine marking by male bank voles (Clethrionomys glareolus Schreber, 1780; Microtidae, Rodentia) in relation to their social rank. Can J Zool 65:2594–2601. https://doi.org/10.1139/z87-393
Rudin FS, Tomkins JL, Simmons LW (2017) Changes in dominance status erode personality and behavioral syndromes. Behav Ecol 28:270–279. https://doi.org/10.1093/beheco/arw151
Russell WMS, Burch RL (1959) The principles of humane experimental technique. Methuen & Co., London
Santostefano F, Wilson AJ, Araya-Ajoy YG, Dingemanse NJ (2016) Interacting with the enemy: indirect effects of personality on conspecific aggression in crickets. Behav Ecol 27:1235–1246. https://doi.org/10.1093/beheco/arw037
Scales J, Hyman J, Hughes M (2011) Behavioral syndromes break down in urban song sparrow populations: behavioral syndromes break down in urban song sparrow populations. Ethology 117:887–895. https://doi.org/10.1111/j.1439-0310.2011.01943.x
Schielzeth H, Dingemanse NJ, Nakagawa S et al (2020) Robustness of linear mixed-effects models to violations of distributional assumptions. Methods Ecol Evol 11:1141–1152. https://doi.org/10.1111/2041-210X.13434
Schirmer A, Herde A, Eccard JA, Dammhahn M (2019) Individuals in space: personality-dependent space use, movement and microhabitat use facilitate individual spatial niche specialization. Oecologia 189:647–660. https://doi.org/10.1007/s00442-019-04365-5
Schirmer A, Hoffmann J, Eccard JA, Dammhahn M (2020) My niche: individual spatial niche specialization affects within- and between-species interactions. Proc R Soc B 287:20192211. https://doi.org/10.1098/rspb.2019.2211
Schoepf I, Schradin C (2012) Differences in social behaviour between group-living and solitary African striped mice, Rhabdomys pumilio. Anim Behav 84:1159–1167. https://doi.org/10.1016/j.anbehav.2012.08.019
Schradin C, Lamprecht J (2002) Causes of female emigration in the group-living cichlid fish Neolamprologus multifasciatus. Ethology 108:237–248. https://doi.org/10.1046/j.1439-0310.2002.00775.x
Schuett W, Tregenza T, Dall SRX (2010) Sexual selection and animal personality. Biol Rev 85:217–246. https://doi.org/10.1111/j.1469-185X.2009.00101.x
Seyfarth RM, Silk JB, Cheney DL (2014) Social bonds in female baboons: the interaction between personality, kinship and rank. Anim Behav 87:23–29. https://doi.org/10.1016/j.anbehav.2013.10.008
Sih A, Bell AM (2008) Insights for behavioral ecology from behavioral syndromes. Adv Stud Behav 38:227–281
Sih A, Bell AM, Johnson JC, Ziemba RE (2004) Behavioral syndromes: an integrative overview. Q Rev Biol 79:241–277. https://doi.org/10.1086/422893
Stevenson RD, Woods WA (2006) Condition indices for conservation: new uses for evolving tools. Integr Comp Biol 46:1169–1190. https://doi.org/10.1093/icb/icl052
Stiegler J, Lins A, Dammhahn M et al (2022) Personality drives activity and space use in a mammalian herbivore. Mov Ecol 10:33. https://doi.org/10.1186/s40462-022-00333-6
Stoffel MA, Nakagawa S, Schielzeth H (2017) rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol Evol 8:1639–1644. https://doi.org/10.1111/2041-210X.12797
Takola E, Schielzeth H (2022) Hutchinson’s ecological niche for individuals. Biol Philos 37:25. https://doi.org/10.1007/s10539-022-09849-y
Tamashiro KLK, Nguyen MMN, Sakai RR (2005) Social stress: from rodents to primates. Front Neuroendocrinol 26:27–40. https://doi.org/10.1016/j.yfrne.2005.03.001
Tamin T, Morinay J, Germain M et al (2023) Behavioural syndrome between boldness and aggressiveness and link with reproductive success in a wild bird population. Anim Behav 197:27–41. https://doi.org/10.1016/j.anbehav.2022.12.011
Tibbetts EA, Pardo-Sanchez J, Weise C (2022) The establishment and maintenance of dominance hierarchies. Phil Trans R Soc B 377:20200450. https://doi.org/10.1098/rstb.2020.0450
Trappes R, Nematipour B, Kaiser MI et al (2022) How individualized niches arise: defining mechanisms of niche construction, niche choice, and niche conformance. Bioscience 72:538–548. https://doi.org/10.1093/biosci/biac023
Treit D, Fundytus M (1988) Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol Biochem Behav 31:959–962. https://doi.org/10.1016/0091-3057(88)90413-3
van Staaden MJ, Searcy WA, Hanlon RT (2011) Signaling aggression. Adv Genet 75:23–49. https://doi.org/10.1016/B978-0-12-380858-5.00008-3
Verbeek MEM, Boon A, Drent PJ (1996) Exploration, aggressive behaviour and dominance in pair-wise confrontations of juvenile male great tits. Behaviour 133:945–963. https://doi.org/10.1163/156853996X00314
Viitala J (1977) Social organization in cyclic subarctic populations of the voles Clethrionomys rufocanus (Sund.) And Microtus agrestis (L). Ann Zool Fenn 14:53–93
Walsh RN, Cummins RA (1976) The open-field test: a critical review. Psychol Bull 83:482–504. https://doi.org/10.1037/0033-2909.83.3.482
Ward-Fear G, Brown GP, Pearson DJ et al (2018) The ecological and life history correlates of boldness in free‐ranging lizards. Ecosphere 9:e02125. https://doi.org/10.1002/ecs2.2125
Wauters LA, Mazzamuto MV, Santicchia F et al (2021) Personality traits, sex and food abundance shape space use in an arboreal mammal. Oecologia 196:65–76. https://doi.org/10.1007/s00442-021-04901-2
Webber QMR, Albery GF, Farine DR et al (2023) Behavioural ecology at the spatial–social interface. Biol Rev 98:868–886. https://doi.org/10.1111/brv.12934
Webster MM, Ward AJW, Hart PJB (2009) Individual boldness affects interspecific interactions in sticklebacks. Behav Ecol Sociobiol 63:511–520. https://doi.org/10.1007/s00265-008-0685-2
Wilson DS (1998) Adaptive individual differences within single populations. Phil Trans R Soc Lond B 353:199–205. https://doi.org/10.1098/rstb.1998.0202
Wilson ADM, Godin J-GJ (2009) Boldness and behavioral syndromes in the bluegill sunfish, Lepomis macrochirus. Behav Ecol 20:231–237. https://doi.org/10.1093/beheco/arp018
Wilson AJ, de Boer M, Arnott G, Grimmer A (2011) Integrating personality research and animal contest theory: aggressiveness in the green swordtail Xiphophorus helleri. PLoS ONE 6:e28024. https://doi.org/10.1371/journal.pone.0028024
Wilson AJ, Grimmer A, Rosenthal GG (2013) Causes and consequences of contest outcome: aggressiveness, dominance and growth in the sheepshead swordtail, Xiphophorus birchmanni. Behav Ecol Sociobiol 67:1151–1161. https://doi.org/10.1007/s00265-013-1540-7
Wolf M, Weissing FJ (2012) Animal personalities: consequences for ecology and evolution. Trends Ecol Evol 27:452–461. https://doi.org/10.1016/j.tree.2012.05.001
Acknowledgements
This work was supported by the German Research Foundation (DFG) in the framework of the BioMove Research Training Group (DFG-GRK 2118/1). The study was also inspired by discussions with the members of the CRC TRR 212 (NC³) – Project number 316099922. We thank Angela Puschmann and Dr. Annika Schirmer for unflagging logistic and technical support. We thank the reviewers and handling editor for constructive comments on the text.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
FE, JAE and MD designed the study; FE with help of RH collected the data, FE and MD analyzed the data and interpreted the results, FE wrote the draft with support from MD, all authors provided input on the interpretation of results and revised the draft.
Corresponding author
Ethics declarations
Ethical approval
Animal holding and experiments were conducted according to German legislation on animal research under permission of the Landesamt für Umwelt, Verbraucherschutz und Gesundheit, Brandenburg (386-1 and 2347-46-2018). We followed all applicable international, national, and institutional guidelines for use of animals.
Conflict of interest
We confirm that we have no competing interests.
Additional information
Communicated by N. A Dochtermann.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Additional information
Online resource 2 is available at https://doi.org/10.17605/OSF.IO/P42Z5.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Erixon, F., Eccard, J.A., Huneke, R. et al. A behavioral syndrome of competitiveness in a non-social rodent. Behav Ecol Sociobiol 78, 98 (2024). https://doi.org/10.1007/s00265-024-03510-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-024-03510-2