Abstract
Many species exhibit high intraspecific color variation between sexes or ontogenetic phases due to sexual selection or sex-/age-specific differential predation. In crabs, the function and adaptive value of carapace coloration have been investigated mainly in aquatic and/or intertidal species, and it is poorly understood in terrestrial species (Gecarcinidae), which are exposed to different selective pressures. Using digital photography and image analysis, we tested if the coloration of the insular land crab Johngarthia lagostoma in Trindade Island (Brazil) varies according to individuals’ size, sex and ecological processes related to the differential occupation of the available habitats. Three color types were observed (black, purple and yellow), with black crabs being exclusive and predominant in the smaller size classes (carapace width < 30 mm). After this size threshold, yellow crabs dominate throughout ontogeny, while purple individuals are less frequent. Crabs of the three color types occur in both sexes, and the frequency of each type, as well as their brightness and color metrics, was similar between males and females. Black and purple crabs occupy mainly hill areas, but yellow crabs predominate throughout the island. Camouflage by background matching seems to be particularly important for small black crabs at recruitment (sand of the beaches) and resident areas (hills vegetation and soil), where individuals exhibit higher color matching. However, although yellow and purple crabs conceal better against hill and beach backgrounds, respectively, their coloration is probably under neutral selection and has no function for camouflage, since large J. lagostoma crabs are rarely predated in nature.
Significance statement
Intraspecific color variability is common in many animal species and can be linked to different ecological processes. In crabs, the function of body coloration for camouflage has been studied mainly in aquatic species that are exposed to high-predation pressure. However, land crabs also exhibit a remarkable intraspecific color variation, but are exposed to reduced predation pressure especially on oceanic islands, and therefore, the function of coloration remains unexplained. We used digital photography and image analysis to assess the general appearance of an insular land crab species. Our results indicate that small individuals, which are under high predation and cannibalism risk, are black and match background color, while large crabs are purple or yellow, but their coloration probably has no camouflage function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The function and adaptive value of animal external appearance have attracted many researchers of different areas through time (Kettlewell 1955; Cuthill et al. 2017; Caro 2021), with some studies on the diversity of color patterns being conducted since the nineteenth century (Darwin 1859; Wallace 1867). The color expressed by an animal can be linked with many intra- (e.g., sex and group communication) and interspecific (e.g., camouflage and aposematism) signalization processes, as well as being important for thermal regulation and protection against UV radiation (Jablonski and Chaplin 2000; Lindstedt et al. 2009; Stevens and Ruxton 2012; Caro et al. 2016). Therefore, it is expected that individual coloration is under high selection pressure and may be adaptive life history trait (Cuthill et al. 2017).
Many animal species exhibit intraspecific color variation within the same population, both between different ontogenetic phases (Booth 1990) and between individuals of the same age/size (Cuthill et al. 2017). Such color diversity has been observed across different taxonomic groups, from vertebrates (Forsman and Shine 1995; Fowlie and Kruger 2003; Majerus and Mundy 2003; Rudh et al. 2007; Hurtado-Gonzales and Uy 2009; Calsbeek et al. 2010) to invertebrates (Stevens et al. 2014a, b; Hegna et al. 2015; Karpestam et al. 2016; Camacho et al. 2020; Rönkä et al. 2020), being especially important for species inhabiting heterogeneous habitats. At these places, each color type may appear camouflaged against specific portions of the occupied habitat, where individuals may be under lower risk of predation (Jormalainen and Merilaita 1995; Palma and Steneck 2001; Bond and Kamil 2002; Duarte et al. 2018). Therefore, color polymorphic species frequently exhibit broader ecological niches and distribution ranges compared to species with monomorphic populations (Gray and McKinnon 2007; Forsman et al. 2008; McKinnon and Pierotti 2010).
To increase the level of background matching against variable substrates, individuals of many species change color over different timescales (Duarte et al. 2017), including changes through ontogeny (Booth 1990; Wilson et al. 2007; Nokelainen et al. 2019). Ontogenetic color changes were mainly described for crabs (Infraorder Brachyura) in species occupying different habitats (Palma et al. 2003; Todd et al. 2006; Caro 2018; Duarte et al. 2021). The plasticity in crab appearance is generally related to changes in behavior and in the use of available habitats by individuals as they grow (Palma and Steneck 2001). Most studies have reported a higher chromatic variability in juvenile crabs than adults, which are characterized by homogeneous and less diverse color patterns. One possible explanation is that small individuals are associated with highly heterogeneous substrates, where they can conceal against specific portions of the background and/or defeat search images in visual predators (i.e., apostatic selection, see Bond and Kamil 2002; Bond 2007), while adults tend to migrate to less variable substrates (Palma et al. 2003; Todd et al. 2006; Stevens et al. 2014a, b). In some species, this change in habitat use occurs in tandem with modifications in the behavior of the individuals, with small crabs being less active and occupying specific portions of the habitat where they match. However, as they grow, crabs increase mobility and become generalists occupying more than one habitat type, where their homogeneous coloration would provide imperfect (i.e., compromise coloration) but adaptive matching against many background types (Hughes et al. 2019; Nokelainen et al. 2019). Alternatively, adults of some crab species may reach a size refuge from predation and, therefore, exhibiting either cryptic or conspicuous coloration during this phase will not affect the probability of individuals being preyed upon (Palma and Steneck 2001; Krause-Nehring et al. 2010).
In parallel to ontogenetic changes, some species exhibit discrete phenotypic variation because of either genetic differences (color polymorphism) or phenotypic plasticity (color polyphenism), with different color types being observed in individuals of the same ontogenetic phase coexisting in the same geographical area (Caro et al. 2019). The mechanisms maintaining such discrete variation in the population are like those used to explain ontogenetic color changes (i.e., differential occupation among substrates and the result of apostatic selection by visual predators, according to Bond and Kamil 2002; Bond 2007) but can also be a result of sexual selection (i.e., individuals of one sex prefer to mate with a specific color pattern of the opposite sex). However, mating preference for particular color types is generally associated with increasing costs or risks (e.g., increased predation risk), which allows neglected color types to be maintained in the population (Sinervo et al. 2001). The mechanisms and selective forces favoring color polymorphism in natural populations have been exhaustively studied through theoretical and experimental approaches (Bond 2007; Forsman et al. 2008; McKinnon and Pierotti 2010). However, for some animal groups, it is unclear whether such intraspecific color variability has an adaptive value or if it is a trait under selection (Caro 2021).
Color polymorphism during the adult phase has been described for many decapod crustaceans, but most of the studies were focused on intertidal crab species (Palma and Steneck 2001; Krause-Nehring et al. 2010; Stevens et al. 2014a, b; Nokelainen et al. 2019; Duarte et al. 2021), with only few reports for species occupying other environments, such as freshwater (da Silva and Nogueira 2023) and terrestrial habitats (Caro 2018). For example, an ontogenetic color change was qualitatively described for the gecarcinid land crab Cardisoma guanhumi, which inhabits environments adjacent to mangroves in Brazil (Silva et al. 2014). For other gecarcinid genera, only a few records about the existence of color diversity were cited in taxonomic and natural history studies as for Gecarcinus (G. nobilii and G. lateralis, by Perger and Wall 2014), Johngarthia (J. cocoensis and J. planata, by Perger et al. 2011; J. oceanica, by Perger 2019), and Tuerkayana (T. hirtipes and T. celeste, by Ng and Shih 2014). However, actual measurements of coloration using quantitative approaches in land Decapoda were conducted only in the coconut crab Birgus latro from Tanzania, an anomuran species, where adult crabs show blue and red coloration, with the first color type being rare and exhibiting better matching against beach substrates, while the second being more common and concealing to land substrates (Nokelainen et al. 2018). However, considering the absence of predators in most of the islands where coconut crabs inhabit, it has been suggested that the observed color polymorphism in the species does not have a protective function and is under a weak or neutral selection (Caro et al. 2019; Caro 2021).
In oceanic islands, crabs of the family Gecarcinidae exhibit one of the highest degrees of terrestriality in Decapoda (Bliss and Mantel 1968; Burggren and McMahon 1988; Guinot et al. 2018). These animals occupy several habitats over the islands, generally residing in vegetated inland areas, such as hills and mountains, but migrating seasonally to the shore for reproduction (Adamczewska and Morris 2001; Hartnoll et al. 2009, 2010; Laidre 2018). In general, few terrestrial predators of land crabs are established in oceanic islands, with the higher pressure being directed to juveniles (Paulay and Starmer 2011), mainly in anthropized islands due to the introduction of exotic species (e.g., rodents, dogs, and crazy ants) (Paulay and Starmer 2011; Laidre 2018; Veitch et al. 2019; Baumgartner and Ryan 2020). Along with this scenario of exotic species, the suppression of natural habitats in oceanic islands has been the leading cause of the population decline of land crabs, with some species being evaluated as endangered in many different locations (Paulay and Starmer 2011; Pinheiro et al. 2016; Laidre 2018; Perger 2019).
The land crab Johngarthia lagostoma (H. Milne Edwards, 1837) is an endemic species from South Atlantic islands, being found in Fernando de Noronha, Rocas Atoll, and Trindade Island (Brazil), as well as in Ascension Island (UK). Most studies to date have addressed the life history, population, and reproductive parameters of the species mainly in Ascension Island (Hartnoll et al. 2009, 2010) and more recently in Trindade Island (João et al. 2021, 2022). In addition, crabs of different color patterns were qualitatively described for a population in Ascension Island, with small individuals being black, but adults exhibiting yellow or purple coloration (Hartnoll et al. 2009). Here, we examined the coloration patterns of J. lagostoma in Trindade Island (Brazil), evaluating whether this trait is correlated with the size and sex of individuals as well as with ecological processes related to the differential occupation of distinct habitats by crabs. Firstly, we tested whether crabs from the different color categories (i.e., black, purple and yellow) differed on metrics of brightness and color (i.e., saturation and hue), as well as how these metrics varied between sexes and along crab’s ontogeny. We predict that crabs will exhibit an ontogenetic change in appearance, with black individuals being frequent only in small size classes when cryptic coloration is expected to be adaptive due to the high predation pressure to which those individuals are exposed. In fact, no record of predation of both juvenile and adult crabs has been registered in Trindade Island to date, but there is evidence of cannibalism and predation by lizards and birds over juveniles of other Johngarthia species (López-Victoria and Werding 2008). Moreover, since the records of coupling pairs in Trindade Island were only for yellow crabs (João et al. 2021), with no register of either mixed color pairs or purple-purple couples, we expect sex-specific differences in the occurrence of color types, suggesting potential use of coloration for mate selection.
A previous study in Ascension Island presented the hypothesis that yellow crabs would have an advantage over purple individuals in less vegetated habitats, where a light body color characteristic of yellow crabs could reduce the thermal stress mainly during the reproductive migration (Hartnoll et al. 2009). Based on that, we evaluated whether the frequency of yellow and purple crabs differed between sites with distinct altitude range and vegetation physiognomy in Trindade Island (e.g., beaches and hills). We predict that while yellow crabs will exhibit a more generalist distribution across the different sites on the island, purple individuals, which are darker and potentially could retain more heat, will be more frequent in areas less exposed to direct sunlight (e.g., hills), where they would seek refuge in sheltered vegetated habitats.
Finally, we tested whether crabs of different color types exhibit contrasting degrees of background matching against the range of substrates that individuals occupy over the gradient between sand beaches and shrubby vegetated hills. Since small crabs are presumably at a higher risk of predation than adults, we expect high background matching of black individuals against substrates from shore habitats (beach sand and shore vegetation) where they recruit. On the other hand, considering negligible predation pressure over adult crabs, we expect reduced background similarity of purple and yellow crabs against the different substrates, with color variation in adults being a possible result of thermal constraints or a neutral trait without protective function as suggested by B. latro (Caro 2021).
Material and methods
Study area and sampling
We sampled individuals of Johngarthia lagostoma in Trindade Island (20º51″09.4′S, 29º30″8.3′W) during an expedition lasting 2 months (December 2019 to January 2020). Trindade is located in the South Atlantic Ocean, 1200 km from the Brazilian coast (Fig. 1(A)), being considered the most austral point of J. lagostoma distribution (Melo 1996). Trindade is a volcanic island with a total area of approximately 10.5 km2, and one of the emersed points of the Vitória-Trindade submarine chain (Clemente et al. 2018). Despite its rugged relief, some structured plateaus and hills are found on the island, with a maximal altitude of 612 m (Fig. 1(B)). The human occupation is small, with a fixed detachment of the Brazilian Navy and a reduced number of seasonal researchers, totaling a maximum of 40 people.
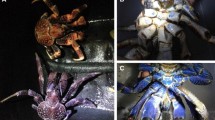
Geographic location of Trindade Island (A), including the four sampling sites with different altitudes (B). Dorsal view of Johngarthia lagostoma crabs of the three different color types (black, purple and yellow — C–E). Photographs representing the five main background types occurring in the sampling sites (F–J), with the predominance of sand (F), sand hill vegetation (G), Cyperus atlanticus shrubs (H), ground (I), and shrubs composed of many vegetal species (J)
J. lagostoma individuals were manually sampled at night with the aid of red-light frontal headlamps in four sites with distinct altitudes, being two beaches (Andradas and Tartarugas — 0 m) and two hills (Príncipe — 136 m; and Desejado — 612 m). In each site, sampling was conducted in six horizontal transects (30 × 2 m = 60 m2), summing up 3600 m2 of sampling area, except for Desejado Hill, where sampling occurred in three transects, totalizing 1800 m2 due to the smaller area available. The same sampling protocol was repeated twice (December 2019 and January 2020) in the same moon phase (crescent moon), with the different sites being sampled on subsequent days. After sampling, crabs were maintained in bags until sunrise to avoid resampling, being further sexed based on the abdominal dimorphism (sub-triangular in males and semi-oval in females) and the number of pleopods (two pairs in males and four pairs in females). While in the field, we used a mechanical caliper (precision 0.05 mm) to measure the carapace width (CW, largest width) and a digital camera to individually photograph the sampled crabs (see details below).
Photography of crabs and backgrounds
We used a digital camera Canon Rebel T5i coupled with an 18–55-mm lens to quantify the color of J. lagostoma crabs and the backgrounds with which they were associated. Backgrounds of each transect were photographed from a fixed distance of 1 m to the substrate every 2 m, totalizing 15 images per transect. Similarly, the sampled crabs were cleaned and placed in plastic containers with a gray background, where they were photographed from a fixed distance (50 cm) under natural light conditions. For both backgrounds and crabs, images were taken in RAW format with manual white balance and fixed aperture settings to avoid overexposure (Stevens et al. 2007). All photographs included a ruler and a gray standard (reflectance of 18%) to control for possible changes in the light levels along the photography section, following current standard procedures (Troscianko and Stevens 2015).
Images were analyzed through custom routines available in the “Multispectral Image Calibration and Analysis Toolbox — MICA” implemented in the “ImageJ” software (Rasband 1997; Troscianko and Stevens 2015). All images were first linearized based on curves modeled from eight Spectralon standards ranging from 2 to 99% of reflectance to correct for camera non-linear responses to light intensity. Next, linearized images were equalized for changes in light conditions using the 18% gray standard and saved as 32-bit multispectral images. Image channels were finally scaled to reflectance values, with an image value of 255 on an 8-bit scale equals 100% reflectance. At the end of this process, crab and background images corresponded to the physical reflectance in three parts of the spectrum (i.e., multispectral images), as red (R), green (G), and blue (B) and could be used for color analysis.
Measuring crab and background color
For each crab image, we defined and selected a region of interest (ROIs) comprising the dorsal part of the entire cephalothorax of the individual. Crabs were qualitatively classified into three color types (black, purple and yellow — Fig. 1(C–E)), based on the color of cephalothorax and pereiopods (Fig. 1(C–E)), as well as according to the size ranges observed for the different types (see “Results”). Black crabs present black cephalothorax and yellow/orange pereiopods (Fig. 1(C)), while purple and yellow individuals exhibit both structures with the same coloration (Fig. 1(D, E)).
Following the methods conducted in previous studies with crabs (Stevens et al. 2014a, b; Stevens et al. 2014a, b; Nokelainen et al. 2017; Duarte et al. 2021), we used normalized reflectance data to calculate brightness and color (i.e., saturation and hue) metrics for each crab image. Brightness is a simple achromatic measure that indicates how dark or bright crabs are across the entire spectrum, being calculated as [(R + G + B) / 3] (Stevens et al. 2014a, b). Saturation indicates the amount of a given color in relation to the white light and was calculated by first transforming the proportional reflectance values in the three-color channels into x–y coordinates of a trichromatic color space (i.e., Maxwell triangle – Kelber et al. 2003). Furthermore, saturation was estimated as the shortest distance between the given color point (i.e., individual coloration) to the achromatic center of the space, where larger distances indicate higher saturation (Kelber et al. 2003). Finally, to analyze hue, we first conducted a principal component analysis (PCA) to identify the main axis of color variation for all crab color types and define a logical color channel (see Nokelainen et al. 2019, and references therein). The proportional reflectance values in the color channels were used in the PCA, with hue being defined as the ratio between the long wavelength (red) and the sum of the medium (green) and short (blue) wavelengths [R / (G + B)].
Background images were classified into five different categories according to the primary substrate recorded in each sample site and occurring in each photograph (Fig. 1(F–J)), namely, sand (SA), which is variable but is predominantly formed by dark (volcanic) sand; sand hill vegetation (SHV), which has a light green coloration; predominance of the shrubby species Cyperus atlanticus (CYA), which has a grayish-green appearance; ground (GRO), which has a dark brown color; and shrubs composed by different vegetal species (SHR), which are uniformly dark green in their coloration. The first two substrates (SA and SHV) were exclusive of beach sites, while the last two (GRO and SHR) were of hill sites. However, CYA was the only substrate occurring in all sampling sites over the island. In each background image, we selected one ROI by drawing one square in a representative area of the primary substrate, following the same analytic procedures used to measure crab color.
Assessing crab background matching
To test the degree of background matching between crab color types and the different backgrounds available in the sampling sites, we first converted the proportional reflectance values in the RGB color channels of crabs and backgrounds into x–y coordinates of a two-dimensional color space, where each color was expressed as a single point (Kelber et al. 2003). Then, we calculated the Euclidean distance between each crab coordinate and the mean of x–y coordinates of each background type, obtaining an estimate of the degree of background matching of crab color types against the different substrates available across the beach-hill gradient in Trindade Island (adapted of Nokelainen et al. 2018 and Duarte et al. 2021). This procedure generates a simple measure of background matching based on color reflectance, regardless of any visual system, since there is no specific information about J. lagostoma predators in Trindade Island.
Statistical analyses
All statistical analyses were undertaken using the R v.4.0.0 environment (R Core Team 2021). First, we used multiple proportion tests (Zar 2010) to assess whether the frequency of the different crab color types differs between sexes and the four sampling sites. Using data for all sampling sites, we used separate two-way analysis of variance (ANOVA) to test whether the size of crabs (CW) and the brightness and color metrics (saturation and hue) of the carapace differ between sexes (male and female) and color types (black, purple and yellow). Finally, we used a linear mixed-effects model to test for differences in the color similarity (i.e., the color distances) between crabs and backgrounds, considering crab color types (black, purple and yellow) and background types (SD, SHV, CYA, GRO, and SHR) as fixed factors and crab identity as a random factor to control for repeated measurements made on the same individual. The model was fitted using the “lmer” function from the “lme4” package (Bates et al. 2015). For all analyses, the normality of residuals was visually checked by q-q plots, and the homogeneity of variances was assessed by the Levene test. Although in some cases variances remained heterogeneous even after data transformation, we preferred to use raw data and parametric analysis given our large sample size, which makes models robust to variance heterogeneity (Underwood 1997). In the case of significant effects, Tukey’s post hoc tests were applied to compare mean contrasts between the different factor levels using the “emmeans” function from the emmeans package (Lenth 2019).
Results
Sex, size, and distribution of crab color types
We collected 431 J. lagostoma individuals (269 males and 162 females) in Trindade Island, with the three color types (black, purple, and yellow) being observed in both sexes. Regardless of color type, the distribution of male and female crabs in the trichromatic color space was very similar, with a clear overlap between them (Fig. 2). Black individuals were exclusive of small crabs, being the predominant color type in size classes < 30-mm CW, where they reach almost 90% of occurrence (Fig. 3A). However, black individuals were the less representative color type when considering the overall population (n = 33, 7.7%). Black coloration in crabs larger than 30-mm CW became progressively less frequent until being no longer registered in size classes > 60-mm CW, where the yellow color type became predominant (Fig. 3A). Purple crabs were less frequent in the entire population (n = 72, 16.7%), with their occurrence varying from 4.2 to 31% in all size classes (Fig. 3A). On the other hand, yellow crabs were dominant in all size classes > 50-mm CW, representing 73.2% of males (n = 197) and 79.6% of females (n = 129) in the overall population. However, the ratio of yellow individuals over the other color types in the population was similar within the sexes (χ2 = 2.24, df = 1, P = 0.523). The frequency of each color type significantly differed among the four sampling sites (black:χ2 = 11.63, df = 3, P = 0.009; purple: χ2 = 24.40, df = 3, P < 0.001; and yellow: χ2 = 38.50, df = 3, P < 0.001) (Fig. 3B), but yellow crabs always predominated over the others regardless of site. Crabs from Andradas Beach and Desejado Hill were mainly yellow (> 92.5%), but the proportion of this color type reduced to 64.2% in Tartarugas Beach and 71.6% in Príncipe Hill, where we registered the highest frequencies of purple (27.7 and 20.2%, respectively) and black (8.0 and 8.3%, respectively) individuals.
Color coordinates in the x–y trichromatic color space (i.e., Maxwell triangle) of male (circles) and female (triangles) Johngarthia lagostoma crabs of different color types (black, purple, and yellow) from the Trindade Island, Brazil. Coordinates were calculated by transforming the normalized reflectance values of the carapace across the three color channels (R, red; G, green; and B, blue) into x–y coordinates of the color space (see “Material and methods” for more details)
Occurrence of Johngarthia lagostoma color types (black, purple and yellow) from the Trindade Island, Brazil, across different size classes (CW, carapace width; A) and sampling sites (Andradas Beach, Tartarugas Beach, Príncipe Hill and Desejado Hill; B). Values on the top of each bar indicate the number sampled for each size class and size
The size (CW) of J. lagostoma crabs significantly differed between sexes and color types, but it was not affected by the interaction between main factors (Table 1). Regardless of the color type, males (mean ± SD: 69.2 ± 1.2 mm CW) were on average larger than females (64.9 ± 1.6 mm CW). Similarly, black crabs (30.3 ± 2.4 mm CW) were on average smaller than the other color types, with purple crabs (61.1 ± 2.0 mm CW) being smaller than yellow crabs (72.8 ± 0.9 mm CW), which were on average the largest J. lagostoma individuals in Trindade Island (Fig. 4A).
Variation in the size (carapace width A), brightness (B), and color metrics (saturation C and hue D) of the carapace of Johngarthia lagostoma color types (black, purple, and yellow) of both female and male crabs in the Trindade Island, Brazil. Squares indicate mean values, and whiskers represent the 95% confidence interval around the mean. Different letters indicate significant differences between factor levels (p < 0.05)
Variation in brightness and color metrics of crab color types
The brightness and color (saturation and hue) of the carapace of J. lagostoma crabs significantly differed between sexes and/or color types (Table 1). Regarding brightness, females exhibited lighter carapace (mean ± SD: 17.5 ± 0.6%) than males (15.4 ± 0.5%). However, the significance of this factor was probably due to a large difference between the brightness of male and female yellow crabs (males: 18.9 ± 0.5%; females: 20.4 ± 0.6%), since this contrast was not evident for black (males: 6.0 ± 0.4%; females: 5.9 ± 0.3%) and purple individuals (males: 5.8 ± 0.3%; females: 5.9 ± 0.6). Regardless of sex, the brightness of the crab’s carapace significantly differed among color types, with yellow crabs (19.5 ± 0.4%) being about three times brighter than black (6.0 ± 0.3%) and purple individuals (5.9 ± 0.3%) (Fig. 4B). Yellow crabs also exhibited more saturated carapace (0.19 ± 0.002) and higher hue values (0.92 ± 0.006) than black (saturation: 0.13 ± 0.008; hue: 0.77 ± 0.02) and purple individuals (saturation: 0.12 ± 0.005; hue: 0.76 ± 0.01), which showed similar color metrics between them (Fig. 4C, D). In conclusion, these results indicate that yellow crabs exhibit a lighter carapace, with a more saturated coloration, which is represented by higher reflectance in long wavelengths (i.e., red).
Background matching of crabs
The degree of background matching, measured as the color distance between crabs and backgrounds, differed according to the substrate type, but such difference depended on the color type (color type × background: F8,423 = 278.74; P < 0.001). Black crabs were better concealed against sand, C. atlanticus vegetation, and the ground of hills than the other substrate types. Similarly, purple crabs were best concealed against the sand substrate but were more conspicuous against shore and hill shrubby vegetation. Finally, yellow crabs exhibited an opposite pattern, being best concealed against the ground of hills, but exhibiting a high color contrast to the sand of beaches (Fig. 5).
Color distance (y-axis, used as a proxy of background matching) between Johngarthia lagostoma color types (black, purple, and yellow) and the main backgrounds found along the beach-hill gradient in Trindade Island, Brazil. Backgrounds were covered mainly by sand (SA), sand hill vegetation (SHV), Cyperus atlanticus shrubs (CA), ground (GRO), and shrubs composed of many vegetal species (SHR). Squares indicate mean values and whiskers represent the 95% confidence interval around the mean. Different letters indicate significant differences between factor levels (p < 0.05)
Discussion
Here, we show that the land crab Johngarthia lagostoma exhibits an ontogenetic change in its general appearance. In the population of Trindade Island (Brazil), black crabs are observed only in small size classes (< 30-mm CW), while large individuals occur in two color types, with a predominance of yellow (81.9%) over purple individuals (18.1%). The three color types recorded here followed the same groups described for Ascension Island (Hartnoll et al. 2009). However, there is no variation in the occurrence of color types between sexes, with a similar proportion of black, purple, and yellow individuals in both male and female crabs. The ontogenetic color change in J. lagostoma is probably adaptive since the coloration of small black crabs highly matches that of the sand of the beaches, where individuals recruit and where predation pressure is arguably more intense. However, while purple and yellow color types considerably conceal against different backgrounds along the beach-hill gradient, the color of adult crabs probably has no adaptive function for camouflage and is possibly under neutral selection, since the predation pressure over those individuals in Trindade is very low or even absent.
Although sex-specific color variation is common to different animal taxa (Forsman 1995; Forsman and Appelqvist 1999; Joron 2005), both the frequency (black: n = 20 males and 13 females; yellow: n = 197 males and 129 females; and purple: n = 52 males and 20 females) and the general appearance of color types are similar between male and female J. lagostoma crabs. In crustaceans, there are few reports of color sexual dimorphism, with some cases related to sexual maturation (Pinheiro and Taddei 2000; Baldwin and Johnsen 2012) and differential patterns of habitat use and predation risk between sexes (Merilaita and Jormalainen 1997; Jensen and Egnotovich 2015). For terrestrial crabs, there is some evidence of sex-specific antipredator behavior (e.g., Epigrapsus notatus by Liu and Jeng 2005), but there is no information on whether this correlates to sex-specific color patterns. Alternatively, females of the semiterrestrial fiddler crab Tubuca capricornis exhibit lighter and more variable carapaces than males of similar size, with color used as a visual cue during courtship (Detto et al. 2008). In this sense, J. lagostoma couples at Trindade Island are mainly composed of yellow individuals, without pre- or post-copulatory behavior registered to date (João et al. 2021). In Ascension Island, only one couple formed by purple and yellow crabs was registered in a presumed courtship embrace (Hartnoll et al. 2006), but this was not observed anymore. The predominance of reproductive pairs of yellow crabs may simply result from the higher probability of encounters between yellow receptive individuals than purple crabs, which are less frequent in the population of Trindade. Similar findings were observed for the coconut crab Birgus latro, on which the blue and red color types were equally distributed between males and females (Caro and Morgan 2018; Nokelainen et al. 2018), and no pre-copulatory behavior has been observed as well as any evidence of preference for some color in couple formations (Helfman 2008). However, without conducting controlled experiments, we cannot exclude a possible role of individual coloration or brightness in the visual selection of sexual partners on both J. lagostoma and B. latro.
The ontogenetic color change observed in J. lagostoma reflects a differential degree of background concealment between small and large crabs. In many crab species, the juvenile phase is the most vulnerable period to predation (Krause-Nehring et al. 2010); therefore, it is when a higher selection for camouflage is expected (Caro 2018). Adults of insular land crabs are rarely predated due to their large size and their role as top-consumers, especially at oceanic islands. Still, juveniles are highly exposed to predation by other native or invasive species (Paulay and Starmer 2011), as well as by cannibalism and aggression from large conspecifics (Bliss et al. 1978; López-Victoria and Werding 2008; Vannini et al. 2003). Our results indicate that the black coloration restricted to small crabs may reduce predation or provide camouflage against the substrate of sand beaches, Cyperus atlanticus vegetation, and hill ground, where the color distance between crabs and backgrounds was significantly smaller. In fact, after the planktonic larval phase, J. lagostoma zoea metamorphoses into benthic megalopa, which settles at the island’s beaches and searches for specific backgrounds (e.g., sand and rocks) to recruit, as observed for both Ascension (Hartnoll et al. 2014) and Trindade Island (MAA Pinheiro et al. unpublished data). On Trindade Island, the supralittoral zone of both Andradas and Tartarugas beaches is covered by Cyperus atlanticus vegetation, forming an extensive and unique habitat after the sand zone. Black crabs exhibited the highest color match against sand and C. atlanticus vegetation, where protection against predation will increase. The highest density of juvenile black crabs on Trindade Island (30%, according to João et al. 2023) is observed at Príncipe Hill. This site is covered mainly by ground and C. atlanticus vegetation, being possibly another important location on the island for black crabs to occupy and hide from potential predators. Therefore, C. atlanticus vegetation appears to be a crucial habitat for crab juveniles in Trindade Island, by providing effective camouflage and offering shading and humidity for the individuals (Vannini et al. 2003).
Although many crab species exhibit a high level of background matching during the juvenile phase, most studies to date have shown that small crabs are also more variable than large individuals in terms of general appearance, which would provide them differential camouflage in heterogeneous habitats or impair search image formation by predators (Nokelainen et al. 2019; Duarte et al. 2021; Troscianko et al. 2021). Our data indicate the opposite for J. lagostoma since small crabs are represented exclusively by a single color type (black), while large crabs are more variable, represented by yellow and purple individuals. This can be explained because juvenile crabs are less mobile than adults, spending a long time sheltered under the vegetation and hidden in crevices (Vannini et al. 2003; Liu and Jeng 2005), which restrict the individuals from being exposed to different types of substrates. In contrast, during the reproductive migration, large J. lagostoma individuals move through many habitats on the island (Hartnoll et al. 2009), for which a higher variability in brightness and color could be advantageous for potential camouflage (Duarte et al. 2021). However, the high variability in adult crab appearance may result from the relaxation of selection pressures for individuals to maintain a cryptic coloration (Bliard et al. 2020), especially when color has no protective function. We believe that the second hypothesis supports better the case of J. lagostoma adults since the predation pressure over these crabs is practically nonexistent in all islands they inhabit (Andrades et al. 2019).
Considering only the results generated by our study, we do not have any evidence supporting that yellow and purple color types of J. lagostoma are real morphotypes and a case of color polymorphism (i.e., if they are genetically determined and fixed phenotypes) or if they arise as different stable phenotypes following an ontogenetic color change from small black crabs. This point can only be clarified through experiments observing the growth of black crabs. If after molting, black individuals change mainly to purple, this would be indicative of the purple color type being an intermediate form, from which yellow crabs would originate after other molting events. However, if black crabs change to both color types or mainly to yellow, this would be indicative that purple and yellow crabs are fixed phenotypes, and the higher chance of originating a yellow individual would occur due to the higher frequency of the allele determining this color type in the population. Overall, large gecarcinid crabs have very slow growth, and each molt event could occur within intervals of some years (Hartnoll et al. 2006). Because of that, performing color change experiments along the ontogeny of J. lagostoma is challenging. The difference in the average size between the adult color types, with purple crabs being smaller than yellow individuals, could lead us to think about the possibility of purple crabs working as an intermediate stage. Still, purple and yellow crabs are similarly frequent in intermediate size classes (40–60 mm CW), but yellow individuals dominate the large classes. However, this difference can be simply due to sampling and statistical bias. Considering that the distribution of crabs’ body size follows a normal distribution, it is expected that very large or very small individuals are less likely to be sampled than mid-size crabs. Those odds are even smaller for purple crabs, which are much less frequent in the population than yellow individuals. Therefore, based on the data we have about the size distribution and the occurrence of J. lagostoma color types across the island, we can argue that purple and yellow crabs arise in the population at similar size, occupy the same habitats, and occur across all size classes larger than 30 mm, but exhibit an extremely differential predominance in the island.
As observed for juvenile black crabs, adult J. lagostoma showing both yellow and purple coloration also exhibited different levels of color matching against backgrounds, with yellow and purple crabs matching substrates of hill and beach habitats, respectively. Similar results were registered for the coconut crab B. latro on Pemba Island (Tanzania), where red individuals predominate in the population and are concealed against land substrates, while blue crabs are rare and better match the coloration of beach backgrounds (Nokelainen et al. 2018). However, in different islands inhabited by B. latro, the two color types share the same habitat and are not predated, suggesting that the body coloration of this species has no protective function and can be considered neutral traits without correlation with ecological aspects (Caro and Morgan 2018). The color polymorphism in this species is possibly determined by a simple Mendelian variation, with the frequencies of both color types being maintained in balance in the population (Caro 2021). We believe that the color variation observed in J. lagostoma adults can be similarly explained, with color types maintained in the population by genetic variation, since yellow and purple crabs are distributed in Trindade Island in a proportion close to 3:1 (yellow/purple). Nonetheless, the predominance of yellow crabs in the population of both Trindade Island and Ascension Island (Hartnoll et al. 2009) may suggest that in case body coloration is a neutral character, the high occurrence of this type results simply from a higher allelic frequency in the population. Considering that our study does not test all behavioral and morphological traits that could be correlated with body coloration, future studies are necessary to understand the adaptive function of coloration in adult J. lagostoma and to describe if the different color types result from discrete or continuous color change from black crabs.
Based on the hypothesis proposed by Hartnoll and colleagues (2009) in Ascension Island, yellow J. lagostoma crabs would have an advantage over purple individuals during the reproductive migrations, since the first could be more resistant to thermal stress due to their lighter carapace. There is no available information about the relationship between carapace color and heat retention of J. lagostoma color types. Still, darker individuals of Menippe mercenaria, for example, exhibit thicker carapaces and consequently larger heat retention than lighter crabs (Melnick et al. 1996). Similarly, fiddler crabs (Leptuca pugilator) become lighter when exposed to high temperatures, with brightness change being a mechanism that individuals use to control corporal temperature and reduce thermal stress (Silbiger and Munguia 2008). In Trindade Island, the ratio between yellow and purple crabs is higher in Andradas Beach, a reproductive area, and Desejado Hill, a residential area of high altitude (João et al. 2023), suggesting that mainly yellow crabs would participate in large displacements during migration events. However, outside of the reproductive season, when this study was performed, crabs remained hidden during most of the day, exhibiting a crepuscular/nocturnal behavior with peaks of activity concentrated during periods of low light intensity. Furthermore, despite the expectation that purple crabs might seek refuge in protected vegetated areas if they were more susceptible to overheating, they were more commonly found in Tartarugas Beach, an open sandy area with direct sunlight. Based on these observations, there is currently no supporting evidence for the thermal stress hypothesis proposed by Hartnoll and colleagues (2009) in the context of Trindade Island. However, it is advisable to conduct studies during the reproductive season when adult crabs are more active and exposed to direct heat (Adamczewska and Morris 2001). Future studies should investigate whether yellow and purple J. lagostoma crabs engage in migrations and whether their choice of open or vegetated areas on the island changes during migration events. Additionally, direct measurements of body heating in both color types should be carried out to shed further light on this topic.
To date, our work is the first to quantitatively describe different color patterns of a land crab species. Although there is a lack of descriptive studies for other species of the family, it seems that the existence of color variation in adult individuals is probably a common phylogenetic trait conserved on many gecarcinids (Ng and Shih 2014; Perger and Wall 2014; Silva et al. 2014; Perger 2019). In C. guanhumi, for example, during ontogeny, the color variation observed is that the small crabs present dark color patterns and large crabs present light color patterns (Silva et al. 2014), which is like the pattern we describe here for J. lagostoma. However, there is no information about any sex or age-related color variation, as well as any study about the functional significance of body coloration for all other gecarcinid crab species, which opens an interesting line of research to investigate in the future. Another important gap is to compare the general appearance of gecarcinid crabs that occupy insular and continental areas, where they exhibit different degrees of terrestriality (Guinot et al. 2018) and are exposed to distinct levels of predation pressure (Vermeij and Dudley 2000). Despite the lower risk of predation on insular crabs, the increasing number and diversity of exotic species in oceanic islands has added new potential predators to these environments (Pinheiro et al. 2016), which has already impacted local crab populations (Ascension Island Government 2015; Pinheiro et al. 2016; Laidre 2018; Perger 2019). In this scenario, the apparent neutral or generalist coloration of land crabs can become a conspicuous trait and increase the chance of capture by the new predators that may have different visual systems (Delhey and Peters 2017). This is of particular concern for the conservation of insular land crabs, since many species are already endangered, exhibit high endemism, and form isolated populations (Paulay and Starmer 2011; Rodríguez-Rey et al. 2016; Freire et al. 2021). Therefore, it is urgently necessary to implement conservation strategies to quantify and mitigate the impact of invasive species in the communities of oceanic islands (Veitch et al. 2019).
Data availability
All data was submitted as electronic supplementary material.
References
Adamczewska AM, Morris S (2001) Ecology and behavior of Gecarcoidea natalis, the Christmas Island red crab, during the annual breeding migration. Biol Bull 200(3):305–320. https://doi.org/10.2307/1543512
Andrades R, Jackson AL, Macieira RM, Reis-Filho JA, Bernardino AF, Joyeux J-C, Giarrizzo T (2019) Niche-related processes in island intertidal communities inferred from stable isotopes data. Ecol Indic 104:648–658. https://doi.org/10.1016/j.ecolind.2019.05.039
Ascension Island Government (2015) Johngarthia lagostoma action plan. In: The Ascension Island Biodiversity Action Plan. Ascension Island Government, Georgetown (Ascension Island), pp 1–5
Baldwin J, Johnsen S (2012) The male blue crab, Callinectes sapidus, uses both chromatic and achromatic cues during mate choice. J Exp Biol 215(7):1184–1191. https://doi.org/10.1242/jeb.067512
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67(1):1–48. https://doi.org/10.18637/jss.v067.i01
Baumgartner NR, Ryan SD (2020) Interaction of red crabs with yellow crazy ants during migration on Christmas Island. Math Biosci 330:108486. https://doi.org/10.1016/j.mbs.2020.108486
Bliard L, Paquet M, Robert A, Dufour P, Renoult JP, Grégoire A, Crochet PA, Covas R, Doutrelant C (2020) Examining the link between relaxed predation and bird coloration on islands. Biol Lett 16(4):4–8. https://doi.org/10.1098/rsbl.2020.0002
Bliss DE, Mantel LH (1968) Adaptations of crustaceans to land: a summary and analysis of new findings. Am Zool 8(3):673–685. https://doi.org/10.1093/icb/8.3.673
Bliss DE, Van Montfrans J, Van Montfrans M, Boyer JR (1978) Behavior and growth of the land crab Gecarcinus lateralis (Fréminville) in southern Florida. Bull Am Museum Nat Hist 160:112–152
Bond AB (2007) The evolution of color polymorphism: crypticity, searching images, and apostatic selection. Annu Rev Ecol Evol Syst 38(1):489–514
Bond AB, Kamil AC (2002) Visual predators select for crypticity and polymorphism in virtual prey. Nature 415(6872):609–613. https://doi.org/10.1038/415609a
Booth CL (1990) Evolutionary significance of ontogenetic colour change in animals. Biol J Linn Soc 40(2):125–163. https://doi.org/10.1111/j.1095-8312.1990.tb01973.x
Burggren WW, McMahon BR (1988) Biology of the land crabs. Cambridge University Press, New York
Calsbeek B, Hasselquist D, Clobert J (2010) Multivariate phenotypes and the potential for alternative phenotypic optima in wall lizard (Podarcis muralis) ventral colour morphs. J Evol Biol 23(6):1138–1147. https://doi.org/10.1111/j.1420-9101.2010.01978.x. http://www.ncbi.nlm.nih.gov/pubmed/20406342
Camacho C, Sanabria-Fernández A, Baños-Villalba A, Edelaar P (2020) Experimental evidence that matching habitat choice drives local adaptation in a wild population. Proc R Soc B Biol Sci 287(1927):20200721. https://doi.org/10.1098/rspb.2020.0721
Caro T (2018) The functional significance of coloration in crabs. Biol J Linn Soc 124(1):1–10. https://doi.org/10.1093/biolinnean/bly021/4951350
Caro T (2021) When animal coloration is a poor match. Evol Ecol 35(1):1–13. https://doi.org/10.1007/s10682-020-10084-8
Caro T, Morgan VM (2018) Correlates of color polymorphism in coconut crabs Birgus latro. Zoology 129:1–8. https://doi.org/10.1016/j.zool.2018.06.002
Caro T, Sherratt TN, Stevens M (2016) The ecology of multiple colour defences. Evol Ecol 30(5):797–809. https://doi.org/10.1007/s10682-016-9854-3
Caro T, Cluff E, Morgan VM (2019) Colour polymorphism and protective coloration in coconut crabs. Ethol Ecol Evol 31(6):514–525. https://doi.org/10.1080/03949370.2019.1626488
Clemente EDP, Oliveira FS, Machado MR, Schaefer CEGR (2018) Fracionamento da Matéria Orgânica dos Solos da Ilha da Trindade. Geogr Dep Univ Sao Paulo 36:48–62. https://doi.org/10.11606/rdg.v36i0.147796
Cuthill IC, Allen WL, Arbuckle K, Caspers B, Chaplin G, Hauber ME, Hill GE, Jablonski NG, Jiggins CD, Kelber A et al (2017) The biology of color. Science 357:eaan0221. https://doi.org/10.1126/science.aan0221
da Silva AR, Nogueira CS (2023) From color to shape: ontogenetic shifts in traits of the freshwater crab Dilocarcinus pagei (Brachyura: Trichodactylidae). Can J Zool 101(8):658–671. https://doi.org/10.1139/cjz-2023-0039
Darwin C (1859) On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. London, Murray, p 247
Delhey K, Peters A (2017) Conservation implications of anthropogenic impacts on visual communication and camouflage. Conserv Biol 31(1):30–39. https://doi.org/10.1111/cobi.12834
Detto T, Hemmi JM, Backwell PRY (2008) Colouration and colour changes of the fiddler crab, Uca capricornis: a descriptive study. PLoS One 3(2):e1629. https://doi.org/10.1371/journal.pone.0001629
Duarte RC, Flores AAVV, Stevens M (2017) Camouflage through colour change: mechanisms, adaptive value and ecological significance. Philos Trans R Soc B Biol Sci 372(1724):20160342. https://doi.org/10.1098/rstb.2016.0342
Duarte RC, Stevens M, Flores AAV (2018) The adaptive value of camouflage and colour change in a polymorphic prawn. Sci Rep 8(1):16028. https://doi.org/10.1038/s41598-018-34470-z
Duarte RC, Dias GM, Flores AAV, Stevens M (2021) Different ontogenetic trajectories of body colour, pattern and crypsis in two sympatric intertidal crab species. Biol J Linn Soc 132(1):17–31. https://doi.org/10.1093/biolinnean/blaa168
Forsman A (1995) Opposing fitness consequences of colour pattern in male and female snakes. J Evol Biol 8(1):53–70. https://doi.org/10.1046/j.1420-9101.1995.8010053.x. http://doi.wiley.com/https://doi.org/10.1046/j.1420-9101.1995.8010053.x
Forsman A, Appelqvist S (1999) Experimental manipulation reveals differential effects of colour pattern on survival in male and female pygmy grasshoppers. J Evol Biol 12:391–401
Forsman A, Ahnesjö J, Caesar S, Karlsson M (2008) A model of ecological and evolutionary consequences of color polymorphism. Ecology 89(1):34–40. https://doi.org/10.1890/07-0572.1
Forsman A, Shine R (1995) The adaptive significance of colour pattern polymorphism in the Australian scincid lizard Lampropholis delicata. Biol J Linn Soc 55:273–291. http://onlinelibrary.wiley.com/doi/https://doi.org/10.1111/j.1095-8312.1995.tb01066.x/abstract
Fowlie MK, Kruger O (2003) The evolution of plumage polymorphism in birds of prey and owls: the apostatic selection hypothesis revisited. J Evol Biol 16(4):577–583. https://doi.org/10.1046/j.1420-9101.2003.00564.x
Freire AS, Teschima MM, Brandão MC, Iwasa-Arai T, Sobral FC, Sasaki DK, Agostinis AO, Pie MR (2021) Does the transport of larvae throughout the south Atlantic support the genetic and morphometric diversity of the Sally Lightfoot Crabs Grapsus grapsus (Linnaeus, 1758) and Grapsus adscensionis (Osbeck, 1765) (Decapoda: Grapsidae) among the o. J Mar Syst 223:103614. https://doi.org/10.1016/j.jmarsys.2021.103614
Gray SM, McKinnon JS (2007) Linking color polymorphism maintenance and speciation. Trends Ecol Evol 22(2):71–79. https://doi.org/10.1016/j.tree.2006.10.005
Guinot D, Ng NK, Moreno PAR (2018) Review of grapsoid families for the establishment of a new family for Leptograpsodes Montgomery, 1931, and a new genus of Gecarcinidae H. Milne Edwards, 1837 (Crustacea, Decapoda, Brachyura, Grapsoidea MacLeay, 1838). Zoosystema 40(26):547–604. https://doi.org/10.5252/zoosystema2018v40a26
Hartnoll RG, Baine MSP, Grandas Y, James J, Atkin H (2006) Population biology of the black land crab, Gecarcinus ruricola, in the San Andres Archipelago, Western Caribbean. J Crustac Biol 26(3):316–325. https://doi.org/10.1651/C-2640.1
Hartnoll RG, Broderick AC, Godley BJ, Saunders KE (2009) Population structure of the land crab Johngarthia lagostoma on Ascension Island. J Crustac Biol 29(1):57–61. https://doi.org/10.1651/08-2992.1
Hartnoll RG, Broderick AC, Musick S, Godley BJ, Pearson M, Stroud SA, Saunders KE (2010) Reproduction in the land crab Johngarthia lagostoma on Ascension Island. J Crustac Biol 30(1):83–92. https://doi.org/10.1651/09-3143.1
Hartnoll RG, Régnier-McKellar C, Weber N, Weber SB (2014) Return to the land; the stages of terrestrial recruitment in land crabs. Crustaceana 87(5):531–539. https://doi.org/10.1163/15685403-00003294
Hegna RH, Galarza JA, Mappes J (2015) Global phylogeography and geographical variation in warning coloration of the wood tiger moth (Parasemia plantaginis). J Biogeogr 42(8):1469–1481. https://doi.org/10.1111/jbi.12513
Helfman GS (2008) Copulatory behavior of the coconut or robber crab Birguslatro (L.) (Decapoda Anomura, Paguridea, Coenobitidae) 1). Crustaceana 33(2):198–202. https://doi.org/10.1163/156854077x00106
Hughes A, Liggins E, Stevens M (2019) Imperfect camouflage: how to hide in a variable world? Proc R Soc B Biol Sci 286(1902):20190646. https://doi.org/10.1098/rspb.2019.0646
Hurtado-Gonzales JL, Uy JAC (2009) Alternative mating strategies may favour the persistence of a genetically based colour polymorphism in a pentamorphic fish. Anim Behav 77(5):1187–1194
Jablonski NG, Chaplin G (2000) The evolution of human skin coloration. J Hum Evol 39(1):57–106. https://doi.org/10.1006/jhev.2000.0403
Jensen GC, Egnotovich MS (2015) A whiter shade of male: color background matching as a function of size and sex in the yellow shore crab Hemigrapsus oregonensis (Dana, 1851). Curr Zool 61(4):729–738. https://doi.org/10.1093/czoolo/61.4.729
João MCA, Kriegler N, Freire AS, Pinheiro MAA (2021) Mating strategies of the endangered insular land crab Johngarthia lagostoma (H. Milne Edwards, 1837). Invertebr Reprod Dev 65(4):256–267. https://doi.org/10.1080/07924259.2021.1961885
João MCA, Duarte RC, da Silva LSB, Freire AS, Pinheiro MAA (2022) Sexual maturity of an endemic insular land crab: priority information toward the conservation of Johngarthia lagostoma. Biol Bull 243(1):14–27. https://doi.org/10.1086/720581
João MCA, Duarte RC, Freire AS, Kriegler N, Pinheiro MAA (2023) Population biology of the endangered land crab Johngarthia lagostoma (H. Milne Edwards 1837) in the Trindade Island Brazil: Identifying crucial areas for future conservation strategies. Mar Ecol 00:e12778. https://doi.org/10.1111/maec.12778
Jormalainen V, Merilaita S (1995) Differential predation on sexes affects colour polymorphism of the isopod Idotea baltica (Pallas). Biol J Linn Soc 55:45–68
Joron M (2005) Polymorphic mimicry, microhabitat use, and sex-specific behaviour. J Evol Biol 18(3):547–556
Karpestam E, Merilaita S, Forsman A (2016) Colour polymorphism protects prey individuals and populations against predation. Sci Rep 6:22122. https://doi.org/10.1038/srep22122
Kelber A, Vorobyev M, Osorio D (2003) Animal colour vision - behavioural tests and physiological concepts. Biol Rev 78(1):81–118. https://doi.org/10.1017/S1464793102005985
Kettlewell H (1955) Selection experiments on industrial melanism in the Lepidoptera. Heredity 9:323–342
Krause-Nehring J, Matthias Starck J, Richard Palmer A (2010) Juvenile colour polymorphism in the red rock crab, Cancer productus: patterns, causes, and possible adaptive significance. Zoology 113(3):131–139. https://doi.org/10.1016/j.zool.2009.09.002
Laidre ME (2018) Coconut crabs. Curr Biol 28(2):R58–R60. https://doi.org/10.1016/j.cub.2017.11.017
Lenth R (2019) Emmeans: estimated marginal means, aka least-squares means. R package version 1.3.5. https://cran.r-project.org/package=emmeans
Lindstedt C, Lindström L, Mappes J (2009) Thermoregulation constrains effective warning signal expression. Evolution 63(2):469–478. https://doi.org/10.1111/j.1558-5646.2008.00561.x
Liu HC, Jeng MS (2005) Reproduction of Epigrapsus notatus (Brachyura: Gecarcinidae) in Taiwan. J Crustac Biol 25(1):135–140. https://doi.org/10.1651/C-2499
López-Victoria M, Werding B (2008) Ecology of the endemic land crab Johngarthia malpilensis (Decapoda: Brachyura: Gecarcinidae), a poorly known species from the tropical eastern Pacific. Pac Sci 62:483–493. https://doi.org/10.2984/1534-6188(2008)62[483:EOTELC]2.0.CO;2
Majerus ME, Mundy NI (2003) Mammalian melanism: natural selection in black and white. Trends Genet 19(11):585–588. https://doi.org/10.1016/j.tig.2003.09.003
McKinnon JS, Pierotti MER (2010) Colour polymorphism and correlated characters: genetic mechanisms and evolution. Mol Ecol 19(23):5101–5125. https://doi.org/10.1111/j.1365-294X.2010.04846.x
Melnick CA, Chen Z, Mecholsky JJ (1996) Hardness and toughness of exoskeleton material in the stone crab, Menippe mercenaria. J Mater Res 11(11):2903–2907. https://doi.org/10.1557/JMR.1996.0367
Melo GAS (1996) Manual de identificação dos Brachyura (caranguejos e siris) do litoral brasileiro. Plêiade, São Paulo, p 476
Merilaita S, Jormalainen V (1997) Evolution of sex differences in microhabitat choice and colour polymorphism in Idotea baltica. Anim Behav 54(4):769–778. https://doi.org/10.1006/anbe.1996.0490
Ng PKL, Shih H (2014) The systematics of the land crabs of the Discoplax hirtipes (Dana, 1851) species-group (Crustacea: Decapoda: Brachyura: Gecarcinidae), with description of a new species from the eastern Indian Ocean. Raffles Bull Zool 30(30):109–135
Nokelainen O, Hubbard N, Lown AE, Wood LE, Stevens M (2017) Through predators’ eyes: phenotype-environment associations in shore crab coloration at different spatial scales. Biol J Linn Soc 122(4):738–751. https://doi.org/10.1093/biolinnean/blx101
Nokelainen O, Stevens M, Caro T (2018) Colour polymorphism in the coconut crab (Birgus latro). Evol Ecol 32(1):75–88. https://doi.org/10.1007/s10682-017-9924-1
Nokelainen O, Maynes R, Mynott S, Price N, Stevens M (2019) Improved camouflage through ontogenetic colour change confers reduced detection risk in shore crabs. Funct Ecol 33:654–669. https://doi.org/10.1111/1365-2435.13280
Palma AT, Steneck RS (2001) Does variable coloration in juvenile marine crabs reduce risk of visual predation? Ecology 82(10):2961–2967
Palma AT, Orrego C, Arriagada M (2003) Crypsis in early benthic phases of Brachyuran Decapod crustaceans in central Chile. Rev Chil Hist Nat 76(2):149–156. https://doi.org/10.4067/S0716-078X2003000200002
Paulay G, Starmer J (2011) Evolution, insular restriction, and extinction of oceanic land crabs, exemplified by the loss of an endemic Geograpsus in the Hawaiian Islands. PLoS One 6(5):e19916. https://doi.org/10.1371/journal.pone.0019916
Perger R (2019) A new species of Johngarthia from Clipperton and Socorro Islands in the Eastern Pacific Ocean (Crustacea: Decapoda: Gecarcinidae). Pacific Sci 73(2):285–304. https://doi.org/10.2984/73.2.9
Perger R, Wall A (2014) The description of a new species of the Neotropical land crab genus Gecarcinus Leach, 1814 (Crustacea, Decapoda, Brachyura, Gecarcinidae). Zookeys 435:93–109. https://doi.org/10.3897/zookeys.435.7271
Perger R, Vargas R, Wall A (2011) Johngarthia cocoensis, a new species of Gecarcinidae MacLeay, 1838 (Crustacea, Decapoda, Brachyura) from Cocos Island Costa Rica. Zootaxa 68(2911):57–68. https://doi.org/10.11646/zootaxa.2911.1.4
Pinheiro MAA, Santana W, Rodrigues ES, Ivo CTC, Santos LCM, Torres RA, Boos H, Neto JD (2016) Avaliação dos caranguejos gecarcinídeos (Decapoda: Gecarcinidae). In: Pinheiro MAA, Boos H (eds) Livro Vermelho dos Crustáceos do Brasil: Avaliação 2010–2014. Sociedade Brasileira de Carcinologia – SBC, Porto Alegre, pp 167–181
Pinheiro MAA, Taddei FG (2000) Chromatic alteration in Arenaeus cribrarius (Lamarck) (Crustacea, Portunidae): an indicator of sexual maturity. Rev Bras Zool 17(4):945–951. https://doi.org/10.1590/s0101-81752000000400006
R Core Team (2021) R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.r-project.org/
Rasband (1997) Image J. National Institutes of Health, Bethesda (USA). https://imagej.nih.gov/ij/
Rodríguez-Rey GT, Hartnoll RG, Solé-Cava AM (2016) Genetic structure and diversity of the island-restricted endangered land crab, Johngarthia lagostoma (H. Milne Edwards, 1837). J Exp Mar Bio Ecol 474:204–209. https://doi.org/10.1016/j.jembe.2015.10.016
Rönkä K, Valkonen JK, Nokelainen O, Rojas B, Gordon S, Burdfield-Steel E, Mappes J (2020) Geographic mosaic of selection by avian predators on hindwing warning colour in a polymorphic aposematic moth. Ecol Lett 23(11):1654–1663. https://doi.org/10.1111/ele.13597
Rudh A, Rogell B, Höglund J (2007) Non-gradual variation in colour morphs of the strawberry poison frog Dendrobates pumilio: genetic and geographical isolation suggest a role for selection in maintaining polymorphism. Mol Ecol 16(20):4284–4294. https://doi.org/10.1111/j.1365-294X.2007.03479.x
Silbiger N, Munguia P (2008) Carapace color change in Uca pugilator as a response to temperature. J Exp Mar Bio Ecol 355(1):41–46. https://doi.org/10.1016/j.jembe.2007.11.014
Silva C, Schwamborn R, Oliveira JL (2014) Population biology and color patterns of the blue land crab, Cardisoma guanhumi (Latreille 1828) (Crustacea: Gecarcinidae) in the Northeastern Brazil. Brazilian J Biol 74(4):949–958. https://doi.org/10.1590/1519-6984.01913
Sinervo B, Bleay C, Adamopoulou C (2001) Social causes of correlational selection and the resolution of a heritable throat color polymorphism in a lizard. Evolution (N Y) 55:2040–2052
Stevens M, Ruxton G (2012) Linking the evolution and form of warning coloration in nature. Proc R Soc B 279(November 2011):417–426. https://doi.org/10.1098/rspb.2011.1932
Stevens M, Párraga CA, Cuthill IC, Partridge JC, Troscianko TS (2007) Using digital photography to study animal coloration. Biol J Linn Soc 90(2):211–237
Stevens M, Lown AE, Wood LE (2014a) Camouflage and individual variation in shore crabs (Carcinus maenas) from different habitats. PLoS One 9(12):e115586. https://doi.org/10.1371/journal.pone.0115586
Stevens M, Lown AE, Wood LE (2014b) Color change and camouflage in juvenile shore crabs Carcinus maenas. Front Ecol Evol 2:1–14. https://doi.org/10.3389/fevo.2014.00014
Todd PA, Briers RA, Ladle RJ, Middleton F (2006) Phenotype-environment matching in the shore crab (Carcinus maenas). Mar Biol 148(6):1357–1367. https://doi.org/10.1007/s00227-005-0159-2
Troscianko J, Stevens M (2015) Image Calibration and Analysis Toolbox - a free software suite for objectively measuring reflectance, colour and pattern. Methods Ecol Evol 6(11):1320–1331
Troscianko J, Nokelainen O, Skelhorn J, Stevens M (2021) Variable crab camouflage patterns defeat search image formation. Commun Biol 4:287. https://doi.org/10.1038/s42003-021-01817-8
Underwood A (1997) Experiments in ecology: their logical design and interpretation using analysis of variance. Cambridge University Press, Cambridge
Vannini M, Berti R, Cannicci S, Innocenti G (2003) Cardisoma carnifex (Brachyura): where have all the babies gone? J Crustac Biol 23(1):55–59. https://doi.org/10.1163/20021975-99990316
Veitch CR, Clout MN, Martin AR, Russel JC, West CJ (2019) Island invasives: scaling up to meet the challenge. IUCN, International Union for Conservation of Nature, Gland (Switzerland)
Vermeij G, Dudley R (2000) Why are there so few evolutionary transitions between aquatic and terrestrial ecosystems? Biol J Linn Soc 70(4):541–554. https://doi.org/10.1006/bijl.1999.0415
Wallace A (1867) Mimicry and other protective resemblances among animals. Westminster Rev 1:1–43
Wilson D, Heinsohn R, Endler JA (2007) The adaptive significance of ontogenetic colour change in a tropical python. Biol Lett 3(1):40–43. https://doi.org/10.1098/rsbl.2006.0574
Zar JH (2010) Bioestatistical analysis. Prentice Hall, New Jersey
Acknowledgements
We are thankful to the Brazilian Navy (1st District), SECIRM (Inter-ministerial Secretariat for Marine Resources), and PROTRINDADE (Research Program of the Trindade Island) in the person of the commanders L. Felipe S. Santos and C. C. Vitória Regia, who guaranteed the presence of the authors in the Trindade Island and helped with the project logistic. The authors thank Isis Batistela, Nicholas Kriegler and Vanessa Martins for helping during the field sampling. We also thank Tim Caro and one anonymous referee for their helpful comments on the manuscript.
Funding
MCAJ and RCD thanks to “Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP” by the master (FAPESP 2019/16581–9) and postdoctoral (FAPESP 2019/01934–3; 2022/00946–0) fellowships, respectively. MAAP thanks “Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq” due to the financial support provided by “Oceanic Island Crabs” Project (CNPq no. 404224–2016), which guaranteed this study, as well as by the Research Productivity Fellowship granted to ASF and MAAP (CNPq no. 311994/2016–4 and no. 305957/2019–8, respectively).
Author information
Authors and Affiliations
Contributions
MCAJ, RCD, and MAAP conceived the idea and design the study. MCAJ sampled and photographed the crabs. MCAJ analyzed all the images and data. MCAJ wrote the first version of the manuscript with the supervision of RCD and MAAP. All authors contributed to the revisions and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
All samples were conducted following permission and rules of Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) and supported by Sistema de Autorização e Informação da Biodiversidade (SISBIO no. 65446).
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by T. Breithaupt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
João, M.C.A., Duarte, R.C., Freire, A.S. et al. Intraspecific color diversity and camouflage associated with ontogeny in an insular land crab. Behav Ecol Sociobiol 77, 120 (2023). https://doi.org/10.1007/s00265-023-03394-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-023-03394-8