Abstract
The underlying mechanisms connecting correlated behaviors in wild populations remain largely unknown. Food-caching behavior is a prime example of an adaptive, compulsive-like behavior with a strong underlying innate drive—it starts after early development and is critical for survival—and individuals of some species rigorously and continuously cache up to tens of thousands of individual food items each season. Another behavior whose base processes may share similar underlying innate drive is nest building, as it involves repeatedly bringing material to the nest site often in a fixed pattern. There are various hypotheses attempting to explain inter- and intra-specific variation in nest characteristics, traditionally considering fitness-related consequences of such variation. Apparent non-functional nest variation remains largely unexplored but may have an association with other innately driven behaviors unrelated to nest building but potentially associated via a shared mechanism, such as food caching. Here, we show that individual variation in food hoarding is associated with differences in nest size in mountain chickadees (Poecile gambeli): individuals that cache more food also build bigger nests. Both behaviors are highly repeatable within individual females, but variation in nest size does not seem to have fitness consequences in our system. This finding suggests a possible connection in which the properties of one adaptive behavior may spillover and influence the outcome of another more neutral behavior, likely controlled by the same general underlying mechanism.
Significance statement
Food storing and some aspects of nest-building behavior are highly repetitive actions that appear compulsive-like in nature. We show that these two behaviors are correlated in a wild avian food-caching species, the mountain chickadee: individual females in a wild population that cache more food also build larger nests. Both behaviors were highly repeatable within individual birds across time and climatic condition. Food storing is highly adaptive in chickadees, whereas nest size appears to have little effect on offspring fitness in this system. Therefore, our data suggest that these two compulsive-like behaviors may be controlled by the same general mechanism and the strong innate drive of adaptive food caching may spillover to potentially impact neutral nest building.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patterns of within-individual behavior that remain consistent across complex varying environments are of great interest to ecology and evolution (Sih et al. 2004a, b; Uher 2011). These suites of correlated behaviors, behavioral syndromes, are often composed of multiple repeatable behaviors that share a similar underlying drive (i.e., boldness; aggression) (Sih et al. 2004a, b; van Oers et al. 2004). There are a growing number of examples of such syndromes observed across wild systems (Sih et al. 2004a). Despite this, the underlying mechanisms and evolutionary implications of these behaviors remain largely unexplored (Sih et al. 2004a, b). A consequence of individuals exhibiting consistent behaviors across heterogeneous situations is that (i.e., bold or shy) individuals may experience context-dependent tradeoffs (Sih et al. 2004b; van Oers et al. 2004). How such tradeoffs are mediated on a mechanistic level is largely unknown (Sih et al. 2004a, b; van Oers et al. 2004). Additionally, whether one or more of the focus behaviors is under strong selective pressure may shape the interaction, both on a mechanistic and evolutionary scale, of the behaviors within a suite (Sih et al. 2004a, b).
One behavior that is critical to survival and repeated across years in highly variable contexts is food caching in non-human animals (Vander Wall 1990). Food caching appears to have evolved as an adaptation for sedentary species residing in relatively harsh and variable environments (Vander Wall 1990). Several avian species are well-known scatter-hoarders (e.g., Corvids, Parids, Picids), where individual birds may store tens or even hundreds of thousands of individual food items during late summer and throughout the fall for later consumption (Pravosudov 1985; Vander Wall 1990; Pravosudov and Roth 2013). Individuals rely on these caches when food is scarce for overwinter survival (Vander Wall 1990; Clayton 1992; Clayton and Dickinson 1999; Pravosudov and Clayton 2002; Pravosudov and Roth 2013; Sonnenberg et al. 2019) and in some cases (e.g., in Canada and Siberian jays) for reproduction (Derbyshire et al. 2019; Sutton et al. 2019). The underlying motivational drive of long-term caching behavior appears highly rigid and compulsive-like—individuals start caching soon after becoming independent (Pravosudov 2006) and will, during the appropriate season, continue caching available food until supplies are depleted (Pravosudov and Roth 2013). Some species (e.g., Parids, Corvids, and Picids) will even cache non-food items, such as stones, in captive conditions once food resources are depleted (Kilham 1963; Clayton 1992; Clayton et al. 1994; Bugnyar et al. 2007) or begin caching early in development before retrieval behavior initiates (Suddendorf and Busby 2003).
Caching drive varies across species, populations, and environments, with some species showing sensitivity to pilfering, resource type, presence of conspecifics, and body condition (Lucas et al. 1993; Clayton et al. 2005; de Kort et al. 2007; Pravosudov 2008). However, Parids appear to be especially driven to cache food and will cache every few minutes throughout the day over the entire duration of the long-term caching period (Pravosudov 1985, 2006), caching double the amount they need to consume to survive the winter (Pravosudov 1985). In addition, food-caching Parids, unlike some Corvids, do not reduce their caching rates in different social settings (i.e., when observed by potential pilferers) (Pravosudov 2008; Pravosudov et al. 2010) and actually increase their caching rates when caches were experimentally pilfered (Lucas et al. 2001). There is indirect evidence that baseline motivation to cache is heritable as individuals from harsher winter climates cache significantly more under controlled lab conditions compared to those from milder environments (Pravosudov and Clayton 2002; Freas et al. 2012), and these differences remained present when birds were hand-reared in identical captive environments (Roth et al. 2012). Altogether, these data strongly suggest that the baseline drive to cache can be described as compulsive. The result of such strong compulsive drive is the caching of much more than food than is necessary for survival (Pravosudov 1985) which is likely highly adaptive. Moreover, there is no evidence that this overcaching behavior constitutes long-term future planning in caching species (Suddendorf and Corballis 2007). Harsh winter environments may frequently create conditions when some cache locations may not be accessible (e.g., snow storms) or disappear entirely (e.g., tree falls, deep snow). These same conditions may cause short-term variation in caching intensity, but the overall drive likely remains. Creating many more caches than is needed can provide better assurance against risk of starvation—and in small-bodied birds, risk of starvation is high (Pravosudov 2006). Evolving strong, compulsive-like drive to cache appears to be an effective solution to this problem.
Small Parid species such as mountain chickadees (Poecile gambeli) are well-known for exhaustively caching available resources during the fall months when food items naturally used for caching (e.g., seeds of various pine species) are abundant (Haftorn 1992; Pravosudov, 1985; Clayton 1992; Croston et al. 2016). Birds will also visit supplementary feeders hundreds to thousands of times a day in addition to natural sources to retrieve items to cache (Croston et al., 2016). However, caching rates of naturally available food peak in the fall, regardless of supplementation (Pravosudov 2006). The sheer number of detected daily caches and the peak of caching, specifically during the periods of over-abundant food, suggests that caching drive is mostly independent from hunger state and may be more dependent on innate impulse, as it is impossible for these small birds to consume all these items in a 24-h period (Clayton 1992; Pravosudov 2006; Croston et al. 2016). Additionally, these food caches are generally consumed weeks and months after the date in which they were made, furthering this assumption (Vander Wall 1990; Pravosudov and Roth 2013). There is, however, no evidence that caches are recovered and used across years (Vander Wall 1990). Caches are a reliable food source and are retrieved in order to cope with uncertain climatic conditions over the winter months (Vander Wall 1990). Mountain chickadees residing in harsh environments, where winter conditions persist for longer periods and are more extreme, appear to require more food stores to survive compared to populations from milder areas (Pravosudov and Roth 2013; Croston et al. 2016; Sonnenberg et al. 2019). Chickadees from such environments have been shown to cache more both in the wild and in captive laboratory settings, reflecting this need (Freas et al. 2012; Croston et al. 2016).
Nest building has long been suspected of being controlled purely by innate drive and fixed genetic mechanisms (Mainwaring et al. 2014; Hall et al. 2015; Anholt 2020), though recent studies show the effect of experience in some species (Walsh et al. 2011, 2013; Breen et al. 2016; Camacho-Alpízar et al. 2021). Often, the process of nest construction requires the collection and subsequent incorporation of hundreds of items into a cohesive structure and could potentially be under the influence of a strong innate drive with genetic underpinnings, as it involves highly repetitive actions throughout the nest-building period (Mainwaring et al. 2014; Anholt 2020). In fact, nest building in mice has been shown to have a strong genetic basis, and inbred lines can be selected for excessive nest-building behavior, which is characterized in part by highly repetitive, compulsive-like material gathering (Greene-Schloesser et al. 2011; Mitra and Bult-Ito 2021). The size of a nest approximately reflects the total number of gathering trips. In some ways, collecting nest materials resembles food caching, as individuals make repeated trips to gather and store materials. There is a plethora of evidence suggesting that both intra- and inter-specific variation in nest structure and composition may also be related to local climate and experience (Britt and Deeming 2011; Crossman et al. 2011; Mainwaring et al. 2014; Camacho-Alpízar et al. 2021). However, some differences in nest structures appear to have little to no apparent fitness consequences, as demonstrated in mountain chickadees (Sonnenberg et al. 2020). For example, large differences in nest depth (which is a direct reflection of the overall nest size associated with the amount of material female birds brought to the nestbox) are not associated with reproductive outcome or local climate across years with drastically different conditions (Sonnenberg et al. 2020).
Such seemingly non-functional variation in nest size suggests a possible link between unrelated behaviors that could have a similar underlying innate mechanism of control in which highly adaptive behaviors may spill over into other fitness-neutral behaviors associated with the same mechanism. Different behaviors resulting from similar underlying mechanisms could directly influence each other, especially if one is under strong selection while the other is not. For example, strong selection for an increased food-caching drive may spillover to nest building and result in an explanation of nest size that remains unresolved by environmental variation during the breeding months (Sonnenberg et al. 2019, 2020). We addressed this question via the association between food-caching propensity and nest size within individuals at our long-term field system in the northern Sierra Nevada, USA (Kozlovsky et al. 2018).
Previously, we found that individual female mountain chickadees built consistently sized nests across years, but that the population showed large variation in nest size (as measured by nest depth in standard-sized nestboxes), both within and across elevations that was not explained by local climate and resulted in no detectable fitness differences (Sonnenberg et al. 2020). As such, we hypothesize that food-caching and nest-building behaviors might be associated through a partially shared innate mechanism involving compulsive-like behaviors. Here, we (a) investigated trait (caching propensity and nest size) repeatability and (b) compared winter food-caching propensity with nest size in females across years.
Methods
Subjects and site
All data for this study were collected 2016–2020 at Sagehen Experimental Forest (Sagehen Creek Field Station, University of California, Berkeley) in Sierra Nevada, USA, where we have studied individually marked (color bands and passive integrated transponder (PIT)-tags) mountain chickadees since 2014 (Kozlovsky et al. 2018) at two elevation sites, referred to as high (ca. 2400 m) and low (ca. 1900 m). It was not possible to record data blind because our study involved focal animals in the field.
Food-caching propensity
We used feeder visitation rates over the 4-day annual spatial cognitive testing task as a proxy for caching propensity (Croston et al. 2016). During such testing, PIT-tagged birds are expected to learn a location of a single rewarding feeder within an 8-feeder spatial array (Croston et al. 2016). The spatial array is equipped with modular microcontrollers connected to antennae built into the feeder perches that monitor all visitation (Croston et al. 2016). During testing, we counted the number of trials each bird completes over the 4-day task. A trial starts when a bird visits any feeder in the array and ends when it visits the correct rewarding feeder (Croston et al. 2016). Chickadees obtain one seed per trial and then fly away from the array with the seed to consume or cache it; as such, one trial is equal to one seed collected from the feeder array. Chickadees complete hundreds of trials during the 4 days of testing; therefore, the number of trials is a good representation of food-caching propensity as a chickadee needs only a small fraction of these seeds to fulfill its metabolic requirements and most of the collected seeds are likely cached (Pravosudov 1985; Croston et al. 2016). A small portion of observed differences in visitation may be related to necessary food consumption; however, such differences appear to be relatively minor at this scale. Therefore, we used the total number of trials completed during the 4-day task as a proxy for caching propensity. Data across 5 years of testing was used for this study and was collected during winter months (2015-2016: November 30, 2016-2017: March 8th, 2017–2018: March 30, 2018–2019: April 8 and 2019–2020: January 20).
Nest measurements
Approximately 350 identically sized nest boxes (Sonnenberg et al. 2020) were monitored from April to July during 3 years of the study (2018–2020) across both elevations. Monitoring included checking each nest box for the status of nest construction, egg number, and hatch date during the nest-building, egg-laying, incubation, and post-hatch periods. Nest boxes were checked approximately one to two times a week. We used nest depth to estimate nest size, as it captures the approximate material amount deposited in each box, by measuring the depth at each of the four corners of the nest box with a metric ruler and then computing the mean of those values (Sonnenberg et al. 2020). Females are the sole nest builders in this species and so nest measurements reflect an individual female’s behavior. Mountain chickadees rarely lay two clutches of eggs in a year and so only first of the season nests were measured and included in analyses. All nests were measured after the onset of incubation which marks the end of nest building in mountain chickadees (Sonnenberg et al. 2020).
Statistical analyses
All data were analyzed using R statistical software (R v.4.0.3, R Core Team 2020). For all analyses, we combined females from high and low elevations as we were specifically interested in individual rather than elevation level variation of these behaviors and their potential associations.
Repeatable behaviors
The repeatability of both nest depth and caching propensity in individual females was calculated using the R package “rptR” (Stoffel et al. 2017). Repeatability scores were calculated using a Gaussian distribution and parametric bootstrapping (nboot = 1500) to obtain 95% confidence intervals for each estimate. Repeatability estimates were calculated for females that participated in at least 2 years of the study. A total of 83 females were included in the analysis with 52 females detected in two nesting years and 31 females detected in all 3 years. Of these females, a total of 15 participated in spatial cognitive testing in more than 1 year (sampled across 2 years: n = 10, 3 years: n = 5). A second repeatability estimate for caching propensity was calculated for the entire participating population of chickadees across 5 years of comparable study. A total of 183 individuals participated in two or more years of testing (sampled across 2 years: n = 109, 3 years n = 47, 4 years = 12, 5 years = 10).
Nest building and caching
To determine the relationship between nest depth and food-caching propensity, mean nest depth was used as the dependent variable and mean total number of trials completed over the 4-day cognitive task served as the independent variable in a simple linear regression. There was a total of 57 females included in the model and the metrics of females that were detected in more than 1 year were averaged across years (1 year: n = 42, 2 years: n = 10, 3 years: n = 5). As 74% of all included females only had data for both caching and nest size for a single year, we averaged the data across the years for the remaining 26% as a more complex model seemed inappropriate (Silk et al. 2020). The data met all assumptions for the linear regression and images were created using ggplot2 (Wickham 2016; Zuur and Ieno 2016).
Results
Repeatability of nest size and food caching
Nest depth was significantly repeatable across 3 years in females (n = 83, R = 0.629, SE = 0.064, CI = [0.494, 0.736], p < 0.001, Fig. 1). These results were consistent with our previously published estimates (Sonnenberg et al. 2020: (R = 0.58; CI: 0.36, 0.73; p < 0.001)).
Food-caching propensity, measured by the total number of trials completed during the 4-day cognitive task, was also significantly repeatable within individual females across years (n = 15, R = 0.541, SE = 0.177, CI = [0.135, 0.83], p = 0.006, Fig. 2). Caching propensity was also found to have significant repeatability for the entire tested population across 5 years (n = 183, R = 0.303, SE = 0.055, CI = [0.196, 0.413], p < 0.001, Fig. 3).
Nest size and food caching
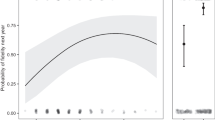
The mean nest depth was significantly and positively associated with food-caching propensity across the 3 years of study (n = 57, beta = 0.35, F1,55 = 7.76, adjusted R2 = 0.11, p = 0.007, Fig. 4).
Discussion
Overall, our data support the hypothesis that food caching and some aspects of nest-building propensity are positively associated in mountain chickadees. Females exhibited high repeatability in both nest size and food-caching propensity across 3 years, despite marked differences in local climate between years (Kozlovsky et al. 2018; Sonnenberg et al. 2020). Furthermore, females that cached more food tended to build bigger nests (e.g., brought more nest materials).
Our findings suggest that variation in nest size may be linked to differences in food-caching motivation, likely as mechanistic spillover from a highly adaptive behavior to a potentially neutral one. We previously reported that variation in nest size was not associated with differences in female breeding experience, local climate, or reproductive success, but was decidedly consistent across years within individual females (Sonnenberg et al. 2020). If there is a similar underlying mechanism relating caching and some aspects of nest building, the behavior currently under stronger selective pressure (e.g., caching) may be influencing the outcome of the other (e.g., nest size). In fact, there may be other nesting characteristics more important to offspring fitness that are unrelated to nest size, allowing nest size to be influenced by another trait. For example, nest site selection or increased female attentiveness while on the nest may be more significant to offspring fitness than nest size in obligate secondary cavity-nesting species (Bouvier et al. 2014; Sofaer et al. 2020; Sonnenberg et al. 2020). Thus, nest size has the potential of being indirectly modified by another behavior that is closely related through a shared mechanism without causing negative fitness consequences. In fact, the majority of evidence showing local climate’s impact on nest structure in closely related taxa concerned non-caching species that may lack this underlying mechanistic connection to caching (Britt and Deeming 2011; Deeming et al. 2012). An alternative explanation is that increased food-caching propensity may be associated with improved individual condition prior to the breeding period, which may result in such individuals being more able to construct larger nests. We do not think this is a viable explanation for the findings presented here as we previously showed that large individual variation in nest size across different years was not associated with annual climatic variation and lacked clear fitness consequences (Sonnenberg et al. 2020). This result held true for both experienced females and first year breeding females (Sonnenberg et al. 2020).
The idea of spillover between seemingly unrelated behaviors is not uncommon (Sih et al. 2004b; Johnson and Sih 2005). Behavioral syndromes have been hypothesized to be genetically correlated which can imply a similar mechanism of control (Sih et al. 2004a, b; Bell et al. 2009). Highly repetitive behaviors, such as food caching, can be described as compulsive-like as they are rigidly repeated, ritualistic, and often exceed the maximum fitness requirements. Such behaviors have an overabundance of underlying innate drive that may be decoupled from daily motivational state. This high level of baseline motivational drive may be a byproduct of selection, as for some traits, the repetitive nature of the behavior is highly beneficial to survival. If two behaviors (i.e., nest building and caching) share a mechanistic connection, the high baseline drive may spillover from one behavior into the other. There are numerous other compulsive-like behaviors exhibited in wild systems that incur fitness benefits (Eilam et al. 2006). For example, territory maintenance in a number of mammal species, in which they systematically retrace territory boundaries and repeatedly mark specific locations, comes with fitness benefits, including increased mate choice and decreased predation risk (Eilam et al. 2006). These repeated, ritualistic behaviors could easily be described as compulsive and benefit the performer through the maintenance of a large territory for foraging, mate attraction, and reproduction (Eilam et al. 2006). It also links two behaviors, locomotion and scent marking, which appear quite different mechanistically (Eilam et al. 2006). However, there are examples of how compulsive spillover can lead to less adaptive outcomes. For example, the aggressive spillover hypothesis proposed in several arachnid species provides an explanation as to why female spiders may kill and eat potential mates despite costly fitness consequences (Johnson 2013). Apparent compulsive female aggression overwhelms courtship behavior, resulting in either driving the male away or consuming the male before copulation can take place (Johnson 2013).
Our results also provide an excellent provocation for future work into the underlying mechanisms relating correlated behaviors. For example, one would assume that if the multiple behaviors in a suite are mechanistically related, then that could affect the outcome of one by directly manipulating the other. This may not be the case for this particular example as these behaviors are temporally separated (aka: caching takes place in the fall and winter months, where nest building takes place in the spring) and altering caching behavior by manipulating the immediate environment (e.g., physical or social environment) may not directly impact the shared mechanism controlling the overall caching motivation. However, it may be possible in future to precisely manipulate the underlying baseline drive, by either increasing or decreasing the innate motivational state. As this study only provides the very preliminary description of this syndrome, future experimental work of this nature would be required in order to actually tease apart the causal connection between nest building and caching.
Overall, we provide novel data linking two behaviors with different functions, food caching and nest building. These data suggest that one adaptive compulsive behavior, food caching, has the potential to affect, or spillover, into other unrelated behaviors. It remains possible that the association between food-caching and nest-building propensities found in our study are simply due to chance; however, this finding warrants more investigation. More data are needed to test if other disparate behaviors may be similarly associated with food-caching drive. Additionally, our results may provide a means to investigate the mechanistic underpinnings of detrimental compulsive behaviors in humans, such as obsessive–compulsive disorder, using food-caching species as a model.
Data availability
All data analyzed for this study are included in this article and its corresponding supplementary materials.
References
Anholt RRH (2020) Evolution of epistatic networks and the genetic basis of innate behaviors. Trends Genet 36:24–29
Bell AM, Hankison SJ, Laskowski KL (2009) The repeatability of behaviour: a meta-analysis. Anim Behav 77:771–783
Bouvier JC, Muller I, Génard M, Lescourret F, Lavigne C (2014) Nest-site and landscape characteristics affect the distribution of breeding pairs of European rollers Coracias garullus in an agricultural area of southeastern France. Acta Ornithol 49:23
Breen AJ, Guillette LM, Healy SD (2016) What can nest-building birds teach us? Compar Cogn Behav Rev 11:83–102
Britt J, Deeming DC (2011) First-egg date and air temperature affect nest construction in Blue Tits Cyanistes caeruleus, but not in great tits Parus major. Bird Study 58:78–89
Bugnyar T, Stöwe M, Heinrich B (2007) The ontogeny of caching in ravens, Corvus corax. Anim Behav 74:757–767
Camacho-Alpízar A, Eckersley T, Lambert CT, Balasubramanian G, Guillette LM (2021) If it ain’t broke don’t fix it: breeding success affects nest-building decisions. Behav Process 184:104336.
Clayton NS (1992) The ontogeny of food-storing and retrieval in marsh tits. Behaviour 122:11–25
Clayton NS, Dickinson A (1999) Motivational control of caching behaviour in the scrub jay, Aphelocoma coerulescens. Anim Behav 57:435–444
Clayton NS, Griffiths D, Bennett ATD (1994) Storage of stones by jays Garrulus glandarius. Ibis 136:331–334
Clayton NS, Dally J, Gilbert J, Dickinson A (2005) Food caching by western scrub-jays (Aphelocoma californica) is sensitive to the conditions at recovery. J Exp Psychol Anim B 31:115–124
Crossman CA, Rohwer VG, Martin PR (2011) Variation in the structure of bird nests between northern Manitoba and southeastern Ontario. PLoS ONE 6:e19086.
Croston R, Kozlovsky DY, Branch CL, Parchman TL, Bridge ES, Pravosudov VV (2016) Individual variation in spatial memory performance in wild mountain chickadees from different elevations. Anim Behav 111:225–234
Dall SRX, Bell AM, Bolnick DI, Ratnieks FLW (2012) An evolutionary ecology of individual differences. Ecol Lett 15:1189–1198
de Kort SR, Correia SPC, Alexis DM, Dickinson A, Clayton NS (2007) The control of food-caching behavior by western scrub-jays (Aphelocoma californica). J Exp Psychol Anim B 33:361–370
Deeming DC, Mainwaring MC, Hartley IR, Reynolds SJ (2012) Local temperature and not latitude determines the design of blue tit and great tit nests. Avian Biol Res 5:203–208
Derbyshire R, Norris DR, Hobson KA, Strickland D (2019) Isotopic spiking and food dye experiments provide evidence that nestling Canada Jays (Perisoreus canadensis) receive cached food from their parents. Can J Zool 97:368–375
Eilam D, Zor R, Szechtman H, Hermesh H (2006) Rituals, stereotypy and compulsive behavior in animals and humans. Neurosci Biobehav R 30:456–471
Finley J, Ireton D, Schleidt WM, Thompson TA (1983) A new look at the features of mallard courtship displays. Anim Behav 31:348–354
Freas CA, LaDage LD, Roth TC, Pravosudov VV (2012) Elevation-related differences in memory and the hippocampus in mountain chickadees, Poecile gambeli. Anim Behav 84:121–127
Fresneau N, Kluen E, Brommer JE (2014) A sex-specific behavioral syndrome in a wild passerine. Behav Ecol 25:359–367
Greene-Schloesser DM, Van der Zee EA, Sheppard DK, Castillo MR, Gregg KA, Burrow T, Foltz H, Slater M, Bult-Ito A (2011) Predictive validity of a non-induced mouse model of compulsive-like behavior. Behav Brain Res 221:55–62
Guillette LM, Healy SD (2015) Nest building, the forgotten behaviour. Curr Opin Behav Sci 6:90–96
Haftorn S (1992) Ontogeny of food storing in titmice Parus spp. Ibis 134:69–71
Hall ZJ, Meddle SL, Healy SD (2015) From neurons to nests: nest-building behaviour as a model in behavioural and comparative neuroscience. J Ornithol 156:133–143
Henschel JR, Skinner JD (1991) Territorial behaviour by a clan of spotted hyaenas Crocuta crocuta. Ethology 88:223–235
Johnson JC (2013) Debates: challenging a recent challenge to the aggressive spillover hypothesis. Ethology 119:811–813
Johnson JC, Sih A (2005) Precopulatory sexual cannibalism in fishing spiders (Dolomedes triton): a role for behavioral syndromes. Behav Ecol Sociobiol 58:390–396
Kilham L (1963) Food storing of red-bellied woodpeckers. Wilson Bull 75:227–234
Kozlovsky DY, Branch CL, Pitera AM, Pravosudov VV (2018) Fluctuations in annual climatic extremes are associated with reproductive variation in resident mountain chickadees. R Soc Open Sci 5:171604.
Lee CT (1973) Genetic analyses of nest-building behavior in laboratory mice (Mus musculus). Behav Genet 3:247–256
Lucas JR, Peterson LJ, Boudinier RL (1993) The effects of time constraints and changes in body mass and satiation on the simultaneous expression of caching and diet-choice decisions. Anim Behav 45:639–658
Lucas JR, Pravosudov VV, Zielinski DL (2001) A reevaluation of the logic of pilferage effects, predation risk, and environmental variability on avian energy regulation: the critical role of time budgets. Behav Ecol 12:246–260
Mainwaring MC, Hartley IR, Lambrechts MM, Deeming DC (2014) The design and function of birds’ nests. Ecol Evol 4:3909–3928
Mitra S, Bult-Ito A (2021) Bidirectional behavioral selection in mice: a novel pre-clinical approach to examining compulsivity. Front Psychiatry 12:716619.
Pravosudov VV (1985) Search for and storage off food by Parus cinctus lapponicus and P. borealis (Paridae). Zool Zh 64:1036–1043
Pravosudov VV (2006) On seasonality in food-storing behaviour in parids: do we know the whole story? Anim Behav 6:1455–1460
Pravosudov VV (2008) Mountain chickadees discriminate between potential cache pilferers and non-pilferers. Proc R Soc Lond B 275:55–61
Pravosudov VV, Clayton NS (2002) A test of the adaptive specialization hypothesis: population differences in caching, memory, and the hippocampus in black-capped chickadees (Poecile atricapilla). Behav Neurol 116:515–522
Pravosudov VV, Roth TC II (2013) Cognitive ecology of food hoarding: the evolution of spatial memory and the hippocampus. Annu Rev Ecol Evol S 44:173–193
Pravosudov VV, Roth TC, LaDage LD (2010) Chickadees are selfish group members when it comes to food caching. Anim Behav 80(2):175–180
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org
Roth TC, LaDage LD, Pravosudov VV (2010) Learning capabilities enhanced in harsh environments: a common garden approach. Proc R Soc Lond B 277:3187–3193
Roth TC, LaDage LD, Freas CA, Pravosudov VV (2012) Variation in memory and the hippocampus across populations from different climates: a common garden approach. Proc R Soc Lond B 279:402–410
Sih A, Bell A, Johnson JC (2004a) Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol 19:372–378
Sih A, Bell A, Johnson J, Ziemba R (2004b) Behavioral syndromes: an integrative overview. Q Rev Biol 79:241–277
Silk MJ, Harrison XA, Hodgson DJ (2020) Perils and pitfalls of mixed-effects regression models in biology. PeerJ 8:e9522.
Sofaer HR, Nagle L, Sillett TS, Yoon J, Ghalambor CK (2020) The importance of nighttime length to latitudinal variation in avian incubation attentiveness. J Avian Biol 2020:e02319
Sonnenberg BR, Branch CL, Pitera AM, Bridge E, Pravosudov VV (2019) Natural selection and spatial cognition in wild food-caching mountain chickadees. Curr Biol 29:670–676
Sonnenberg BR, Branch CL, Benedict LM, Pitera AM, Pravosudov VV (2020) Nest construction, ambient temperature and reproductive success in a cavity-nesting bird. Anim Behav 165:43–58
Stoffel MA, Nakagawa S, Schielzeth H (2017) RptR: Repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol Evol 8:1639–1644
Suddendorf T, Busby J (2003) Mental time travel in animals? Trends Cogn Sci 7:391–396
Suddendorf T, Corballis MC (2007) The evolution of foresight: what is mental time travel, and is it unique to humans? Behav Brain Sci 30:299–313
Sutton AO, Strickland D, Freeman NE et al (2019) Autumn freeze-thaw events carry over to depress late-winter reproductive performance in Canada jays. Royal Society Open Science 6:181754
Szechtman H, Ahmari SE, Beninger RJ, Eilam D, Harvey BH, Edemann-Callesen H, Winter C (2017) Obsessive-compulsive disorder: insights from animal models. Neurosci Biobehav R 76:254–279
Uher J (2011) Individual behavioral phenotypes: an integrative meta-theoretical framework. why “behavioral syndromes” are not analogs of “personality.” Dev Psychobiol 53:521–548
van Oers K, Drent PJ, de Goede P, van Noordwijk AJ (2004) Realized heritability and repeatability of risk-taking behaviour in relation to avian personalities. Proc R Soc Lond B 271:65–73
Vander Wall SB (1990) Food hoarding in animals. University of Chicago Press, Chicago
Walsh PT, Hansell M, Borello WD, Healy SD (2010) Repeatability of nest morphology in African weaver birds. Biol Lett 6:149–151
Walsh PT, Hansell M, Borello WD, Healy SD (2011) Individuality in nest building: do Southern masked weaver (Ploceus velatus) males vary in their nest-building behaviour? Behav Process 88:1–6
Walsh PT, Hansell M, Borello WD, Healy SD (2013) Are elaborate bird nests built using simple rules? Avian Biol Res 6:157–162
Whitehouse CM, Lewis MH (2015) Repetitive behavior in neurodevelopmental disorders: clinical and translational Findings. Behav Anal 38:163–178
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-Verlag, New York.
Wolf M, Weissing FJ (2012) Animal personalities: consequences for ecology and evolution. Trends Ecol Evol 27:452–461
Zuur AF, Ieno EN (2016) A protocol for conducting and presenting results of regression-type analyses. Methods Ecol Evol 7:636–645
Acknowledgements
Thanks to Hannah Lansverk who was involved in data collection and generously provided insightful revisions to the manuscript. Comments by two anonymous reviewers significantly improved the MS.
Funding
Research presented in this article was supported by the National Science Foundation (NSF) via grants IOS1856181 and IOS 2119824t o VVP.
National Science Foundation,IOS1856181,Vladimir V. Pravosudov,National Science Foundation,IOS2119824,Vladimir V. Pravosudov
Author information
Authors and Affiliations
Contributions
BRS and VVP developed the ideas. BRS, CMB, AMP, LMP, and VKH participated in data collection. VKH, AMP, and VVP processed RFID data from cognitive testing; BRS analyzed the data. All authors co-wrote the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This research has followed all the guidelines provided by the Institutional Animal Care and Use Committee of the University of Nevada, Reno (Protocol No. 00603), as well as any federal guidelines (California Department of Fish and Wildlife Permit D-0011776516–4).
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by N. Clayton
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sonnenberg, B.R., Branch, C.L., Pitera, A.M. et al. Food-hoarding and nest-building propensities are associated in a cavity-nesting bird. Behav Ecol Sociobiol 76, 14 (2022). https://doi.org/10.1007/s00265-021-03114-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-021-03114-0