Abstract
Size-assortative mating is a pattern of non-random pairing among individuals that has been presumed to arise due to the enhanced reproductive success that may accrue from mating with an individual of similar size. Its proximal mechanism may be female choice for similarly sized mates and/or large-male advantage during bouts of direct male-male competition. The hallmark for the occurrence of size-assortative mating is a significant correlation between female and male body sizes in mated pairs. In this study, we investigated the mating pattern of the emerald glass frog, Espadarana prosoblepon, whose mating system is purportedly based on female choice and therefore is a suitable study system for testing hypotheses of size-assortative mating. We specifically tested whether E. prosoblepon males found in amplexus were larger than solitary males, indicating a large-male advantage in mating and whether either larger males or size-matched pairs of frogs had a higher proportion of their eggs fertilized, consistent with a benefit to size-assortative mating. We found no evidence for any of these relationships in E. prosoblepon despite a positive correlation between female size and clutch size. Males in amplexus were not larger than unmated males, and male size did not predict the proportion of fertilized eggs. Our evidence thus indicates that the mating pattern of E. prosoblepon is random with respect to body size, in conformity with a growing body of evidence that body size is likely not a significant factor influencing mating patterns in anurans.
Significance statement
Size-assortative mating occurs when similarly sized individuals mate together more often than expected by chance. Studies addressing this question test for a correlation between female and male sizes within mating pairs, but few studies test whether mating with similarly sized individuals provides proximate benefits. Size-assortative mating should occur in species where mate choice is possible to occur, in which individuals can discriminate and choose to mate with similarly sized individuals. In frogs, many previous studies have searched for size-assortative mating, but evidence for its occurrence or selective advantage remains scant. We used the emerald glass frog, Espadarana prosoblepon, as a study system to test the predictions of size-assortative mating. We found that both mating preference and fertilization success are random with respect to body size.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In sexually reproducing animals, mating patterns may reflect preferences for phenotypic traits, such as age, body size, body condition, or coloration, that may correlate with increased individual reproductive success (Crespi 1989; Andersson 1994; Shine et al. 2001). If individuals choose mates that more closely resemble themselves on the basis of these traits, they are said to exhibit assortative mating. Assortative mating patterns have been reported for many different taxa (Andersson et al. 1998; Harari et al. 1999; Reynolds and Fitzpatrick 2007), with almost half of these being reports of assortative mating based on body size (Jiang et al. 2013). This size-assortative mating, hereafter referred to as “SAM,” thus specifically refers to a mating pattern in which individuals mate preferentially with similarly sized individuals (Arak 1983; Arnqvist et al 1996).
SAM should occur when the choosy sex in a species (often the female) demonstrably prefers to mate with similarly sized individuals, which requires that the individual is able to discriminate and choose among available mates based on size (Green 2019). Consequently, true SAM should only occur in species with mating systems with demonstrable mate choice. Surprisingly, few studies arguing the existence of SAM have actually demonstrated these principles (Green 2019).
SAM is, however, not the only way a correlation between the sizes of males and females in mated pairs may be generated. In cases where larger males gain access to females by keeping smaller males away (Crespi 1989), intrasexual competition provides an advantage to larger individuals during mating acquisition. As a result, larger males may be able to mate with females of any size, whereas smaller males may only be able to retain and mate with smaller females, if they are able to mate at all. In these cases, assortative mating is merely “apparent” (Arnqvist et al. 1996), rather than true.
Frogs are particularly attractive subjects for studies of SAM (Green 2019) and have many traits that have been thought to lead to its occurrence. Many species of frogs have prolonged breeding seasons during which males call to attract receptive females, allowing ample opportunity for female mate choice (Wells 1977). A positive relationship between female body size and number of eggs is well documented in frogs (Han and Fu 2013; Nali et al. 2014), leading to the idea that males might increase their reproductive success if they were to mate with larger females. Reciprocally, it has been thought that females could achieve a selective advantage if they mate with larger males, assuming that testis size, and thus greater sperm production capacity, enables larger males to fertilize a greater proportion of the eggs within a single clutch (Kusano et al. 1991; Jennions and Passmore 1993; Emerson 1997). Alternatively, because females are larger than males in most frog species (Nali et al. 2014), it has also been suggested that there may be an optimal female/male size ratio for maximum fertilization of eggs (Davies and Halliday 1977; Bourne 1993) and that the inverse relationship between male size and the dominant frequency of his advertisement call (Ryan 1988; Gerhardt 1994) may be a trait that allows females to detect the body sizes of available males (Robertson 1990; reviewed in Ryan and Keddy-Hector 1992). Finally, there have been numerous suggestions that female size-based mate choice coupled with male-male competition could generate a considerable large-male advantage, resulting in greater mating success by larger males (Arak 1983; Howard and Kluge 1985; Márquez-M de Orense and Tejedo-Madueño, 1990; Sullivan et al. 1995; Wells 2007; Liao and Lu 2011).
However, the evidence that any of these mechanisms, either alone or in combination, indeed does result in SAM remains scant to non-existent. There is, as yet, no evidence that the ratio of male to female size has any influence on fertilization success (Briggs 2008; Fan et al. 2013; Chajma and Vojar 2016; Dittrich et al. 2018). Similarly, evidence for female preferences for larger males based on the dominant frequency of their call is sparse compared to studies showing female preferences for traits such as call rate or call duration (Wells 2007). Evidence for a positive association between testis size and the amount of sperm produced is limited to only a few anuran families (Byrne et al. 2002; Lüpold et al. 2020). Thus, the expectation that SAM should arise because large size may confer increased reproductive success is assumed, but seldom tested. It is not sufficient to test for a correlation between male and female size among mated pairs without also examining whether or not amplectant pairs more similar in size actually have higher reproductive success. SAM requires not only that larger individuals, of either sex, be most desirable as mates, but that smaller individuals should also be desirable as mates by other small individuals.
The emerald glass frog, Espadarana prosoblepon (Anura: Centrolenidae), is an arboreal species found in Central and South America from Honduras to western Ecuador (Basto-Riascos et al. 2017). Males call at night from vegetation surrounding small streams and initiate amplexus by jumping onto a female’s back should she approach. Pairs remain in amplexus for up to 5 h (Jacobson 1985) before depositing their eggs either on leaves overhanging streams or in patches of moss (Jacobson 1985; Basto-Riascos et al. 2017; Ortiz-Ross et al. 2020). Using amplectant pairs of E. prosoblepon found in the wild, we tested for evidence of SAM or any of its postulated predictions. Accordingly, we investigated (1) whether female choice yields a pattern of SAM via a demonstrable preference for mating with males of similar size, (2) whether there is large-male advantage in these frogs that would yield a pattern of size-disproportionate mating (Green 2019), in which males found in amplexus would be larger than males that failed to obtain a mate, and (3) whether fertilization success depended upon either male size or the relative sizes of females vs. males in mated pairs.
Materials and methods
Our study took place at Las Cruces Biological Station in San Vito de Coto Brus, Puntarenas Province, Costa Rica (8° 47′ 04.07″ N, 82° 57′ 36.55″ W, 1200 m.a.s.l.). The mean annual rainfall in this area is ~ 4000 mm, with a pronounced dry season between the months of January and March, and a rainy season beginning towards the end of May until November. The study site encompassed an area of approximately 500 m2 adjacent to a 60 m segment of a small stream (“Culvert Creek”). The vegetation within the study area consisted largely of Heliconia plants, gingers, giant ferns, and palms that served as calling and perch sites for E. prosoblepon.
During the 2018 and 2019 onset of the rainy seasons (June–July), we conducted nightly visual encounter surveys for frogs starting at 2000 h. Males are found calling on top of leaves or other vegetation along streams or water-holding ditches. Most males in this population are found within 2 m from each other with an average nearest neighbor distance of 2.36 ± 2.13 m (range = 0.1–7.9 m, median = 1.35 m). In some particular locations, five or more males are found within 1 m from each other (this study), and they may move actively (> 1 m) throughout the night (Jacobson 1985). Males reliably stop vocalizing after midnight, after which no additional pairs in amplexus can be found (JGV pers. obs.), enabling us to easily distinguish between successful males in amplexus and unsuccessful males that remained solitary (males who stayed at their calling sites). To avoid re-sampling of individuals, all frogs, including males and females found in amplexus as well as solitary males, were marked before release. Solitary males were measured and marked immediately upon capture. We investigated whether there is large-male advantage in E. prosoblepon in June and July 2018, by comparing the snout-vent length (SVL) of males found in amplexus with SVLs of solitary males using a t-test. In 2018, frogs were given a unique 2-digit toe-clip number, but we abandoned this method in 2019 and instead marked the frogs with visible implant alpha tags (Northwest Marine Technology, Inc.). All individuals were returned to their point of capture within 24 h.
Once a pair in amplexus was located, we captured it using a small plastic bag and immediately transferred it to a semi-natural enclosure (Goyes Vallejos and Ramirez-Soto 2020) within our study site. The enclosure consisted of a steel frame with subdivisions of 38 × 50 × 75 cm in size (four total) surrounded by plastic mesh. Each subdivision was provided with leaf litter at the bottom, a water bowl, and a fern hung from a bamboo post located at the corner of the enclosure to provide a perch and oviposition substrate. The bamboo post doubled as hiding refuge. Mated pairs remained in the enclosure overnight until after oviposition. The following night, we measured SVL of the frogs in each pair to the nearest mm using calipers and counted the total number of eggs in each clutch. It was not possible to record data blind because our study involved focal animals in the field.
To determine whether E. prosoblepon exhibited size-assortative mating, we evaluated the relationship between male and female SVLs in mated pairs using Pearson’s product moment correlation. To avoid Simpson’s paradox in our analysis, which may result from pooling non-equivalent data sets (see Green 2019), we first tested for significant differences in male and female SVLs between years.
To determine fertilization success, in 2019, we cut the fern leaflet holding the egg clutch within 24 h after oviposition and taped it onto a 5 × 7 cm plastic card. Each plastic card was taped vertically to the inside of a funnel that was sealed at the bottom and partially filled with stream water to capture newly hatched tadpoles. We calculated fertilization success as the number of tadpoles produced divided by the total number of eggs in the clutch. Embryos not reaching Gosner stage 14 (Gosner 1960) 3–4 days after oviposition were considered inviable.
We investigated SVL in relation to clutch size and fertilization success. First we tested for a relationship between female SVL and clutch size using a linear regression with number of eggs as the response variable. We then tested whether either male SVL or female/male size ratio was correlated with the number of eggs fertilized per clutch using Pearson’s product moment correlation. Additionally, after inspecting the data for overdispersion, we used two generalized linear models with a logit link function and a quasibinomial. The explanatory variable in the first model was male SVL, and female/male size ratio in the second model, with fertilization success as the response variable for both models.
We performed all statistical analysis using R 2.6.2 (R Development Core Team 2019). All tests for normality, including inspection of residual plots, were met, and alpha was set at 0.05. All averages were recorded to two decimal points and one standard deviation.
Results
We captured and measured a total of 68 pairs of E. prosoblepon in amplexus, 13 in 2018 and 55 in 2019. We found no significant differences in SVL between years of either females (mean = 26.03 mm in 2018, mean = 25.66 mm in 2019, t15.72 = 0.87, P = 0.39) or males (mean = 23.69 mm in 2018, mean = 23.30 mm in 2019, t88.5 = 1.41, P = 0.16), justifying our pooling of the amplexus pair data from 2018 and 2019 for analysis.
SVLs of 29 solitary males we captured in 2018 averaged 23.75 ± 1.24 mm (range = 20.72–26.09 mm), whereas SVLs of 13 males caught in amplexus that same year averaged 23.56 ± 1.58 mm (range: 21.59–27.27). We found no significant difference in body size between the solitary males and the males found in amplexus (t18.89 = 0.38, P = 0.70; Fig. 1).
Box-and-whisker plots of SVL (mm) of male Espadarana prosoblepon in 2018, comparing 13 males found in amplexus versus and 29 “solitary” males. Boxes indicate the median, first, and third quartiles of the distribution of SVLs, and whiskers indicate the minimum and maximum values. Outliers are portrayed as dots
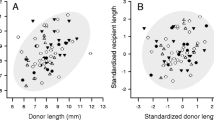
We did not find any significant correlation between female and male SVL of amplectant pairs captured in 2018 (r = 0.10, P = 0.75, n = 13), 2019 (r = 0.12, P = 0.38, n = 55), or both years combined (r = 0.12, P = 0.31, n = 68; Fig. 2a). Among all amplectant pairs, female SVL averaged 25.73 ± 1.19 mm (range = 22.70–28.93 mm), whereas male SVL averaged 23.35 ± 1.38 mm (range: 20.10–27.88). Females within a pair were more often larger than their mates; female SVL/male SVL ratios among mated pairs ranged from 0.88 to 1.26, for an overall mean female SVL/male SVL ratio of 1.10 (P < 0.001; Fig. 2b).
Comparisons of female versus male body size (measured as snout-vent length) in Espadarana prosoblepon. a Pearson’s product moment correlation and linear regression between female and male SVL. b Histogram showing the frequency distribution of E. prosoblepon size ratios (female SVL/male SVL). Light gray bars indicate pairs in amplexus in which males were bigger than the females, and dark gray bars indicate pairs in which females were larger than males. The dashed line indicates a 1:1 size ratio
Clutch size averaged 25.15 ± 5.26 eggs (range = 14–41 eggs, n = 54). We found a significant, positive relationship between female size and number of eggs laid (F2,51 = 2.501, P = 0.03; Fig. 3). Fertilization success ranged between 7 and 100% (mean = 88.1%, SD = 21%, n = 37). We did not find a significant effect of male body size on the proportion of eggs fertilized per clutch (P = 0.63; Fig. 4). The correlation between female SVL/male SVL ratio and fertilization success was not significant (r = − 0.04, P = 0.82). Moreover, our model did not detect a significant effect of size ratio on the proportion of fertilized eggs, suggesting that closely size matched pairs do not have higher fertilization success (P = 0.50, Fig. 5).
Discussion
We found no evidence that body size has any effect on any aspect of mating or reproduction in E. prosoblepon aside from a significant size-dependent female fecundity relationship. Larger males did not mate with larger females, smaller males did not mate with smaller females, and there was no evidence for a large-male advantage in mating. Overall, pairs encountered naturally in amplexus within our study site were no more similar in size than expected by chance, and pairs with a size ratio closer to one did not produce clutches with higher fertilization success. Hence, the mating pattern of E. prosoblepon in our study population seems to be completely random with respect to body size, with potentially all sexually mature males having an equal probability of mating.
In general, large-male advantage resulting in size-disproportionate mating has been associated with male-male physical competition for mate retention (Green 2019) and has been observed in a few arboreal tropical frogs with reproductive habits similar to E. prosoblepon (Bastos and Haddad 1996; Briggs 2008). However, although physical combat between male E. prosoblepon has been reported, these male-male contests are rare (Jacobson 1985), and we observed no instances of males attempting to displace conspecifics already in amplexus. This could explain, in part, why males in amplexus were no larger than solitary males. Males of E. prosoblepon do defend territories (Savage 2002), but once a male clasps a female, it is the female who chooses the oviposition site, which may be several meters away from the male’s territory (Jacobson 1985). Thus, any opportunity for another male competitor to displace a male already in amplexus may be negligible.
With male-male competition for mate retention unlikely in E. prosoblepon, mate choice should be a strictly female prerogative. However, the lack of any significant correlation between female size and male size in amplectant pairs in E. prosoblepon indicates that females do not exercise any choice of mate on the basis of body size. Nor do they need to, as all males can successfully fertilize a clutch, irrespective of their size. Furthermore, as pairs more similar in size do not have higher fertilization rates, and thus, females do not receive proximate benefits from mating with similarly sized males, there appears to be no selective advantage in doing so, despite previous suggestions that there should be (Bourne 1993; Lu et al. 2016). Numerous studies have now also refuted the idea that there is an optimal size ratio for mated pairs of frogs that correlates with higher fertilization rates in the wild (e.g., Dickerson et al. 2004; Friedl and Klump 2005; Fan et al. 2013; Mobley et al. 2014; Chajma and Vojar 2016; Székely et al. 2018). Therefore, the expectation that a significant correlation between male and female size in amplexus yields fitness benefits in the form of higher reproductive success is misleading.
Although male frogs, in general, exhibit an inverse correlation between body size and the dominant frequency of their advertisement call (Gingras et al. 2013; Tonini et al. 2020), the relationship may not necessarily be very exact at an individual level. Among individual males of E. prosoblepon, a relationship between size and dominant frequency is not evident (Jacobson 1985), and thus, females may be unable to determine a male’s body size based on his call. In some populations of E. prosoblepon, as in many other species of frogs (Wells 2007), male mating success is correlated with longer chorus tenure, which is not correlated with male body size (Basto-Riascos et al. 2017). In reality, males with longer chorus tenure may be more successful due to the overall reproductive effort (number of nights calling) instead of female choice. Therefore, male size appears to be inconsequential for mate choice in E. prosoblepon. In other species of glass frogs, females prefer males already attending clutches (Valencia-Aguilar et al. 2020), but egg attendance behavior in E. prosoblepon is not only short (< 1 day) but performed exclusively by females (Jacobson 1985; JGV and ADH-F unpubl. data). We have observed females repeatedly mating with the same male over 2–3 weeks, and males exhibited high site fidelity. Thus, as has been seen in the strawberry poison frog, Oophaga pumilio (Meuche et al. 2013), it is possible that the mating system of E. prosoblepon is defined simply by mate proximity rather than female choice.
Our results are consistent with numerous recent studies that find little evidence for SAM in anuran amphibians (e.g., Friedl and Klump 2005; Wogel et al. 2005; Green 2015; Zhang et al. 2020). SAM can only arise if individuals can discriminate and choose mates of similar size and can only be perpetuated if this choice results in higher reproductive success for both sexes. For example, this situation exists in the paternally mouth-brooding fish, Sphaeramia nematoptera, where females prefer to mate with males capable of successfully mouth-brooding the totality of their offspring, and males prefer to mate with females possessing a capacity to produce more eggs (Rueger et al. 2016). However, significant, credible correlations between female and male body sizes in mated pairs are very rare among frogs (Green 2019), and most such reports are for explosive breeders in which male-male competition is intense, and there is little opportunity for female choice. The idea that SAM arises where the largest individuals in a population are assumed to be the most attractive as mates fails to consider that assortment—should it occur—must involve the entire range of body sizes within the population. Correlations between female and male body sizes, even if significant, should not be over-interpreted and should be accompanied by empirical tests evaluating the proximate benefits for both sexes.
Our results indicating that E. prosoblepon in a montane forest in Costa Rica exhibits random pairing with regard to body size does not exclude the possibility that other factors may be involved. Ecological context is an inescapable adjunct to the process of pair formation in animals and may add considerable complexity to mating patterns overall. Coloration, call characteristics, parental care, and the intensity of competition have all been found to influence mate selection in certain anuran amphibians (Pettitt et al., 2020; Yang and Richards-Zawacki 2021). It is also possible for mate selection criteria to be dynamic and shift in importance depending on conditions. For example, mating preferences based on coloration may be overwhelmed by intense male-male competition in the poison dart frog Oophaga pumilio (Yang and Richards-Zawacki 2021). Similarly, mate choice may change on temporal and/or spatial scales, as seen in some species of anurans with prolonged breeding seasons (Ng et al. 2016; Moura et al. 2021). Site or mate fidelity can also delimit pair formation in different animal systems (Rueger et al. 2018).
Thus, precisely how mate selection takes place in E. prosoblepon remains largely unknown. Male-male competition has been observed on rare occasions in E. prosoblepon, but we have not observed displacement attempts by males even at relatively high densities. Therefore, a role for male-male competition in mate acquisition in E. prosoblepon appears to be minor, but whether or not it can dictate non-random mating patterns remains unknown. We have observed females of E. prosoblepon mating with the same male after the first observation (JGV and ADH-F unpubl. data). However, differentiating site preference from mate preference among females is a difficult proposition requiring experimental manipulation and, therefore, what direct or indirect benefits are derived from such preferences are, so far, not readily discernable. Nevertheless, however it is that females of E. prosoblepon go about selecting mates, body size does not appear to be a factor. Therefore, it remains for additional natural history studies on other aspects of the reproductive behavior of this species to infer its mating system and mate choice patterns.
Availability of data and material
The datasets generated and/or analyzed during the current study are available within the article and its supplementary materials.
Code availability
Not applicable.
References
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Andersson S, Örnborg J, Andersson M (1998) Ultraviolet sexual dimorphism and assortative mating in blue tits. Proc R Soc Lond B 265:445–450
Arak A (1983) Male-male competition and mate choice in anuran amphibians. In: Bateson P (ed) Mate choice. Cambridge University Press, Cambridge, pp 181–210
Arnqvist G, Rowe L, Krupa JJ, Sih A (1996) Assortative mating by size: a meta-analysis of mating patterns in water striders. Evol Ecol 10:265–284
Basto-Riascos MC, López-Caro J, Vargas-Salinas F (2017) Reproductive ecology of the glass frog Espadarana prosoblepon (Anura: Centrolenidae) in an urban forest of the Central Andes of Colombia. J Nat Hist 51:2535–2550
Bastos RP, Haddad CFB (1996) Breeding activity of the neotropical treefrog Hyla elegans (Anura, Hylidae). J Herpetol 30:355–360
Bourne GR (1993) Proximate costs and benefits of mate acquisition at leks of the frog Ololygon rubra. Anim Behav 45:1051–1059
Briggs VS (2008) Mating patterns of red-eyed treefrogs, Agalychnis callidryas and A. moreletii. Ethology 114:489–498
Byrne PG, Roberts JD, Simmons LW (2002) Sperm competition selects for increased testes mass in Australian frogs. J Evol Biol 15:347–355
Chajma P, Vojar J (2016) The effect of size-assortative mating on fertilization success of the common toad (Bufo bufo). Amphibia-Reptilia 37:389–395
Crespi BJ (1989) Causes of assortative mating in arthropods. Anim Behav 38:980–1000
Davies NB, Halliday TR (1977) Optimal mate selection in the toad Bufo Bufo. Nature 269:56–58
Dickerson BR, Willson MF, Bentzen P, Quinn TP (2004) Size-assortative mating in salmonids: negative evidence for pink salmon in natural conditions. Anim Behav 68:381–385
Dittrich C, Rodríguez A, Segev O, Drakulić S, Feldhaar H, Vences M, Rödel MO (2018) Temporal migration patterns and mating tactics influence size-assortative mating in Rana temporaria. Behav Ecol 29:418–428
Emerson SB (1997) Testis size variation in frogs: testing the alternatives. Behav Ecol Sociobiol 41:227–235
Fan XL, Lin ZH, Ji X (2013) Male size does not correlate with fertilization success in two bufonid toads that show size-assortative mating. Curr Zool 59:740–746
Friedl TW, Klump GM (2005) Sexual selection in the lek-breeding European treefrog: body size, chorus attendance, random mating and good genes. Anim Behav 70:1141–1154
Gerhardt HC (1994) The evolution of vocalization in frogs and toads. Annu Rev Ecol Evol S 25:293–324
Gingras B, Boeckle M, Herbst CT, Fitch WT (2013) Call acoustics reflect body size across four clades of anurans. J Zool 289:143–150
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Goyes Vallejos J, Ramirez-Soto K (2020) Causes of embryonic mortality in Espadarana prosoblepon (Anura: Centrolenidae) from Costa Rica. Phyllomedusa 19:83–92
Green DM (2015) Implications of female body-size variation for the reproductive ecology of an anuran amphibian. Ethol Ecol Evol 27:173–184
Green DM (2019) Rarity of size-assortative mating in animals: assessing the evidence with anuran amphibians. Am Nat 193:279–295
Han X, Fu J (2013) Does life history shape sexual size dimorphism in anurans? A comparative analysis. BMC Evol Biol 13:1–11
Harari AR, Handler AM, Landolt PJ (1999) Size-assortative mating, male choice and female choice in the curculionid beetle Diaprepes abbreviatus. Anim Behav 58:1191–1200
Howard RD, Kluge AG (1985) Proximate mechanisms of sexual selection in wood frogs. Evolution 39:260–277
Jacobson SK (1985) Reproductive behavior and male mating success in two species of glass frogs (Centrolenidae). Herpetologica 41:396–404
Jennions MD, Passmore NI (1993) Sperm competition in frogs: testis size and a ‘sterile male’ experiment on Chiromantis xerampelina (Rhacophoridae). Biol J Linn Soc 50:211–220
Jiang Y, Bolnick DI, Kirkpatrick M (2013) Assortative mating in animals. Am Nat 181:E125–E138
Kusano T, Toda M, Fukuyama K (1991) Testes size and breeding systems in Japanese anurans with special reference to large testes in the treefrog, Rhacophorus arboreus (Amphibia: Rhacophoridae). Behav Ecol Sociobiol 29:27–31
Liao WB, Lu X (2011) Proximate mechanisms leading to large male-mating advantage in the Andrew’s toad, Bufo andrewsi. Behaviour 148:1087–1102
Lu X, Ma X, Fan L, Hu Y, Lang Z, Li Z, Fang B, Guo W (2016) Reproductive ecology of a Tibetan frog Nanorana parkeri (Anura: Ranidae). J Nat Hist 50:2769–2782
Lüpold S, de Boer RA, Evans JP, Tomkins JL, Fitzpatrick JL (2020) How sperm competition shapes the evolution of testes and sperm: a meta-analysis. Phil Trans R Soc B 375:20200064
Márquez-M de Orense R, Tejedo-Madueño M (1990) Size-based mating pattern in the tree frog Hyla arborea. Herpetologica 46:176–182
Meuche I, Brusa O, Linsenmair KE, Keller A, Pröhl H (2013) Only distance matters–non-choosy females in a poison frog population. Front Zool 10:29
Mobley KB, Abou Chakra M, Jones AG (2014) No evidence for size-assortative mating in the wild despite mutual mate choice in sex-role-reversed pipefishes. Ecol Evol 4:67–78
Moura RR, Gonzaga MO, Pinto SN, Vasconcellos-Neto J, Requena GS (2021) Assortative mating in space and time: patterns and biases. Ecol Lett 24:1089–1102. https://doi.org/10.1111/ele.13690
Nali RC, Zamudio KR, Haddad CFB, Prado CPA (2014) Size-dependent selective mechanisms on males and females and the evolution of sexual size dimorphism in frogs. Am Nat 184:727–740
Ng TP, Williams GA, Davies MS, Stafford R, Rolán-Alvarez E (2016) Sampling scale can cause bias in positive assortative mating estimates: evidence from two intertidal snails. Biol J Linn Soc 119:414–419
Ortiz-Ross X, Thompson ME, Salicetti-Nelson E, Donnelly MA (2020) Oviposition site selection in three glass frog species. Copeia 108:333–340
Pettitt BA, Bourne GR, Bee MA (2020) Females prefer the calls of better fathers in a Neotropical frog with biparental care. Behav Ecol 31:152–163
R Development Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org
Reynolds RG, Fitzpatrick BM (2007) Assortative mating in poison-dart frogs based on an ecologically important trait. Evolution 61:2253–2259
Robertson JGM (1990) Female choice increases fertilization success in the Australian frog, Uperoleia laevigata. Anim Behav 39:639–645
Rueger T, Gardiner NM, Jones GP (2016) Size matters: male and female mate choice leads to size-assortative pairing in a coral reef cardinalfish. Behav Ecol 27:1585–1591
Rueger T, Gardiner NM, Jones GP (2018) Site fidelity facilitates pair formation in aggregations of coral reef cardinalfish. Oecologia 186:425–434
Ryan MJ (1988) Constraints and patterns in the evolution of anuran acoustic communication. In: Fritzsch B, Hethington T, Ryan M, Wilczynski W, Walkowiak W (eds) The Evolution of the Amphibian Auditory System. John Wiley, New York, pp 37–677
Ryan MJ, Keddy-Hector A (1992) Directional patterns of female mate choice and the role of sensory biases. Am Nat 139:S4–S35
Savage JM (2002) The amphibians and reptiles of Costa Rica: a herpetofauna between two continents, between two seas. University of Chicago Press, Chicago
Shine R, O’connor D, LeMaster MP, Mason RT (2001) Pick on someone your own size: ontogenetic shifts in mate choice by male garter snakes result in size-assortative mating. Anim Behav 61:1133–1141
Sullivan BK, Ryan MJ, Verrell PA (1995) Female choice and mating system structure. In: H. Heatwole and B. K. Sullivan (eds) Amphibian biology. Surrey Beatty, Baulkham Hills, Australia, pp 469–517
Székely D, Székely P, Denoël M, Cogălniceanu D (2018) Random size-assortative mating despite size-dependent fecundity in a Neotropical amphibian with explosive reproduction. Ethology 124:218–226
Tonini JFR, Provete DB, Maciel NM, Morais AR, Goutte S, Toledo LF, Pyron RA (2020) Allometric escape from acoustic constraints is rare for frog calls. Ecol Evol 10:3686–3695
Valencia-Aguilar A, Zamudio KR, Haddad CFB, Bogdanowicz SM, Prado CPA (2020) Show me you care: female mate choice based on egg attendance rather than male or territorial traits. Behav Ecol 31:1054–1064
Wells KD (1977) The social behaviour of anuran amphibians. Anim Behav 25:666–693
Wells, KD (2007) The ecology and behavior of amphibians. University of Chicago Press, Chicago
Wogel H, Abrunhosa PA, Pombal JP (2005) Breeding behaviour and mating success of Phyllomedusa rohdei (Anura, Hylidae) in south-eastern Brazil. J Nat Hist 39:2035–2045
Yang Y, Richards-Zawacki CL (2021) Male–male contest limits the expression of assortative mate preferences in a polymorphic poison frog. Behav Ecol 32:151–158
Zhang L, Sheng Y, Yuan X, Yu F, Zhong X, Liao J, Liu Z, Chen W (2020) Proximate mechanisms responsible for random mating by size in the Himalayan toad Duttaphrynus himalayanus. Anim Biol 71:183–195
Acknowledgements
We thank Darko Cotoras, Rodolfo Quiros Flores, and Scott Walter for their invaluable support (logistic and otherwise). We thank Karim Ramirez-Soto for his help during fieldwork. We thank two anonymous reviewers whose comments greatly improved our manuscript.
Funding
Funding for this study was provided by the US National Science Foundation (grant number HRD-1712757) and the Organization for Tropical Studies which supported JGV, JG, ADHF, and RV during the NSF Louis Stokes Alliances for Minority Participation Research Experience for Undergraduate Program in 2018 and 2019 at Las Cruces Biological Station, Costa Rica.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This research was conducted under the Ministerio del Ambiente y Energía de Costa Rica (MINAE) and Sistema Nacional de Areas de Conservación (SINAC) Scientific Research Permit Number R-SINAC-PNI-ACLAP-031–2018/2019 and followed the guidelines of the Animal Behavior Society (ABS) for the treatment of animals in behavioral research.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by K. Summers.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Goyes Vallejos, J., Gomez, J., Hernández-Figueroa, A.D. et al. Fertilization success suggests random pairing in frogs with regard to body size. Behav Ecol Sociobiol 75, 140 (2021). https://doi.org/10.1007/s00265-021-03081-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-021-03081-6