Abstract
Previous research has shown that competition, familiarity, diet, and relatedness can all influence aggregation patterns in garter snakes. We controlled for these factors and examined social aggregation patterns in juvenile Eastern garter snakes (Thamnophis sirtalis sirtalis). We assayed snakes individually for consistent individual differences in boldness and sociability. We then placed snakes in groups of 10 in an enclosure with four shelters and observed their social interactions over a period of 8 days. We demonstrate that the snakes actively seek social interaction, prefer to remain with larger aggregates, and associate nonrandomly with specific individuals or groups. We show that their social interaction patterns are influenced by individual boldness, sociability, and age. The snakes’ social networks were perturbed twice a day by “shuffling” their locations. Despite these disturbances, the snakes eventually re-formed their preferred social environment. Aggregation and exploration patterns also varied across time, with most activity occurring later in the day. These results highlight the complexity of snake sociality and may have important implications for conservation efforts.
Significance statement
Snakes are often considered nonsocial animals, but this is inaccurate. We used social network analyses to compare juvenile Eastern garter snakes’ group behaviors to their individual personalities. Our research demonstrates that these snakes actively seek out social interaction and prefer to join and remain with larger groups and that their social interaction patterns are influenced by consistent individual differences in boldness and sociability. Our work contributes to a sparse but growing body of literature on sociability in reptiles. This work is important for changing perceptions among the scientific community and the public as a whole. As a consequence, it may aid efforts in developing appropriate conservation techniques that consider sociability patterns in relocated reptiles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although it is recognized that almost all animal species must interact socially at various times, the majority of research on sociality has focused on a small percentage of animal species (Bonnet et al. 2002; Pawar 2003). Fortunately, this taxonomic bias has started to shift, with researchers examining social cognition in a wider array of taxa (Hatchwell 2010; Wilkinson et al. 2010; Edsinger and Dölen 2018). Reptiles are often considered asocial and, as such, have often been ignored in studies of social cognition (Doody et al. 2013). This bias is exacerbated by the fact that in some reptile species, social interactions are hidden, due to their secretive nature, and that social communication is often conducted via invisible chemical cues. These hidden social interactions have been termed “Cryptic Sociality” (Hatchwell 2010; Clark et al. 2012), and though they are more difficult to study, they provide an important piece of the evolutionary puzzle for understanding sociality across taxa (Burghardt 1977; Clark et al. 2012; Doody et al. 2013).
Among reptiles, snakes are often considered the least social (Doody et al. 2013). However, research on sociality in snakes has uncovered parental care in pit vipers (Greene et al. 2002) and African pythons (Python natalensis; Alexander 2018), coordinated hunting in Cuban boas (Chilabothrus angulifer; Dinets 2017), social grouping in sea snakes (Emydocephalus annulatus; Shine et al. 2005), and deception in red-sided garter snakes (Thamnophis sirtalis parietalis; Mason and Crews 1985). Additionally, research on snake aggregation has revealed complex patterns of conspecific recognition (Burghardt 1983; Yeager and Burghardt 1991; Clark et al. 2012).
Snakes additionally form aggregations (see Graves and Duvall 1995 for a review), most commonly for mating (Luiselli 1996; Shine et al. 2003) and, especially in colder climates, for hibernation (Carpenter 1953; Larsen et al. 1993). Other evidence suggests that certain snake species aggregate for the purpose of giving birth (Reichenbach 1983), and some snakes may use communal shedding grounds (Loughran et al. 2015). Research on snake aggregation has also been able to tease apart specific aggregation patterns. For example, fieldwork on aggregations of timber rattlesnakes (Crotalus horridus) within den sites demonstrated kin recognition (Clark et al. 2012), and social network analysis of aggregations of Arizona black rattlesnakes (Crotalus cerberus) demonstrated nonrandom association patterns, including more interactions than would be expected by chance in male, female, and juvenile snakes, as well as selective associations with preferred conspecifics (Schuett et al. 2017). Studies of aggregation patterns in the lab have demonstrated that snakes are capable of conspecific recognition (Burghardt 1983; Graves and Halpern 1988), as well as examining the particular information they may use for determining where, and with whom, they should aggregate (Heller and Halpern 1982; Lyman-Henley and Burghart 1994).
Instances of accidental aggregation, resulting from mutual attraction to resource-rich locations, are likely under some conditions (Gregory 2004). However, it seems more likely that many social interactions are the result of aggregation benefits. Grouping has numerous possible benefits (Krause and Ruxton 2002), and for snakes, these benefits include thermoregulation (Graves and Duvall 1987), protection from water loss (Nobel and Clausen 1936), and protection from predation (Graves and Duvall 1995). On the other hand, aggregation has some unique costs for snakes: snakes cannot share food and, among communally housed neonate and juvenile snakes, food fights can be both common and deadly (Yeager and Burghardt 1991). Furthermore, predators may be attracted to aggregations of snakes (Shine et al. 2001), and heat kleptoparasitism from other male snakes is a purported strategy used by she-male red-sided garter snakes (T. sirtalis parietalis) to warm up after emerging from hibernation (Shine et al. 2012).
We hypothesized that in a laboratory setting, in which the environment was held constant and resources such as food and water were plentiful, juvenile Eastern garter snakes (Thamnophis sirtalis sirtalis) would nonetheless actively seek out social interaction. Additionally, like other strategies that have both costs and benefits, we expected to see individual variability in exploiting of the safety of the group (Jolles et al. 2017): since aggregating may require temporarily leaving the safety of a shelter to seek a group to join, some snakes may choose to avoid the risk and remain in their current shelter.
Eastern garter snakes inhabit a wide geographic area ranging from Canada to the Southern United States (Rossman et al. 1996). As a result of this extensive range, there is large variability in their behavioral patterns. For example, northern populations communally hibernate for many months while southern populations have much longer active periods and, in some cases, may not hibernate at all (Rossman et al. 1996). As a result of communal hibernation, northern populations of Eastern garter snakes interact socially in the fall when they return to their hibernation sites and again in the spring when they emerge from hibernation to mate. Additionally, pregnant females have been found to aggregate in the summer at birthing sites (Reichenbach 1983; Rossman et al. 1996). Eastern garter snakes will also follow the scent of conspecifics (a behavior known as trailing; Heller and Halpern 1981). Unlike many species that derive benefits in both safety and foraging from social interactions (Krause and Ruxton 2002), garter snakes often aggregate at times of vulnerability but cannot share food and therefore hunt and eat independently (Lyman-Henley and Burghardt 1994; Rossman et al. 1996).
Building on earlier work that examined aggregation in snakes (Dundee and Miller 1968; Heller and Halpern 1982; Burghardt 1983), we placed groups of 10 snakes in an arena that contained four shelters and tracked their movements and social interactions. In this group context, we used social networking statistics to quantify the snakes’ social interactions, and additionally, we measured their boldness in the group environment. We also assayed each snake independently for consistent individual differences (sometimes called “animal personality”). We tested for sociability, and—based on Mayer et al.’s (2016) procedure using keelback snake hatchlings (Tropidonophis mairii)—we also tested their boldness. As such, we had both group- and individual-context measures of sociability and boldness. To our knowledge, few tests of individual differences in sociability have been performed on snakes. However, propensity to approach a shelter that smells like a conspecific has been used to assess sociability in a species of lizard (Lacerta vivipara; Cote and Clobert 2007). Based on this work, we used likelihood to approach conspecific odor to assess sociability in our snakes. We based our sociability test paradigm on an experiment by Graves and Halpern (1988), which was designed to determine if garter snakes could detect the difference between conspecific and heterospecific skin lipids. Individual assay results were compared to behavior in the group experiment.
Methods
Subjects and housing
Three gravid female Eastern garter snakes were collected from field sites in Ontario, Canada, in July 2017. These snakes were transported to the laboratory where they were housed individually and gave birth shortly after arriving in the lab. Our sample consisted of 30 snakes that were born to these wild-caught mothers and 10 additional snakes of approximately the same age (from a single litter) purchased from a local breeder. The total sample size was 40 juvenile snakes.
As both familiarity and kinship can influence social patterns (Hoss et al. 2015), we primarily tested snakes in familiar groups of siblings (hereafter referred to as batches or Batch 1, 2, 3, and 4). As such, snakes were communally housed in their testing groups, which were also sibling groups. Although all snakes were communally housed in their sibling groups to begin with, it was necessary to divide the older batches as they outgrew their living quarters (e.g., Batches 3 and 4 were split into two tanks of five snakes each). As a result of communal housing, all the snakes in Batches 1, 3, and 4 were familiar with each other at the time of testing. Due to uneven litter sizes, Batch 2 was an exception to this pattern. The snakes from Batch 2 were of mixed parentage and were housed in two separate groups. Eight of the snakes were housed in one aquarium. This aquarium contained seven siblings and one non-sibling that was accidentally placed in the tank before the snakes were individually marked. The other two snakes, siblings from a different mother, were housed separately. Therefore, in Batch 2, 2 of the 10 snakes were both unrelated and unfamiliar to the majority of their batch mates at the time of testing.
Throughout the time the snakes were housed in our facility, they had access to belly heat (30 °C) provided by heat tape (THGHeat) placed under their aquariums. The housing room was maintained at 22 °C, with a 12-h light cycle from 7 am to 7 pm. Shelters were placed on both the warm and cool sides of the aquariums, and snakes were fed a mixed diet of chopped night-crawlers (Pagonis Live Bait, Toronto) and defrosted chopped cod fillets (Great Value) twice a week, with vitamin supplements (Zilla) provided as necessary. Clean water was provided daily. All snakes were sexed at the end of the experiment, with the exception of two snakes that died.
Apparatus
The aggregation experiment was conducted in a 73.7-cm2 tabletop enclosure made of 35.6-cm-high polyethylene walls (Fig. S1; all supplementary tables and figures are found in Online Resource 1), which was placed in the snakes’ housing room. The center of the enclosure was covered with 12 sandstone squares (GlassCoasterStore.com). Four black plastic reptile shelters (14 cm × 10.2 cm × 5 cm, Cornel’s World) were placed, one along the center of each wall. The snakes were tested in four batches of 10 snakes. Due to the large size of the snakes in Batch 4, it was necessary to increase the shelter size for this batch (to 15 cm × 14 cm × 6 cm) in order to allow comparable-sized aggregations to form. White paper towels were placed underneath each shelter for ease of cleaning. Small plastic water dishes were placed in each corner of the enclosure. For the batches with larger snakes, small triangles of plastic mesh were taped to the four top corners of the enclosure to prevent the snakes from escaping. A DSLR camera (Canon EOS Rebel T5i) was mounted above the enclosure such that the entire testing arena was visible. The camera was connected to a laptop which recorded a 5184 × 3456 pixel image of the testing arena every 5 s for the 12 h of light on each day of the experiment (from 7 am to 7 pm). Pictures were sufficiently detailed such that individual snake markings (described below) could be identified in each image.
The individual boldness assays were conducted in a 54.6-cm2 testing arena made from 24-cm-high PVC boards, with a clear sheet of acrylic placed on top to prevent escapes (Fig. S2A). A black plastic reptile shelter (the same kind used in the aggregation experiment) was placed against the center of one wall. Trials were video recorded using a camcorder (Panasonic HC-V700) mounted above the arena such that the entire testing arena was visible.
The arena for individual sociability assays consisted of a Styrofoam box (40.6 cm × 45.7 cm × 33 cm) with two black plastic reptile shelters (of the same kind as used in the aggregation experiment) placed at 45° angles in the far corners opposite the start location (Fig. S2B). A clear sheet of acrylic was used to cover the arena. Trials were recorded using a camcorder (Panasonic HC-V700) mounted above the arena such that the entire arena was visible. Snakes were placed into the arena through a small door cut out of the wall opposite the shelters, which was covered after the snake entered the arena. To create a social stimulus, we extracted skin lipids from shed juvenile snake skins based on a process described by Graves and Halpern (1988; see Online Resource 1). The skin lipid mixture was produced from pooled snake sheds taken from both male and female snakes. For each sociability assay, 10 drops of the lipid solution were placed on a piece of 4-μm filter paper which was then placed at the entrance to one of the shelters, designated the social shelter. As a control, 10 drops of dichloromethane were placed on a second piece of filter paper, placed under the entrance of the other shelter, designated the Control Shelter. The sides of the Social and Control Shelters were pseudo-randomized across individuals.
Procedure
Aggregation experiment
Snakes were tested in the aggregation experiment in four batches of 10 individuals each. Each snake was a member of only one batch and was only tested once. Batch 1 consisted of 10 siblings with identified parentage, purchased from a local breeder. The remaining three batches were born in the lab to wild-caught female snakes (adult female B, adult female C, and adult female D). Batch 2 consisted of two snakes born from adult female B, seven snakes born from adult female C, and one snake born from adult female D. Batch 3 consisted of 10 siblings born from adult female C, and Batch 4 consisted of 10 siblings born from adult female D. The paternity of these three batches was not known. The snake demographics of each batch were as follows: Batch 1: five males, three females, two unsexed, age at testing = 6 months; Batch 2: five males, five females, age at testing ≈ 7 months; Batch 3: four males, six females, age at testing = 8 months; and Batch 4: five males, five females, age at testing = 9 months.
Before starting the experiments, nontoxic nail polish (Adrianne K) was used to individually mark each snake with one, two, or three colored dots on their head. Dot patterns were unique within each batch of 10 snakes. As dot patterns were lost during shedding, it was necessary to monitor the snakes closely during the testing period to ensure that dot pattern identities remained consistent. Snakes were re-marked after each shedding. Monitoring the snake identities involved checking the snakes every day, recording any defining features of the snakes, and weighing the snakes each week. For the duration of each batch’s testing, the combination of these practices allowed us to reliably maintain the identities of each snake. Subjects remained in the aggregation experiment enclosure for 8 days. In order to ensure that each batch experienced 8 full days and to allow for a period of habituation, snakes were placed into the enclosure the day before the experiment began and removed on the morning of day 9. Subjects were weighed before entering the experiment and again upon its completion. Due to a camera malfunction, data from parts of day 6 and day 7 were lost for some batches, and therefore, these days were excluded from analysis.
To control for the effects of differences in diet on aggregation patterns (Lyman-Henley and Burghardt 1994), snakes were fed an all-worm diet beginning at least 1 week prior to the start of the experiment. Feedings also occurred on days 3 and 7 of the experiment, at which time the snakes were removed from the enclosure and individually fed chopped worms. This was done to mitigate the effects of food competition on aggregation patterns (Yeager and Burghardt 1991). During their removal for feeding, the experimental apparatus was cleaned with 70% isopropyl alcohol and then washed and dried thoroughly. After the apparatus was cleaned, the shelters, paper towels, and water dishes were replaced.
On each full day of the experiment, twice a day, an experimenter would briefly raise each shelter and record the locations of the snakes using a handheld video camera. Additionally, after recording locations, all the snakes were removed from the enclosure, the shelters and paper towels were replaced, the water was changed, and the areas around the shelters were cleaned with 70% isopropyl alcohol and then washed and dried thoroughly. The snakes’ positions were then shuffled. To shuffle the snakes, we either released them all in the center of the enclosure (Central) or placed them in particular shelters based on four different predetermined release patterns (Forced; see Table S1). For Central releases, we placed the snakes into a container and then simultaneously released them into the center of the arena. For the Forced release, we positioned each snake’s head at the entrance of the predetermined shelter and allowed it to slither in. Snakes readily entered the shelters, although on occasion they left before the rest of the snakes were placed into their respective shelters. These predetermined release patterns were designed to test if snakes had a preferred group size. We hypothesized that the snakes would demonstrate group stability in dense release patterns and transitivity in dispersed release patterns. However, these release patterns had no effect on grouping patterns and, as such, were not considered in our analysis.
Individual behavioral assays
Individual behavioral assays for boldness and sociability were conducted following the aggregation experiment. Snakes were tested three times on each assay, and each trial lasted 20 min. Snakes were not tested on days when they were fed, and each snake completed their assays within a single week. For each batch, the time between their individual behavioral assays and their aggregation assay varied, but all testing (individual and group) occurred within a 1- to 3-month period. Due to camera malfunctions, data for five trials were lost (four individual boldness trials and one individual social trial). Additionally, two snakes died after they had completed the aggregation experiment but before they could complete their individual assays. Snakes with missing trial data were excluded from all analyses that included data from these assays.
For the boldness assay, snakes were initially placed into the shelter in the same way as in the aggregation experiment. We recorded the proportion of the session that the snake spent out of the shelter. For the sociability assay, snakes were gently placed into the test arena through the entrance hole. We recorded the proportion of time the snake spent in or on top of the shelter containing conspecific odor as a proportion of total time spent in or on both shelters.
Statistical analysis
We used mixed effect models to account for the repeated measures nature of our analysis. For all statistical tests that considered batches, we used mixed effect linear models or generalized linear mixed effect models with a random intercept for Subject nested in Batch (analysis 1, henceforth referred to as “Method A1”) or with Batch as a random slope and Subject as a random intercept (analysis 2, henceforth referred to as “Method A2”). When the time of interest was not on the scale of days (for example comparing mornings to afternoons), we added Testing Day as a random intercept (analysis 3, henceforth referred to as “Method A3”). To compare exploration rates to those expected by chance, we used Pearson’s chi-squared test (analysis 4, henceforth referred to as “Method A4”; see also Supplementary Materials). We also estimated the repeatability of individual differences in sociability and boldness. To do this, we used mixed effect linear models and examined the consistency of the variance explained by subject (a random intercept) across time/trials (analysis 5, henceforth referred to as “Method A5”). This was done using the r package rptR (Stoffel et al. 2017). To confirm our repeatability findings, we also used Kendall’s test of concordance to examine whether or not the snakes had similar ranks in their social preferences across testing (analysis 6, henceforth referred to as “Method A6”). When analyzing data from individual tests (in which there were no groups), we used repeated measures linear (or general linear) models (analysis 7, henceforth referred to as “Method A7”).
Due to the nature of our measures, the distributions of the variables were rarely normal. As such, we used transformations when it resulted in normalizing the residuals in our models. We used arcsine transformations for proportional variables, and for all other variables, we used the Tukey ladder of powers to find a transformation with the closest approximation to normality (Tukey 1977). In situations in which it was necessary to compare distributions, we used two-sample Kolmogorov-Smirnov tests (KS test) to determine if the distributions were sampled from the same population (Sprent and Smeeton 2001; analysis 8, henceforth referred to as “Method A8”).
In the aggregation experiment, we only sampled points in which a snake made a choice (moved from one zone to another). When appropriate, we based our analyses on these individual choices (e.g., how long a snake stayed in a shelter after transitioning into the shelter). Along with asking questions about these individual choices, we also used social network statistics to quantify behavior in the group experiment. We used a variety of social network measures to describe the snakes’ grouping patterns, including transitivity, closeness centrality, network degree, weighted degree, association preference, and homophily between familiar subgroups of siblings in Batch 2. Each of these measures is described in the “Results” section. In our data, we had complete knowledge of all snakes’ locations (at all times) and therefore used a simple-ratio index to quantify associations for our analysis.
Analysis
In the aggregation experiment, positions of all snakes were manually coded from the DSLR images each time they moved from one zone of the enclosure to another. Each of the four shelters and the area around each of the four water dishes were designated as a zone; separate zones were also defined for the area around each shelter and the central portion of the enclosure (Fig. S1). Each time a snake’s head completely crossed a zone boundary, we recorded the frame number, identity of the snake, and the new zone it occupied using a custom ethologger program. Due to the nature and quantity of our data, completely blind methods were neither possible nor completely necessary. For example, it was not possible to know which snakes were social and which were nonsocial during coding. However, to minimize observer bias, all behavioral data were recorded electronically, and the ethologger program used for data analysis was designed to minimize data analysis ambiguity.
Traversing an environment is a common metric for measuring boldness in animals (Chiszar et al. 1976; Wilson et al. 1993). Therefore, in the boldness assay, we manually coded from the videos whenever the snake was outside the shelter. For the sociability assay, we coded the times each snake entered or climbed on top of each of the two shelters. Preliminary analysis of the social assay data suggested that snakes partly lost interest in the social shelter by their second trial and completely by their third trial. Scores on sociability assay trials were not significantly repeatable (Radj | trial = 0.04, 95% CI = [0.00, 0.27], p = 0.34; Method A5), and the snakes were not consistent in their ranks (Kendall’s Wt = 0.35, p = 0.38). We therefore excluded trials 2 and 3 and evaluated sociability based on the results of the first sociability assay only. Boldness scores were based on data from all three boldness assays.
Across all our data, we found that older snakes were also heavier (r = 0.83, t(36) = 8.80, p < 0.001); we therefore considered only the effects of weight and not age in our analyses.
Analyses were conducted in R (R Core Team 2017) using the psych, DescTools, lme4, nlme, asnipe, rcompanion, rptR, assortnet, and igraph packages, and in Mathematica (version 10.0; Wolfram Research).
Results
Individual behavioral assays
Male and female snakes did not differ in weight (KS test; D = 0.32, p = 0.26; Method A8). Time spent out of the shelter differed between individuals and decreased across the three boldness assay trials that each snake completed (Fig. S3; effect of individual: F(37,70) = 5.20, p < 0.001; effect of trial: F(2,70) = 14.60, p < 0.001; Method A7). Heavier snakes were bolder (F(1, 34.9) = 6.16, p = 0.018; Method A5). Boldness assay scores were significantly repeatable (Radj | trial = 0.57, 95% CI = [0.37, 0.72], p < 0.001; Method A5), and snakes were similar in their boldness ranks across trials (Kendall’s Wt = 0.66, p < 0.001; Method A6). We therefore averaged all three scores to give each snake a single boldness score. Males (M = 0.34, SD = 0.23) were marginally bolder than females (M = 0.19, SD = 0.17; t(32) = 2.07, p = 0.046).
In our sociability assay, snakes spent more time in or on the social shelter than the control shelter (M = 65%, SD = 31%; compared to 50%: t(33) = 2.68, p = 0.011). Sociability scores correlated negatively with boldness scores (r = −0.38, t(31) = −2.32, p = 0.014) but not with weight (r = 0.26, t(35) = 1.58, p = 0.062) or sex (D = 0.18, p = 0.84; Method A8).
Group/aggregation experiment
In the group assay, the snakes spent most of their time sheltering. On average, snakes spent 94% (SD = 0.01) of their time in one of the four shelters. Males spent more time sheltering than females (D = 0.42, p = 0.001; Method A8). Time sheltering did not correlate with boldness scores from the individual assays (r = −0.25, t(32) = −1.45, p = 0.08), sociability scores (r = 0.22, t(35) = 1.35, p = 0.09), or weight (r = −0.15, t(36) = −0.89, p = 0.19).
Boldness changed between the individual and group assay. In the group assay, we defined boldness as the amount of time spent outside the shelter. Since we measured the time spent outside a shelter both when alone (in the boldness assay) and in a group, we defined Δboldness as the difference between these values, a possible proxy for social effects on boldness (e.g., Guayasamin et al. 2017). To examine Δboldness, we first converted both boldness scores (from the individual assay and from the group context) into z-scores. Each snake then had two boldness z-scores which we subtracted from each other to provide a single change-in-boldness score. Snakes spent less time out of the shelter when in a group than when alone (Δboldness: M = 0.17, SD = 0.21), with a larger effect in males (M = 0.25, SD = 0.22) than females (M = 0.09, SD = 0.17; t(32) = 2.49, p = 0.018; Fig. S4).
In the group context, snakes actively searched for a large aggregate. As snakes explored the arena, they frequently visited shelters, and the duration of their visits correlated with the number of individuals already present in that shelter (Fig. 1). That is, snakes were more likely to stay for longer in a shelter that already contained a large number of snakes (F(9,4646) = 387.95, p < 0.001). We did not find a main effect for Batch, indicating that the propensity to stay in a shelter that already had a large number of snakes was consistent across batches (F(3,36) = 1.11, p = 0.36). However, there was a significant interaction between Batch and the number of snakes already in the shelter (F(3,4651) = 11.43, p < 0.001; Method A2). To assess this interaction, we examined the model slopes for each batch and found that Batch 4 was significantly different from the other three batches (all p’s < 0.001) whereas Batches 1, 2, and 3 did not significantly differ from each other (all p’s > 0.85). Batch 4 (the oldest batch) had a much shallower positive slope, suggesting that the number of snakes already in a shelter was less influential in their sheltering decisions.
Mean duration of visits to a shelter as a function of the number of snakes already in that shelter. Dashed lines show linear model estimates and the inset text gives the model’s slope (see text for details). Error bars show ± SEM. The gray line in each panel shows the number of times each event occurred (right y-axis)
To assess general preferences for aggregate size across batches, we used a similar model with number of snakes in a shelter (aggregate size) converted to a categorical variable. We then used planned comparisons to compare the time spent in each different-sized aggregate to the grand mean of time spent in all aggregates (deviation contrasts). These comparisons revealed that, overall, snakes spent significantly less time than average in shelters with two or fewer snakes (all p’s < 0.05). Time spent in shelters with three to eight snakes was no different from the average (all p’s > 0.09). In summary, when a snake entered a shelter, it was more likely to stay for longer if the shelter already contained a larger number of snakes. These results suggest that snakes may seek a specific population density when sheltering and that their preferred density may change with age.
Time-of-day effects
Snakes were shuffled twice a day in the group experiment, being removed from their shelters and either all released into the center of the arena or placed into specific shelters (Table S1), and we therefore compared behavior across the three segments of each day: from lights-on to the morning shuffle (morning), from the morning to the afternoon shuffle (midday), and from the afternoon shuffle to lights-out (afternoon). Snakes spent more time exploring in the afternoon than in the morning or midday (Fig. 2; ANOVA, day as a random variable; effect of Batch: F(3,83) = 1.97, p = 0.12, effect of day segment: F(2,83) = 16.50, p < 0.001; Method A2). Post hoc tests revealed that significantly more snakes explored in the afternoon than in either the morning or midday (p’s < 0.001) whereas morning and midday did not significantly differ (p = 1.00). Snakes tended to stay slightly longer when we placed them in shelters than when we released them into the center of the arena and they could choose a shelter (F(1,592) = 10.04, p = 0.002; Method A3). However, independently of whether or not the snake chose the shelter, snakes spent more time in their first post-release shelter in midday than in the afternoon (F(1,592) = 41.69, p < 0.001; Method A3). Overall, this suggests that our manipulated release patterns had a slight effect on determining the length of stay in a shelter, but time of day and social exploration were more important factors. The slight effect of forcing snakes into a shelter most likely occurred because we tended to place snakes in larger groups than the groups that occurred after a central release.
Mean number of snakes out of shelter for each segment of the day, by batch (B1–B4). Data are averaged across all 8 days of the experiment. a, morning; b, midday; c, afternoon. Horizontal black lines give the levels expected by chance (see Online Resource 1). Error bars show ± SEM
Outside the shelters, when snakes did explore, they tended to explore together, except in the afternoon. We estimated the mean number of individuals expected to be outside the shelters at any one time in the absence of any coordination between the snakes (see Online Resource 1). In the mornings, in two of the batches, when any snakes were outside the shelters, the number out was significantly larger than expected (Fig. 2, bars denoted A; Batch 1: χ2 = 152.86, p < 0.001; Batch 2: χ2 = 10.85, p = 0.09; Batch 3: χ2 = 5.41, p = 0.49; Batch 4: χ2 = 37.97, p < 0.001; Method A4). All batches displayed coordination in midday (Fig. 2, bars denoted B; Batch 1: χ2 = 24.04, p = 0.001; Batch 2: χ2 = 21.97, p = 0.003; Batch 3: χ2 = 32.35, p < 0.001; Batch 4: χ2 = 18.93, p = 0.008; Method A4), and only one did in the afternoon (Fig. 2, bars denoted C; Batch 1: χ2 = 1.20, p = 0.99; Batch 2: χ2 = 6.86, p = 0.44; Batch 3: χ2 = 18.07, p = 0.01; Batch 4: χ2 = 1.78, p = 0.97; Method A4). These results suggest that snakes coordinated the times when they left the shelters, mostly in midday, less so in the morning, and rarely in the afternoons.
This coordinated activity may be, in part, caused by the disturbance a snake’s exit has on their aggregate; this disturbance may induce other snakes to leave the shelter as well. To test if this was the case, we observed all instances in which a snake exited from a shelter and coded any subsequent snake exits that followed within 1 min. These exits were coded based on whether the following snake came from the same shelter as the disturbance (coded as 1) or not (0). Means of the values for each individual were calculated and compared to a null distribution. The null distribution consisted of the expected values if exits had occurred at the same times but from randomly selected shelters, taking into account the distribution of snakes across shelters at that moment. We found that, in Batches 1–3, snakes that followed were no more likely than chance to have come from the same shelter as the disturbance (Batch 1: D = 0.37, p = 0.07; Batch 2: D = 0.33, p = 0.15; Batch 3: D = 0.33, p = 0.14; Method A8). For Batch 4, who aggregated less than the other batches overall (see above), we found that following snakes were significantly less likely than chance to come from the same shelter as a recent disturbance (D = 0.52, p = 0.003; Method A8). These results strongly suggest that the coordination in our snakes’ exploration times was not solely a consequence of disturbances within shelters.
Social interactions
We next constructed association networks in which individual snakes were the nodes, and edges (i.e., connections between individuals) represented social interactions. Edge weights (i.e., the thickness of an edge) represented the proportion of frames a snake spent with another individual (either in the same shelter or both outside the shelters). As snakes’ positions were shuffled twice a day, we constructed both averaged daily networks and separate networks for each segment of the day (morning, midday, and afternoon; Fig. S5). For each network, we calculated both network-level and individual-level measures: the weighted degree of each snake (the summed weights of all its edges, in this case a metric similar to “time spent with other snakes”), the weighted density of the network (the summed weights of all edges), transitivity (or connectedness, the extent to which nodes in the network cluster), and closeness centrality (a measure of the lengths of the shortest paths between individuals).
Due to the physical density of our snakes in the arena and the duration of the experiment, all our networks eventually became completely connected all-to-all networks (Fig. S5D). However, as snakes spent much of their time under shelter, these networks tended to be cliquish, with high transitivity values (Fig. S6; grand mean transitivity = 0.93, SD = 0.01; morning, 0.91, SD = 0.04; midday, 0.94, SD = 0.03; afternoon, 0.93, SD = 0.03). Morning networks were slightly less cliquish, as they also contained more instances of isolated individuals (i.e., individuals that spent the entire morning alone in a shelter), or pairs (Fig. S5A, S7A). As snakes moved more later in the day, midday and afternoon networks were always fully connected (Fig. S7). During these more active segments, as snakes sought the aggregate, centrality scores converged to the mean (Fig. S6B), as networks became more connected (Fig. S7B). The centrality distribution of morning networks was significantly different from those for midday (Fig. S6B; D = 0.33, p = 0.03; Method A8) and afternoon (D = 0.4, p = 0.003; Method A8) networks; there was no significant difference between the midday and afternoon distributions (D = 0.23, p = 0.27; Method A8).
We used a permutation test to examine the patterns of interaction in our networks (using the asnipe package in R; see Farine 2013). All batches had association patterns that were significantly different from chance (Table S2; all p values but one <0.05). To examine preferences in association patterns, we looked at the variability in association patterns for each snake (McDonald and Hobson 2018). In this analysis, the proportion of time that each snake spent with their batch mates (known as “Observed Edge Weight Density” and referred to as “O”) was compared to an expected value (known as “Expected Edge Weight Density” and referred to as “E”). The expected value represents a null hypothesis with an even distribution of association preferences (i.e., the snake spends an equal amount of time with all associated batch mates). To test if the snakes were showing a significant preference for associating with particular individuals, we calculated the Observed Edge Weight Density for all the snakes across the morning, midday, and afternoon networks. Across all time periods, all the snakes demonstrated at least some bias in association patterns (an “O” less than their “E”). To quantify the general pattern, we calculated bias-corrected confidence intervals for all snakes and compared them to the “E” for a fully connected network. The results suggested that the snakes had preferential association patterns, but the effect was stronger in the morning (Mo = 0.869, CI[0.861, 0.873], E = 0.889) than in the midday (Mo = 0.881, CI[0.879, 0.882], E = 0.889) or the afternoon (Mo = 0.882, CI[0.880, 0.883], E = 0.889). Taken together, the permutation analysis and the association analysis suggested that juvenile garter snakes were choosing to interact with particular individuals or particular groups of individuals. These interaction patterns were not driven by sex as neither sex preferentially interacted with either the same or the opposite sex (MRQAP on mean daily network; all p’s for batches by segment >0.18), and males and females had nearly identical average network densities (Welch’s t-test; Mmale = 2.85, Mfemale = 2.81, t(35.6) = −0.42, p = 0.68). For Batch 2, in which there were two related snakes that were both unfamiliar and unrelated (subgroup 1) to the majority (subgroup 2), we examined network homophily (the tendency for individuals to associate with others that are like them). In this case, we defined homophily as associations between snakes from each subgroup. We examined homophily across all days, as well as just mornings (see below for why we chose morning networks). To quantify homophily, we calculated the assortativity coefficient (Newman 2003), which provides an r-value between 1 and −1 with positive values indicating associations within subgroups and negative values indicating associations between subgroups. Our analysis found a slight tendency to associate with the unfamiliar subgroup (all days: r = −0.13, all mornings: r = −0.04). To test if this was significantly different from a random association pattern, we compared these r-values to the values calculated from 10,000 random networks and found that neither value was significant (all days: p = 0.08, all mornings: p = 0.23). This suggests that the snakes displayed no bias for associating with individuals from either the same or different subgroups. However, we are reticent to make generalizations based on this finding as the sample for this particular analysis was small since three of our four replicates consisted of all related individuals.
Individual weighted degree was significantly repeatable in the morning (Radj | day = 0.16, 95% CI = [0.05, 0.29], p < 0.001; Method A5) and marginally so in the afternoon (Radj | day = 0.07, 95% CI = [0.00, 0.20], p = 0.05; Method A5) but not in midday (Radj | day = 0, 95% CI = [0.00, 0.09], p = 1.00; Method A5). The nonparametric concordance test revealed the same patterns in the consistency of the snakes’ relative social preferences for the morning (Kendall’s Wt = 0.31, p < 0.001; Method A6), midday (Kendall’s Wt = 0.12, p = 0.88; Method A6), and afternoon (Kendall’s Wt = 0.23, p = 0.05; Method A6). These tests further suggest that despite our best efforts to manipulate the snakes’ aggregation patterns by shuffling them, given enough time, the snakes re-formed their chosen networks and were consistent in their social choices.
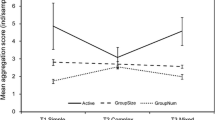
Individual weighted degree was predicted by both sociability and boldness. This was examined using linear mixed effect models that tested the combined influence of sociability and boldness on network degree for each day segment. Snakes that were more social had higher degrees in their networks in the morning (main effect of sociability on degree; F(1,30) = 6.18, p = 0.02; Method A2) but not in midday (F(1, 217) = 0.0002, p = 0.99) or the afternoon (F(217) = 2.09, p = 0.15), suggesting that morning association networks are more stable than those measured later in the day. Since an individual’s degree is likely negatively correlated to its boldness (since most of the snakes remained in the shelters, spending more time in a shelter is likely to increase degree), to test the effect of boldness on degree for a particular day segment, we calculated for each snake a partial boldness score, based on the proportion of time it spent out of the shelter during the other two segments of that day. These partial boldness scores are theoretically independent of an individual’s degree and were entered into the mixed effect linear models (all VIF < 2.00). Boldness had no direct effect on degree in the morning (main effect of boldness; F(1, 31) = 1.97, p = 0.17; Method A2), midday (F(1,190) = 0.014, p = 0.91), or afternoon (F(1,215) = 0.89, p = 0.35). However, we found an interaction between boldness and sociability on an individual’s weighted degree (Fig. 3) such that sociability predicted degree less for bolder snakes in morning networks (interaction effect; F(1, 30) = 5.06, p = 0.03; Method A2). There was no such effect in midday (F(1,218) = 0.0004, p = 0.98) or afternoon (F(1,217) = 1.63, p = 0.20) networks. In other words, in the morning, bold snakes’ connectivity is not affected by their sociability, but shyer snakes that are more social have a higher weighted degree.
Discussion
Students of social behavior distinguish between aggregations that result from shared attraction to an external stimulus (e.g., two snakes accidentally sharing a particularly attractive rock) and those that are formed for social purposes (Krause and Ruxton 2002; also termed “biosocial attraction”; Allee 1934). Here we offer evidence that juvenile Eastern garter snakes actively seek social interaction and vary in how they do so. Our data suggest that (1) snakes actively seek to join and remain part of groups—and prefer larger groups—both when selecting a shelter and when deciding to leave a shelter; (2) snakes display individual variability in boldness and sociability, and these traits influence their aggregation patterns; (3) snakes form nonrandom networks in which they preferentially interact with some conspecifics over others; and (4) aggregation patterns are repeatable over time, and snakes return to their previous distributions after a perturbation.
Snakes seek social interaction
Previous work has suggested that external factors, such as heat, humidity, or diet, can drive aggregation in snakes (Nobel and Clausen 1936; Graves and Duvall 1987; Lyman-Henley and Burghardt 1994). Our data show that juvenile Eastern garter snakes still seek out social interaction when the number and size of shelters, temperature, and availability of food are all held constant. By giving our 10 snakes four shelters, we forced them to at least partially aggregate. Despite this, snakes left their shelters, potentially exposing themselves to danger, explored alternative shelters, and were more likely to remain for longer in shelters that already housed larger groups. This effect disappeared in the oldest snakes, suggesting that social aggregation in garter snakes may be an exclusively juvenile phenomenon, possibly due to larger (and older) snakes being less vulnerable to predation (Shine et al. 2001) or more sensitive to competition. However, age was confounded with parentage in our data, limiting our ability to answer this question. We also note that the larger snakes may have filled the shelters more. If snakes’ preferences for shelters depend on the amount of space or its density, rather than the number of conspecifics in it, this might partially account for our oldest batch showing a diminished preference for larger groups.
We also found that our snakes coordinated the times they spent exploring outside the shelters, especially in the middle of the day, such that they were more likely to be outside the shelters at the same time than would have been expected by chance. We often observed snakes poking their heads out of the shelters, which would have allowed them to monitor how many others were outside at any time. Since snakes spend the vast majority of their time sheltering, seeing other snakes outside is informative. Snakes may use this information to decide when environmental conditions are right to leave shelter and may also prefer to explore in larger groups to dilute predation risk (Krause and Ruxton 2002). Under our laboratory conditions—where temperature, light, and feeding schedules are fixed—snakes co-explored most in midday, when the overall amount of exploration was moderate, and two groups co-explored in the morning when exploration was rare. Snakes spent more time out of shelter and exploring in the afternoon, but they did not coordinate the timing of their exploration. It seems likely that at least some exploration is food-motivated. It has been documented that thermal conditions alter snake foraging (Nelson and Gregory 2000), which is a solitary activity (see below). Our snakes may therefore have preferred to forage in the afternoons. However, our data suggest that although their exploration was not coordinated in the afternoon, this was also the time when they re-formed their preferred social groupings. Since exploration and foraging are not mutually exclusive, this suggests that the snakes’ afternoon explorations served both purposes. Exploration in the morning and midday, when we saw more coordination, may be driven by other motivations separate from foraging, such as social interaction or searching for a basking location.
We found few effects of sex on our juvenile snakes’ behaviors. Snakes showed no preference for associating with the same or the opposite sex, and there was no difference in network degree between sexes. Males spent slightly more time than females in shelters, were bolder, and changed their exploration behavior more between the individual and group contexts.
Association networks
Garter snakes in our aggregation experiment formed nonrandom association networks. In other words, snakes did not merely aggregate in shelters but were more likely to associate with particular subgroups or individuals.
In our experiment, we “shuffled” the snakes twice a day, disrupting their associations. After these perturbations, snakes gradually re-formed their preferred groups. We found the networks observed in the mornings—after the longest time since a shuffle—to be the most repeatable ones, suggesting that they reflect the snakes’ preferred distributions. Reconstituting a preferred network may partly explain why our snakes left their shelters more in midday and the afternoons—after being shuffled—than the mornings (but see also above). Morning networks had repeatable weighted degrees and frequently contained individuals or pairs in separate components (Fig. S7). Additionally, morning networks reflected the effects of individual differences. Individuals’ morning weighted degree correlated with both their sociability—more social snakes had a higher degree—and on an interaction between sociability and boldness such that degree correlated with sociability only in shyer snakes. Neither of these effects was observed at other times of day when snakes were still re-forming their networks.
Individual differences
Though exploration or emergence from a shelter has been used to measure boldness in a wide range of species (van Oers et al. 2004; Cote et al. 2010; Wisenden et al. 2011), including snakes (Mayer et al. 2016), our results suggest that boldness in snakes comprises a slightly different suite of behaviors than in other taxa. Contrary to much of the literature on individual differences, we find that snakes spend decreasing amounts of time outside a shelter across three individual tests in a novel environment (Fig. S3; compare to standard habituation findings, e.g., Tchernichovski et al. 1998), that snakes spend less time outside a shelter when in a group than when alone (Fig. S4; compare, e.g., Guayasamin et al. 2017), and that time spent outside the shelter correlates negatively with time spent near a social stimulus (whereas sociability is often considered independent of boldness in other species; e.g., Jolles et al. 2017; but see, e.g., Bevan et al. 2018). We suggest the following explanation of these results. In most taxa, it is likely that the main driver of exploration is the need to forage. Snakes, however, can satisfy their energy requirements by eating less frequent but larger meals. As such, when foraging is not the primary concern, particularly vulnerable snakes (such as juveniles) may leave their shelters to seek heat, social contact, or to generally decrease environmental uncertainty. Thus, when placed in an environment with no spatial or temporal temperature gradient, in a group, snakes locate their preferred partners or group size and remain with them inside shelters. More social snakes may periodically leave the shelter in search of additional or alternate social contact. The inverse correlation we observe between sociability and boldness suggests a behavioral syndrome, driven by conflicting decisions about sheltering and exploration (Sih et al. 2012): Sociability, in our snakes, appears to primarily occur under shelter; bolder individuals sacrifice the advantages of the group (and shelter) for priority access to resources and therefore appear to be less social.
Since snakes cannot share food and competition for food can be costly (Yeager and Burghardt 1991), snakes offer a fascinating case study on the balancing act between maintaining the protections of grouping and managing competition for food. Finding the perfect balance may be beneficial; for example, in broad-banded water snakes (Nerodia fasciata), young snakes that were housed together but fed separately grew faster than individuals that were housed together and ate together and faster than individuals that were housed separately and fed separately. This occurred despite an equal abundance of food in all conditions (Burghardt 1990). Garter snakes have evolved a number of unique strategies for juggling these challenges of group living. For example, male red-sided garter snakes do not eat during the breeding season (O’Donnell et al. 2004), and Butler’s garter snakes (Thamnophis butleri) prefer to aggregate with individuals that are on a different diet (Lyman-Henley and Burghardt 1994). Our findings suggest that individual differences in sociability are yet another way for snakes to deal with the advantages and disadvantages of food competition and group living.
While food competition is likely of critical importance to grouping behaviors in snakes, we recognize that there are other advantages and disadvantages to grouping that will influence snake behavior (Krause and Ruxton 2002). For example, sheltering in a group may provide thermal benefits, and this effect (and others) may have driven the evolution of a nonspecific bias in favor of social aggregation which continues to affect snakes’ decisions even when temperatures are kept constant, as in our experiment. What is notable, however, is that snakes can also respond differently at an individual level, modulating their sociability. Although we mitigated competition during our experiments, the snakes did have the opportunity to compete for food while they were communally housed. As such, it is possible that observed individual differences in social behavior were the result of differing responses to long-term competition. Future experiments comparing sociability in snakes that have never competed for food to snakes that have may assist in furthering our understanding of the development of social personality in snakes.
Implications
Research using social networks to examine association patterns in Arizona black rattlesnakes (C. cerberus) demonstrated complex patterns of social interactions that echo some of the findings we have reported here (Schuett et al. 2017). Taken together, it appears that the analysis of social interactions in snakes should force us to reconsider common beliefs about snakes being nonsocial and start examining the broader implications of the social patterns observed in these species. Recognizing existing social patterns is of particular importance for conservation efforts. The translocation of reptiles is a common conservation practice used to bolster or re-establish vulnerable populations and/or remove animals from development zones (Dodd and Seigel 1991; Towns et al. 2016). However, the effectiveness of translocation as a conservation tool for reptiles has been questioned (Dodd and Seigel 1991). In one study involving the translocation of common European adders (Viperus berus), translocated males ranged much further than translocated females and resident males. This increased movement after translocation can endanger the snakes as they venture into unknown and/or anthropogenic landscapes (Nash and Griffiths 2018). Our findings suggest that it might be possible to improve the translocation of snakes by considering their social needs or preferences. We demonstrated that young snakes looked for each other and that male snakes spent less time exploring in a group. These results suggest that, in social snake species, releasing snakes in a group might help maintain site fidelity after relocation. An important first step in this process is determining the generalizability of conspecific attraction across snake species. Through our individual sociability testing, we confirmed Graves and Halpern’s (1988) finding that garter snakes are attracted to conspecific skin lipids. This could be a useful procedure for establishing sociability across snake species. Once we understand the generalizability of social attraction in snakes, translocation efforts can be tailored to best ensure the success and development of relocated populations and, subsequently, improve the conservation of our natural habitats.
References
Alexander G (2018) Reproductive biology and maternal care of neonates in southern African python (Python natalensis). J Zool 305:141–148. https://doi.org/10.1111/jzo.12554
Allee E (1934) Recent studies in mass physiology. Biol Rev 9:1–48. https://doi.org/10.1111/j.1469-185X.1934.tb00872.x
Bevan PA, Gosetto I, Jenkins ER, Barnes I, Ioannou CC (2018) Regulation between personality traits: individual social tendencies modulate whether boldness and leadership are correlated. Proc R Soc B 285:20180829. https://doi.org/10.1098/rspb.2018.0829
Bonnet X, Shine R, Lourdais O (2002) Taxonomic chauvinism. Trends Ecol Evol 17:1–3. https://doi.org/10.1016/S0169-5347(01)02381-3
Burghardt G (1977) Of iguanas and dinosaurs: social behavior and communication in neonate reptiles. Am Zool 17:177–190
Burghardt G (1983) Aggregation and species discrimination in newborn snakes. Z Tierpsychol 61:89–101. https://doi.org/10.1111/j.1439-0310.1983.tb01330.x
Burghardt G (1990) Chemically mediated predation in vertebrates: diversity, ontogeny, and information. In: Macdonald DW, Muller-Schwarze D, Natynczuk SE (eds) Chemical signals in vertebrates 5. Oxford University Press, Oxford, pp 475–499
Carpenter C (1953) A study of hibernacula and hibernating associations of snakes and amphibians in Michigan. Ecology 34:74–80. https://doi.org/10.2307/1930310
Chiszar D, Carter T, Knight L, Simonsen L, Taylor S (1976) Investigatory behavior in the plains garter snake (Thamnophis radix) and several additional species. Anim Learn Behav 4:273–278. https://doi.org/10.3758/BF03214049
Clark R, Brown W, Stechert R, Greene H (2012) Cryptic sociality in rattlesnakes (Crotalus horridus) detected by kinship analysis. Biol Lett 8:523–525. https://doi.org/10.1098/rsbl.2011.1217
Cote J, Clobert J (2007) Social personalities influence natal dispersal in a lizard. Proc R Soc Lond B 274:383–390. https://doi.org/10.1098/rspb.2006.3734
Cote J, Fogarty S, Weinersmith K, Brodin T, Sih A (2010) Personality traits and dispersal tendency in the invasive mosquitofish (Gambusia affinis). Proc R Soc Lond B 277:1571–1579. https://doi.org/10.1098/rspb.2009.2128
Dinets V (2017) Coordinated hunting by Cuban boas. Anim Behav Cogn 4:24–29. https://doi.org/10.12966/abc.02.02.2017
Dodd C, Seigel R (1991) Relocation, repatriation, and translocation of amphibians and reptiles: are they conservation strategies that work? Herpetologica 47:336–350. https://doi.org/10.1016/0006-3207(92)91063-X
Doody JS, Burghardt G, Dinets V (2013) Breaking the social-non-social dichotomy: a role for reptiles in vertebrate social behavior research? Ethology 119:95–103
Dundee H, Miller M (1968) Aggregative behavior in habitat conditioning by the prairie ringneck snake, Diadophis punctatus arnyi. Tulane Stud 15:41–58
Edsinger E, Dölen G (2018) A conserved role for serotonergic neurotransmission in mediating social behavior in octopus. Curr Biol 28:3136–3142. https://doi.org/10.1016/j.cub.2018.07.061
Farine D (2013) Animal social network inference and permutations for ecologists in R using asnipe. Methods Ecol Evol 4:1187–1194. https://doi.org/10.1111/2041-210X.12121
Graves B, Duvall D (1987) An experimental study of aggregation and thermoregulation in prairie rattlesnakes (Crotalus viridis viridis). Herpetologica 43:259–264
Graves B, Duvall D (1995) Aggregation of squamate reptiles associated with gestation, oviposition, and parturition. Herpetol Monogr 9:102–119. https://doi.org/10.2307/1466999
Graves B, Halpern M (1988) Neonate plains garter snakes (Thamnophis radix) are attracted to conspecific skin extracts. J Comp Psychol 102:251–253. https://doi.org/10.1037/0735-7036.102.3.251
Greene HW, May PG, Hardy DL, Sciturro JM, Farrell TM (2002) Parental behavior by vipers. In: Schuett GW, Höggren M, Douglas ME, Greene HW (eds) Biology of the vipers. Eagle Mountain Publishing LC, Eagle Mountain, pp 179–206
Gregory PT (2004) Analysis of patterns of aggregation under cover objects in an assemblage of six species of snakes. Herpetologica 60:178–186. https://doi.org/10.1655/02-101
Guayasamin OL, Couzin ID, Miller NY (2017) Behavioural plasticity across social contexts is regulated by the directionality of inter-individual differences. Behav Process 141:196–204. https://doi.org/10.1016/j.beproc.2016.10.004
Hatchwell B (2010) Cryptic kin selection: kin structure in vertebrate populations and opportunities for kin directed cooperation. Ethology 116:203–216. https://doi.org/10.1111/j.1439-0310.2009.01732.x
Heller S, Halpern M (1981) Laboratory observations on conspecific and congeneric scent trailing in garter snakes (Thamnophis). Behav Neural Biol 33:372–377. https://doi.org/10.1016/S0163-1047(81)92136-1
Heller S, Halpern M (1982) Laboratory observations of aggregative behavior of garter snakes, Thamnophis sirtalis. J Comp Physiol Psychol 96:967–983
Hoss S, Deutschman D, Booth W, Clark R (2015) Post-birth separation affects the affiliative behaviour of kin in a pitviper with maternal attendance. Biol J Linn Soc 116:637–648. https://doi.org/10.1111/bij.12604
Jolles JW, Boogert NJ, Sridhar VH, Couzin ID, Manica A (2017) Consistent individual differences drive collective behavior and group functioning of schooling fish. Curr Biol 27:2862–2868. https://doi.org/10.1016/j.cub.2017.08.004
Krause J, Ruxton G (2002) Living in groups. Oxford University Press, New York
Larsen KW, Gregory PT, Antoniak R (1993) Reproductive ecology of the common garter snake Thamnophis sirtalis at the northern limit of its range. Am Midl Nat 129:336–345. https://doi.org/10.2307/2426514
Loughran C, Beck D, Weaver R (2015) Use of communal shedding sites by the northern Pacific rattlesnake (Crotalus oreganus oreganus) in Central Washington state. Northwest Nat 96:156–160
Luiselli L (1996) Individual success in mating balls of the grass snake, Natrix natrix: size is important. J Zool 239:731–740. https://doi.org/10.1111/j.1469-7998.1996.tb05474.x
Lyman-Henley L, Burghardt G (1994) Opposites attract: effects of social and dietary experience on snake aggregation behaviour. Anim Behav 47:980–982. https://doi.org/10.1006/anbe.1994.1131
Mason RT, Crews D (1985) Female mimicry in garter snakes. Nature 316:59–60. https://doi.org/10.1038/316059a0
Mayer M, Shine R, Brown GP (2016) Bigger babies are bolder: effects of body size on personality of hatchling snakes. Behaviour 153:313–323. https://doi.org/10.1163/1568539X-00003343
Nash DJ, Griffiths RA (2018) Ranging behaviour in adders (Vipera berus) translocated from a development site. Herpetol J 28:155–159
Nelson K, Gregory P (2000) Activity patterns of garter snakes, Thamnophis sirtalis, in relation to weather conditions at a fish hatchery on Vancouver Island, British Columbia. J Herpetol 34:32–40
Nobel G, Clausen H (1936) The aggregation behavior of Storeria dekayi and other snakes, with especial reference to the sense organs involved. Ecol Monogr 6:269–316. https://doi.org/10.2307/1943244
O’Donnell R, Shine R, Mason R (2004) Seasonal anorexia in the male red-sided garter snake, Thamnophis sirtalis parietalis. Behav Ecol Sociobiol 56:413–419. https://doi.org/10.1007/s00265-004-0801-x
Pawar S (2003) Taxonomic chauvinism and the methodologically challenged. BioScience 53:861–864. https://doi.org/10.1641/0006-3568(2003)053[0861:TCATMC]2.0.CO;2
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna http://www.R-project.org
Reichenbach N (1983) An aggregation of female garter snakes under corrugated metal sheets. J Herpetol 17:412–413. https://doi.org/10.2307/1563599
Rossman D, Ford N, Seigel R (1996) The garter snakes: evolution and ecology (Animal natural history series, vol. 2). University of Oklahoma Press, Norman
Schuett GW, Clark RW, Repp RA, Amarello M, Smith CF, Greene HW (2017) Social behavior of rattlesnakes: a shifting paradigm. In: Schuett GW, Feldner MJ, Smith CF, Reiserer RS (eds) Rattlesnakes of Arizona, vol 2. Eco Publishers, Rodeo, pp 61–244
Shine R, LeMaster M, Moore I, Olsson M, Mason R (2001) Bumpus in the snake den: effects of sex, size, and body condition on mortality of red-sided garter snakes. Evolution 55:598–604. https://doi.org/10.1111/j.0014-3820.2001.tb00792.x
Shine R, Langkilde T, Mason RT (2003) Confusion within ‘mating balls’ of garter snakes: does misdirected courtship impose selection on male tactics? Anim Behav 66:1011–1017. https://doi.org/10.1006/anbe.2003.2301
Shine R, Shine T, Shine JM, Shine BG (2005) Synchrony in capture dates suggests cryptic social organization in sea snakes (Emydocephalus annulatus, Hydrophiidae). Aust Ecol 30:805–811. https://doi.org/10.1111/j.1442-9993.2005.01524.x
Shine R, Langkilde R, Mason RT (2012) Facultative pheromonal mimicry in snakes: “she-males” attract courtship only when it is useful. Behav Ecol Sociobiol 66:691–695. https://doi.org/10.1007/s00265-012-1317-4
Sih A, Cote J, Evans M, Fogarty S, Pruitt J (2012) Ecological implications of behavioral syndromes. Ecol Lett 15:278–289. https://doi.org/10.1111/j.1461-0248.2011.01731.x
Sprent P, Smeeton N (2001) Applied nonparametric statistical methods, 3rd edn. Chapman & Hall/CRC, Boca Raton
Stoffel M, Nakagawa S, Schielzeth H (2017) rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol Evol 11:1639–1644. https://doi.org/10.1111/2041-210X.12797
Tchernichovski O, Benjamini Y, Golani I (1998) The dynamics of long-term exploration in the rat. Part I. A phase-plane analysis of the relationship between location and velocity. Biol Cybern 78:423–432
Towns DR, Miller KA, Nelson NJ, Chapple DG (2016) Can translocations to islands reduce extinction risk for reptiles? Case studies from New Zealand. Biol Conserv 204:120–127. https://doi.org/10.1016/j.biocon.2016.04.024
Tukey JW (1977) Exploratory data analysis. Addison-Wesley, Reading
van Oers K, Drent PJ, de Goede P, van Noordwijk AJ (2004) Realized heritability and repeatability of risk-taking behaviour in relation to avian personalities. Proc R Soc Lond B 271:65–73
Wilkinson A, Kuenstner K, Mueller J, Huber L (2010) Social learning in a non-social reptile (Geochelone carbonaria). Biol Lett 6:614–616. https://doi.org/10.1098/rsbl.2010.0092
Wilson DS, Coleman K, Clark AB, Biederman L (1993) Shy-bold continuum in pumpkinseed sunfish (Lepomis gibbosus): an ecological study of a psychological trait. J Comp Psychol 107:250–260
Wisenden BD, Sailer CD, Radenic SJ, Sutrisno R (2011) Maternal inheritance and exploratory-boldness behavioural syndrome in zebrafish. Behaviour 148:1443–1456
Yeager C, Burghardt G (1991) Effect of food competition on aggregation: evidence for social recognition in the plains garter snake (Thamnophis radix). J Comp Psychol 105:380–386. https://doi.org/10.1037/0735-7036.105.4.380
Acknowledgments
The authors wish to thank Dr. Ken Maly for developing and performing the lipid extraction methodology used in the paper, Kelley Putzu for the assistance with animal care, and members of the Collective Cognition Lab for the assistance with running the experiment and coding the data. We also thank two anonymous reviewers for their helpful comments on an earlier version of the manuscript.
Funding
This research was funded by the National Science and Engineering Research Council of Canada (NSERC) grant RGPIN-2016-06138 (to NM).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception, design, material preparation, and statistical analyses. The first draft of the manuscript was written by Morgan Skinner. All authors contributed to manuscript editing and further re-writes. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All experimental procedures conformed with the Canada Council on Animal Care guidelines and were approved by the Wilfrid Laurier University Animal Care Committee (AUP R17004). The adult snakes (mothers of the experimental subjects) were collected from the Waterloo, Ontario, area under a Scientific Collector’s Permit from the Ontario Ministry of Natural Resources.
Data availability statement
The datasets generated and analyzed during the current study are available in the Open Science Framework repository, https://osf.io/jx9ve/.
Additional information
Communicated by T. Madsen
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1.01 MB)
Rights and permissions
About this article
Cite this article
Skinner, M., Miller, N. Aggregation and social interaction in garter snakes (Thamnophis sirtalis sirtalis). Behav Ecol Sociobiol 74, 51 (2020). https://doi.org/10.1007/s00265-020-2827-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-020-2827-0