Abstract
Despite evidence that organisms are more likely to exhibit their full range of cognitive abilities under conditions found in nature, studies evaluating cognition under such conditions remain rare, particularly in vertebrate species. Here, we conducted an experiment to evaluate problem-solving and motor self-regulation in free-living arboreal lizards, Anolis sagrei, under natural conditions. We presented lizards with a novel detour problem which challenged individuals to circumvent a transparent barrier in order to obtain a food reward. Individuals varied in their ability to solve the detour problem. Furthermore, those that solved the problem were able to improve their performance across trials by modifying the natural response of attempting to strike the reward through the transparent barrier, providing evidence of motor self-regulation. Solving the problem required individuals to modify their typical foraging behavior, as approaching the prey in a single burst of movement that culminated with an attack was an unsuccessful strategy. Contrary to expectations, our findings provide evidence of motor self-regulation in a visually oriented, sit-and-wait predator under natural conditions, suggesting motor self-regulation is not limited by foraging strategy. Our results also underscore the need to evaluate the cognitive abilities of free-living organisms in the wild, particularly for taxa that perform poorly under laboratory conditions.
Significance statement
Studies of animal cognition have a long history in animal behavior, which, in vertebrate species, has been dominated by experiments conducted under controlled laboratory conditions. Here, we showed that experiments can be taken “outside the box,” from the laboratory into natural conditions, and by doing so overcome some of the obstacles that have hindered our ability to study cognition in species unlikely to remain motivated when removed from the wild. We implemented a modified version of the cylinder task, which provided the stimuli needed for a visually oriented, sit-and-wait foraging lizard to participate in the experiments. Individuals of Anolis sagrei learned to solve the task by modifying what was previously described as a stereotyped prey capture behavior. In addition, individuals decreased the number of times they attempted to strike the prey through the transparent barrier. These findings provide further evidence of behavioral flexibility in anoles and new evidence of motor self-regulation. The latter demonstrates the need to extend our current understanding of potential forces favoring the evolution of cognition beyond those that have been proposed in birds and mammals. More generally, our findings demonstrate the importance of using experimental paradigms that are rooted in an understanding of the natural history of the species of interest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Research evaluating the cognitive abilities of animals has flourished over the last decade, providing new insights into the potential mechanisms and evolutionary processes mediating inter- and intra-specific variation in cognition (Shettleworth 2010). A recurrent prediction of these studies is that the demands of a species’ ecology have shaped the evolution of cognitive traits, including behavioral flexibility and problem-solving (Sol et al. 2005; Shettleworth 2010; Auersperg et al. 2011; Benson-Amram et al. 2016; and references therein). Observation of the full range of cognitive abilities for a species requires studies under conditions where subjects have access to the full repertoire of stimuli available in nature (Morand-Ferron et al. 2015; Pritchard et al. 2016). Thus, in order to observe a species’ full range of cognitive abilities, researchers have called for more studies under conditions where individuals have access to the full repertoire of stimuli available in nature. Two approaches have been taken to achieve this goal. First, and more commonly, experiments are conducted under semi-natural conditions with captive individuals, providing an opportunity for the individuals to potentially experience a larger range of ecologically relevant conditions than under laboratory conditions (e.g., Healy and Hurly 2003; Noble et al. 2012; Schwarz et al. 2017). Second, and relatively rarer, are studies conducted with free-living organisms under natural conditions (e.g., Benson-Amram and Holekamp 2012; Morand-Ferron et al. 2015; Cauchoix et al. 2017; Croston et al. 2017). The latter approach provides an opportunity to measure cognition under the conditions in which selection has shaped the evolution of these traits and under which the ability to solve novel tasks is likely to be a better predictor of behavioral flexibility and innovation (Shettleworth 2010; Morand-Ferron et al. 2015).
Detour tasks, using transparent barriers, have been commonly used to test the ability of animals to access a reward that can be seen but is not directly accessible. Because transparent surfaces are extremely rare in nature, incorporating these surfaces into the paradigm reduces the possibility that previous experience with those surfaces directly or indirectly impacts the ability of organisms to solve the task (Epstein et al. 1984; von Bayern et al. 2009; Griffin and Guez 2014; Logan 2016). This presents a novel problem that can provide insight into an organism’s cognitive abilities (e.g., Boogert et al. 2011; Maclean et al. 2014; Lucon-Xiccato and Bisazza 2017; Szabo et al. 2019). In order to solve the task, the subject must inhibit the impulsive behavior of trying to reach the reward through the transparent surface and instead detour around the barrier to access the reward, what is known as motor self-regulation (Kabadayi et al. 2018). Motor self-regulation underpins self-control and problem-solving abilities (reviewed in Kabadayi et al. 2018). For example, corvids’ performance in a detour-reaching task is associated with absolute and relative brain size (Kabadayi et al. 2016), which are positively correlated with general cognition (Sol et al. 2005). Detour tasks have been mostly used with birds and mammals (reviewed in Kabadayi et al. 2018, but see Lucon-Xiccato and Bisazza 2017; Szabo et al. 2019) and implemented under laboratory conditions. Furthermore, most of the species tested consume a significant portion of non-moving prey items in their diet, because the general expectation is that motor self-regulation should be selected against in species that eat moving prey (Shettleworth 2010).
Detour tasks have been conducted using a variety of methods (reviewed in Kabadayi et al. 2018). A current trend for detour experiments employs the cylinder task, stemming from Boogert et al. (2011), in which subjects are first trained to retrieve a reward from an opaque cylinder and then tested on their ability to retrieve a reward from a transparent cylinder. Trials are only successful in the cylinder task if the subject does not touch the surface of the cylinder during both the training and testing phases (Boogert et al. 2011). Multiple recent studies have employed this procedure (MacLean et al. 2014; Kabadayi et al. 2016; Vernouillet et al. 2016; Szabo et al. 2019; Johnson-Ulrich and Holekamp 2020). However, this procedure has only become popular within the last decade and is far from the only published procedure to test detour performance. Many published detour tasks, especially those evaluating learning, have not employed an opaque training phase (Schiller 1949a; Spigel 1964; Scholes 1965; Scholes and Wheaton 1966; Santos et al. 1999; Wynne and Leguet 2004; Vlamings et al. 2010; Wilkinson et al. 2010), which is included to shape the response of subjects to the transparent cylinder. The requirement of subjects to make no contact with the apparatus during successful trials was also not present in previous paradigms of the detour-reaching task (Diamond 1990; Santos et al. 1999; Amici et al. 2008; Vlamings et al. 2010).
In our experiment, we present free-living naïve lizards with a transparent half-cylinder containing a reward, similar to the apparatus utilized in the studies described above. However, we do not use a training phase and score any trials in which the reward is accessed as successful. This is due to the natural history of Anolis sagrei, a visually oriented, sit-and-wait forager that would be unmotivated to interact with an opaque cylinder blocking the view of a reward. Because of this, we expected incremental improvement as lizards learned to detour, and considered lizards to have successfully completed the task when reaching a pre-defined criterion, as employed in previous detour studies (Schiller 1949b; Spigel 1964; Scholes 1965; Scholes and Wheaton 1966; Boogert et al. 2011).
Compared with endothermic species, the cognitive abilities of reptiles have largely been overlooked (Burghardt 2013). This is surprising when considering their evolutionary history, species richness, and diverse natural history, which is likely to provide valuable insights into the evolution of heavily studied avian and mammalian cognition (Shettleworth 2010). In fact, recent studies have demonstrated that cognitive abilities of reptiles are more complex than recognized historically (Wilkinson et al. 2010; Davis and Burghardt 2011; Leal and Powell 2012; Burghardt 2013; Noble et al. 2014; Szabo et al. 2019; and references therein), raising questions about previous hypotheses addressing the evolution of cognitive traits developed from studies on endothermic animals. For example, it has been demonstrated that having a complex social structure or exploiting a diversity of food resources, two of the main predictors of behavioral flexibility in endothermic species are not predictors of behavioral flexibility in the arboreal lizard Anolis evermanni (Leal and Powell 2012). In a similar vein, the ability to learn by observing the behavior of conspecifics has been shown in the skink Eulamprus quoyii, a mostly solitary lizard, suggesting that the potential for social learning is not limited to highly social species (see also Wilkinson et al. 2010; Davis and Burghardt 2011; Noble et al. 2014). Nevertheless, our ability to study reptile cognition has been hampered by their relatively low metabolic rate, which can limit the number of trials in training and testing phases when food is used as reward and the difficulty of creating laboratory conditions under which reptiles might be expected to behave naturally (Burghardt 2013; Steinberg and Leal 2018). Thus, our current understanding is based primarily on a relatively small sample of the vast diversity of species of reptiles.
In this study, we evaluate if free-ranging individuals of Anolis sagrei can solve a novel detour problem under natural conditions. To do so, we administered a modified cylinder task, in which naïve individuals were presented with a transparent tube containing a reward. The task challenged individuals to circumvent a transparent barrier in order to obtain a food reward, without prior training, providing an opportunity to evaluate problem-solving and motor self-regulation (Kabadayi et al. 2018). Furthermore, by working with free-ranging individuals, we avoid the possibility of previous experience with transparent surfaces, thus presenting a novel problem to the individuals. As a novel problem, the expectation is that motor self-regulation will be exhibited incrementally, as individuals gain experience by interacting with the transparent surface (Vernouillet et al. 2016). Therefore, we predicted that individuals of A. sagrei would solve the detour problem and in doing so would decrease the number of attempts to secure the reward through the transparent surface as they gained experience with the novel problem.
Materials and methods
Study system
We carried out this study from June 19 through July 21, 2016 in Marsh Harbour on the island of Great Abaco, Bahamas. Anolis sagrei (nmale = 10, nfemale = 13) were sampled in a forest fragment of approximately 7500 m2 located within the premises of The Abaco Beach Marina, which holds a high density of lizards.
Anolis sagrei is a small-to-medium-sized, sexually dimorphic (females to 39.5 mm; males to 51.9 mm), arboreal, territorial lizard that inhabits open and semi-shaded forest (Losos 2009). Individuals are most commonly observed within 1.5 m of the ground on the trunks of trees. From these perches, males and females signal to conspecifics in the surrounding habitat and forage for potential prey items, primarily insects (Schoener and Schoener 1982). Anolis sagrei is a sit-and-wait forager, and while foraging, individuals typically assume a survey posture—head-down position with the front limbs extended and head elevated—while scanning the ground for the movement of potential prey (Stamps 1977; Schoener and Schoener 1982). If a prey item is detected, individuals will sprint to the ground and capture the prey at the end of the run, which commonly occurs as one sequence of events (Moermond 1981).
Lizards were located by walking slowly through the forest. Once a lizard was found, it was captured using a noose and its snout–vent length (± 0.1 mm) and weight (± 0.1 g) were measured and recorded (Online Resource 1). It was then marked by attaching two temporary queen bee tags (Bee Works, Ontario, Canada) with a small amount of cyanoacrylate adhesive to each shoulder, providing each individual with a unique color and number combination. Lizards were released at their site of capture, which was marked with flagging tape. We conducted experiments from 0800 to 1800 h, excluding periods of rain. The first trial for each lizard occurred at least 24 h after it was marked.
Testing apparatus
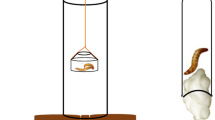
A photo of the testing apparatus is presented in Fig. 1. The apparatus consisted of an acrylic half-cylinder (11.5 L × 8 W × 4 H cm) divided into three segments: a removable transparent center (5 cm) and two equally sized terminal segments (3 cm) that were attached to a green painted wood platform (11.5 L × 10.5 W × 2 H cm). Both ends of the half-cylinder were open and provided access to a live fly maggot used as a reward. The maggot was restrained at the center of the half-cylinder by tethering it to a small piece of dental floss (ca. 1.5 cm) with cyanoacrylate adhesive and then securing the other end between two strong magnets at the center of the platform. The movements of the maggot are very salient to anoles (Fleishman and Pallus 2010), usually eliciting a foraging response. Lizards were able to easily detach the maggot from the dental floss.
The terminal pieces of the half-cylinder were covered by one of two black-white patterns: vertical lines or checkered (Fig. 1). Those patterns provided cues for the lizards to associate a given pattern with access to the reward. However, it should be noted that the development of an association was not a requirement to solve the detour task. Nevertheless, providing this additional cue allows for the evaluation of this possibility, which has been shown before in other species of lizards including anoles (Leal and Powell 2012).
Testing
Lizards were located by walking slowly through the forest. Once a marked lizard was found, we walked slowly towards the lizard’s perch and placed the apparatus perpendicular to the lizard’s line of sight within a meter of the base of its perch. The orientation of the patterns was randomly determined for each trial relative to the lizard’s line of sight; thus, from the lizard’s perspective, a given pattern and the spatial position of an entrance were not correlated. Under field conditions, it was not possible to place the apparatus in the exact same position for every trial. Instead, the apparatus was placed in proximity to the focal individual, as described above, which likely corresponded to the territory of that lizard. Each individual completed 0–10 trials per day, and lizards were tested until they were no longer found or they stopped approaching the apparatus.
Prior to presenting the lizards with the complete half-cylinder, lizards were habituated to the painted wood platform and the pattern sections of the half-cylinder by presenting the apparatus with the reward, but without the central section (Fig. 1a). This allowed lizards to access the reward unimpeded and controlled for the possibility that lack of participation in the experiment was due to neophobia. A total of 23 lizards completed this stage of the experiment after grabbing the reward in seven consecutive trials, exhibiting behavior typical of anoles while foraging. Individuals directly approached the reward, usually as part of a sprint that concluded with the lizard striking the reward, all happening as a single event (Online Resource 1).
After the lizards completed habituation, the central section of the half-cylinder was replaced (Fig.1b), presenting the lizards with the novel detour problem of accessing the reward behind a transparent barrier (Online Resource 2). We defined a trial as a success whenever the lizard accessed the reward. We predicted that the number of touches to the cylinder would decrease as lizards experienced the obstacle presented by the barrier and reached our learning criterion. Thus, our experiment is designed to measure problem-solving by trial-and-error learning with the expectation that motor self-regulation will be exhibited incrementally (Shettleworth 2010). The criterion to solve the detour problem was to reach the reward in 7 of their previous 8 trials, similar to criteria commonly used in learning experiments (e.g., Schiller 1949b; Spigel 1964; Scholes 1965; Scholes and Wheaton 1966; Anderson et al. 2017; Szabo et al. 2019).
A trial lasted a maximum of 15 min, beginning when the apparatus was placed in front of a lizard, and ending when the lizard took the reward or the 15-min period elapsed. Using a stopwatch and a notebook, we recorded all behaviors exhibited by the lizard, including the side by which the lizard entered the apparatus and the number of touches (i.e., the number of times a lizard placed its snout against the outside of the transparent barrier) made during each trial. To reach the reward, lizards had to place most of their body inside the apparatus. Trials in which individuals did not approach the apparatus within one body length are not included in the analysis. We evaluated the strategies employed by individuals in solving the detour problem by determining which cues were correlated with an individual’s access to the reward. Individuals demonstrated preference for access using spatial (i.e., side) cues, pattern cues, or neither. We evaluated this by calculating a bias index as performed in Szabo et al. (2019). The magnitude of the bias index indicates the strength of the bias towards one cue, either spatial (left or right) or pattern (checked or striped). A preference was indicated when the bias index for one cue was greater than the other, and no preference was indicated when indices for each cue were equal. Because we were working with free-living lizards and individuals can move outside the forest fragment while the study was going on, we only counted lizards as having participated in the experiment if they attempted at least seven detour trials. It was not possible to record data blind because we conducted focal observations of animals in the field.
Statistics
All statistical tests are two-tailed and were performed using R statistical software (R Development Core Team 2008) with the package nlme (Pinheiro et al. 2019). A linear mixed effects model was used to test for a decrease in number of touches as individuals proceeded through the detour task. In particular, the model evaluated if the number of touches decreased as a function of trial number, including only individuals that reached criterion in the detour task. Because each individual completed a different number of trials to reach criterion, we scaled trial number for each individual between 0 and 1 by dividing each trial number by the number of the last trial for each individual. Only successful trials in which individuals accessed the reward were included in this analysis. We used a linear mixed effects model with the number of touches as the response variable and scaled trial number as the predictor with individual as a random effect. Our data conformed to all assumptions of this model. The graph was generated using the package ggplot2 (Wickham 2009).
Results
Twenty-three lizards completed habituation (Table 1). Of those, 17 lizards participated in the detour problem, and 9 of those lizards solved the problem. Sex, body size, and mass did not differ between the lizards that solved the detour problem and those that failed (two-sided Fisher’s exact test, n = 17, P = 0.15; two-sided Wilcoxon signed rank sum test, W = 28.5, n = 17, r = 0.178, P = 0.49 and W = 27.5, n = 17, r = 0.199, P = 0.44 respectively). Lizards used three strategies to solve the detour problem. Five lizards solved the problem by entering more often through a given side (i.e., left or right entrance with respect to their line of sight). One lizard entered the half-cylinder more often under a given pattern (i.e., striped or checkered) irrespective of its orientation with respect to the lizard’s perch site. Three lizards showed neither preference (Online Resource 4).
Lizards that ultimately solved the detour problem improved their performance by reducing the number of touches committed during their approach to the reward, rather than through greater persistence (Fig. 2). The latter would have resulted in an increase in touches rather than a decrease. A significant negative fixed effect of scaled trial number on the number of touches was detected with individual as a random effect (Fig. 2, n = 74, estimate ± standard error = − 5.98 ± 2.91, df = 64, P = 0.04), indicating that individuals decreased the number of touches made during trials as they progressed through the detour problem and reached criterion.
The relationship between scaled trial number and the number of touches made by individuals of Anolis sagrei that solved the detour problem. Trial number was scaled for each individual between 0 and 1 by dividing each trial number by the number of the last trial for each individual. Values are the number of touches made by one individual during one trial in solving the detour problem
Discussion
Our findings demonstrate the feasibility of experimentally evaluating the cognitive abilities of free-ranging lizards in nature and of the ability of Anolis sagrei to exhibit motor self-regulation in response to experience with a transparent surface, resulting in a decrease in the number of touches made as they progressed through the detour problem. The ability of A. sagrei to solve the detour problem provides further evidence that the cognitive abilities of lizards, and more specifically Anolis, can provide significant insights into potential factors shaping the evolution of behavioral flexibility and motor self-regulation (Leal and Powell 2012; Burghardt 2013). The solution to the problem required major changes to the highly stereotyped foraging behavior of A. sagrei (Losos 2009), which consists of scanning the habitat for moving prey and approaching them directly in a single burst of movement before striking. This was a behavioral strategy that was ineffective in our experiment because the transparent section of the half-cylinder served as a physical barrier between the reward and the lizard. Instead, lizards moved either towards an entrance before turning to enter the half-cylinder, that is, approached the apparatus at an angle away from the prey, or moved toward the prey and detoured away from it after reaching the apparatus in order to reach an open end. Therefore, regardless of the approach taken by the lizards, the prey was temporarily not visible (i.e., out of the line of sight), potentially resulting in a temporal mismatch between reaching the reward and decision-making.
Behavioral flexibility has received significant attention as a trait that contributes to species’ invasive abilities, particularly in birds (Lefebvre et al. 1997; Sol et al. 2002, 2013), although there are conflicting ideas regarding the degree of flexibility needed for successful invasion (reviewed in Wright et al. 2010). Anolis sagrei is the most invasive anole species (Kolbe et al. 2014), and our findings open the possibility for further work to evaluate the potential contribution of behavioral flexibility to its invasive ability. Future work, comparing the cognitive abilities of multiple species of anoles, including those with different degrees of invasiveness, should shed light on this hypothesis. Alternatively, a study evaluating problem-solving abilities between native and invasive populations of the same species can also shed light on the potential contributions of cognition to exploit novel habitats (Wright et al. 2010). Also, both A. sagrei and A. evermanni are members of the Caribbean anole radiation which is characterized by the independent evolution of ecological forms and convergence in morphological and behavioral traits across those forms (Losos 2009). Behavioral flexibility has been proposed as a contributor to the radiation of clades by facilitating the exploration of novel environments (Sol and Price 2008; Tebbich et al. 2010; Leal and Powell 2012).
Lizards used three strategies to access the reward. The most common strategy was for a lizard to preferentially enter the apparatus through the same side (i.e., right or left with respect to the individual’s line of sight), regardless of the pattern of the entrance (Online Resource 2). The apparatus was not placed in the same spot for every trial, and thus we are unable to disentangle the attendance to allocentric vs. egocentric cues in our experiment. A less common strategy was a preference for entering the half-cylinder through an entrance covered by a given pattern (i.e., vertical lines or checkered) regardless of the side, suggesting the development of an association between access to the reward and one of the distinct patterns at each entrance. Both strategies might be expected of a territorial species, such as A. sagrei, where the ability to associate particular cues or landmarks with ecologically important information can contribute to an individual’s fitness (Ladage et al. 2009; Leal and Powell 2012).
On average, the number of touches to the cylinder made by individuals of A. sagrei that solved the detour problem decreased significantly across trials (Fig. 2). This finding indicates that individuals are not only learning how to reach the reward by achieving our learning criterion but also to avoid using a natural behavior (i.e., striking through the transparent barrier) that is ineffective at securing the reward. Furthermore, as has been shown in corvid species (Kabadayi et al. 2016), although individuals of A. sagrei had no previous experience with transparent surfaces, relatively few presentations were needed to reduce their motor response and improve their ability to access a reward behind a transparent surface (Table 1). This reduction occurred even in the absence of a training phase with the opaque surface, which can shape the behavior of individuals as they interact with a transparent surface and increase the likelihood of accessing the reward without touching the cylinder (Santos et al. 1999; Vlamings et al. 2010; Isaksson et al. 2018). It should be noted that a reduction in the number of touches (i.e., motor self-regulation) is even more surprising when considering that individuals performed the tasks while facing all the potential distractions of a natural environment, including competition for food. The latter should select for quick, impulsive behavior, which is commonly exhibited when multiple individuals are chasing the same moving prey. However, individuals were able to modify the required behavior to solve the task. Nonetheless, as shown in (Fig. 2), individuals varied in their ability to inhibit striking at the reward through the transparent barrier (i.e., individual differences in the magnitude of the decrease in touches), which demonstrates that the paradigm used in this study can also be used to evaluate individual variation in cognitive performance under natural conditions.
The ability of individuals to inhibit the natural response of attempting to access a food reward through a transparent barrier has been measured across a diversity of endothermic species, and the findings suggest that in primates, dietary breadth predicts cognitive performance (MacLean et al. 2014; Kabadayi et al. 2016). However, compared with primates, the dietary breadth of A. sagrei is relatively narrow, suggesting that other aspects of species ecology are also likely to contribute to the ability of species to inhibit natural responses. Furthermore, our findings also challenge the prediction that species like A. sagrei, for which success of prey capture is determined by their ability to surprise moving prey, are unlikely to exhibit motor self-regulation abilities (Shettleworth 2010). This finding provides further evidence of the potential insights that can be gained by studying lizard cognition.
Two factors that are commonly suggested to contribute to individual differences in performance on cognitive tasks are body size and neophobia (Shettleworth 2010; Wright et al. 2010). In lizards, including A. sagrei, body size can be correlated with age, thus potentially influencing previous experience (Noble et al. 2014). Age has been shown to affect behavioral diversity and neophobia in spotted hyenas, both of which are associated with increased problem-solving success (Benson-Amram and Holekamp 2012). The body size of A. sagrei was not significantly different between those individuals that solved the detour problem and those that failed. Furthermore, all the individuals that participated in the detour problem had completed the habituation period by grabbing the maggot from the same apparatus in seven consecutive trials. Therefore, it seems unlikely that differences in body size or neophobia account for our results.
Elucidating the shared ecological demands that have favored the evolution of cognitive traits across species is a long-standing goal of cognitive ecology. Our findings that A. sagrei exhibits motor self-regulation provide further evidence that the cognitive abilities of Anolis lizards, and more generally reptiles, have been underappreciated, as this ability is commonly suggested to underpin higher cognitive processes such as decision making and problem-solving (Kabadayi et al. 2016; Vernouillet et al. 2016). Furthermore, as discussed above, the dietary breadth, foraging behavior, and social structure of A. sagrei are unlike those previously associated with the evolution of motor self-regulation, suggesting that other aspects of species ecology might also contribute to this behavior. One possibility is that motor self-regulation is necessary for the evolution of other cognitive traits, such as behavioral flexibility, including reversal learning, which requires the use of inhibitory responses and has been shown in multiple species of lizard (e.g., Day et al. 1999, Leal and Powell 2012, Szabo et al. 2019 and references there in). Under this scenario, selection favoring the ability to modify pre-existing behaviors would also favor the evolution of motor self-regulation. More studies across a diversity of taxa, particularly with free-living organisms, are needed in order to develop a cohesive framework in which to evaluate the interactions between species ecological demands and the evolution of cognitive traits.
Our findings underscore the potential advantages of conducting cognitive experiments under natural conditions. Captivity causes subjects potential stress and presents conditions that greatly differ from those found in nature, both of which can affect behavioral responses in an unpredictable fashion (Steinberg and Leal 2018). In the case of A. sagrei, this approach also provides an opportunity in which unique factors of the species’ ecology, such as territoriality and spatial awareness, are more likely to be exhibited and to contribute to problem-solving ability.
More generally, the most commonly used version of the cylinder task includes a training phase with an opaque cylinder (see intro for references). This training step likely reduces the number of species that can be tested using the method, for example, sit-and-wait foragers or those that rely on visual motion cues for the detection of prey are unlikely to be able to perform the task due to lack of adequate stimulation. Our modification—elimination of the opaque cylinder phase—allowed us to use the cylinder task with a visually oriented, sit-and-wait forager, A. sagrei. Our adaptation of the paradigm illustrates the utility of using experimental paradigms that are rooted in an understanding of the natural history of the species of interest (i.e., their “umwelt”), which is a critical component for conducting experiments with free-living individuals. Furthermore, it provides a methodology to evaluate problem-solving through motor self-regulation by measuring the reduction in the number of touches made to the cylinder as individuals gain experience by interacting with the transparent surface (Vernouillet et al. 2016). The apparatus used in this study can also be used to evaluate associate and reversal learning under natural conditions in free-living animals by limiting the access to the reward to a given pattern and/or changing the reward contingency, providing an opportunity to evaluate performance of the same individuals across multiple cognitive tasks.
In summary, by implementing a modified version of the cylinder task, we demonstrate the feasibility of experimentally evaluating the cognitive abilities of free-living lizards, opening a new avenue for the study reptile cognition. Lizards have been mostly studied under laboratory settings (Burghardt 2013), potentially limiting our ability to sample the diversity of taxa within this clade and consequently hampering our understanding of the cognitive abilities of a diverse group, which is characterized by using distinct niches and showing a wide range of life-history traits. Therefore, understanding variation in lizard cognition is likely to shed light on current efforts to elucidate shared ecological demands contributing to the evolution of cognitive traits across vertebrates. Furthermore, our results demonstrate that a sit-and-wait forager can modify its foraging strategy relatively quickly in the face of a novel problem, providing further evidence of behavioral flexibility in anoles (Leal and Powell 2012) and of motor self-regulation. Additionally, A. sagrei does not possess the traits previously associated with the evolution of motor self-regulation (MacLean et al. 2014; Kabadayi et al. 2016), suggesting that other aspects of a species’ ecology can also contribute to this ability.
Data availability
The datasets generated during and analyzed during the current study are available in the Dryad repository, https://doi.org/10.5061/dryad.g79cnp5mq.
References
Amici F, Aureli F, Call J (2008) Fission-fusion dynamics, behavioral flexibility, and inhibitory control in primates. Curr Biol 18:1415–1419
Anderson RC, Searcy WA, Peters S, Hughes M, DuBois AL, Nowicki S (2017) Song learning and cognitive ability are not consistently related in a songbird. Anim Cogn 20:309–320
Auersperg AMI, von Bayern AMP, Gajdon GK, Huber L, Kacelnik A (2011) Flexibility in problem solving and tool use of kea and New Caledonian crows in a multi access box paradigm. PLoS One 6:e20231
Benson-Amram S, Dantzer B, Stricker G, Swanson EM, Holekamp KE (2016) Brain size predicts problem-solving ability in mammalian carnivores. P Natl Acad Sci USA 113:2532–2537
Benson-Amram S, Holekamp KE (2012) Innovative problem solving by wild spotted hyenas. Proc R Soc Lond B 279:4087–4095
Boogert NJ, Anderson RC, Peters S, Searcy WA, Nowicki S (2011) Song repertoire size in male song sparrows correlates with detour reaching, but not with other cognitive measures. Anim Behav 81:1209–1216
Burghardt GM (2013) Environmental enrichment and cognitive complexity in reptiles and amphibians: concepts, review, and implications for captive populations. Appl Anim Behav Sci 147:286–298
Cauchoix M, Hermer E, Chaine AS, Morand-Ferron J (2017) Cognition in the field: comparison of reversal learning performance in captive and wild passerines. Sci Rep 7:12945
Croston R, Branch CL, Pitera AM, Kozlovsky DY, Bridge ES, Parchman TL, Pravosudov VV (2017) Predictably harsh environment is associated with reduced cognitive flexibility in wild food-caching mountain chickadees. Anim Behav 123:139–149
Davis KM, Burghardt GM (2011) Turtles (Pseudemys nelsoni) learn about visual cues indicating food from experienced turtles. J Comp Psychol 125:404–410
Day LB, Crews D, Wilczynski W (1999) Spatial and reversal learning in congeneric lizards with different foraging strategies. Anim Behav 57:393–407
Diamond A (1990) Developmental time course in human infants and infant monkeys, and the neural bases of, inhibitory control in reaching. Ann N Y Acad Sci 608:637–676
Epstein R, Kirshnit CE, Lanza RP, Rubin LC (1984) ‘Insight’ in the pigeon: antecedents and determinants of an intelligent performance. Nature 308:61–62
Fleishman LJ, Pallus AC (2010) Motion perception and visual signal design in Anolis lizards. Proc R Soc Lond B 277:3547–3554
Griffin AS, Guez D (2014) Innovation and problem solving: a review of common mechanisms. Behav Process 109B:121–134
Healy SD, Hurly TA (2003) Cognitive ecology: foraging in hummingbirds as a model system. Adv Study Behav 32:325–359
Isaksson E, Utku Urhan A, Brodin A (2018) High level of self-control ability in a small passerine bird. Behav Ecol Sociobiol 72:118
Johnson-Ulrich L, Holekamp KE (2020) Group size and social rank predict inhibitory control in spotted hyaenas. Anim Behav 160:157–168
Kabadayi C, Bobrowicz K, Osvath M (2018) The detour paradigm in animal cognition. Anim Cogn 21:21–35
Kabadayi C, Taylor LA, von Bayern AMP, Osvath M (2016) Ravens, New Caledonian crows and jackdaws parallel great apes in motor self-regulation despite smaller brains. R Soc Open Sci 3:160104
Kolbe JJ, Ehrenberger JC, Moniz HA, Angilletta MJ Jr (2014) Physiological variation among invasive populations of the brown anole (Anolis sagrei). Physiol Biochem Zool 87:92–104
Ladage LD, Riggs BJ, Sinervo B, Pravosudov VV (2009) Dorsal cortex volume in male side-blotched lizards (Uta stansburiana) is associated with different space use strategies. Anim Behav 78:91–96
Leal M, Powell BJ (2012) Behavioural flexibility and problem-solving in a tropical lizard. Biol Lett 8:28–30
Lefebvre L, Whittle P, Lascaris E, Finkelstein A (1997) Feeding innovations and forebrain size in birds. Anim Behav 53:549–560
Logan CJ (2016) How far will a behaviourally flexible invasive bird go to innovate? R Soc Open Sci 3:160247
Losos JB (2009) Lizards in an evolutionary tree: ecology and adaptive radiation of anoles. University of California Press, Berkeley
Lucon-Xiccato T, Bisazza A (2017) Individual differences in cognition among teleost fishes. Behav Process 141:184–195
MacLean EL, Hare B, Nunn CL et al (2014) The evolution of self-control. P Natl Acad Sci USA 111:E2140–E2148
Moermond TC (1981) Prey-attack behavior of Anolis Lizards. Z Tierpsychol 56:128–136
Morand-Ferron J, Hamblin S, Cole EF, Aplin LM, Quinn JL (2015) Taking the operant paradigm into the field: associative learning in wild great tits. PLoS One 10:e0133821
Noble DWA, Byrne RW, Whiting MJ (2014) Age-dependent social learning in a lizard. Biol Lett 10:20140430
Noble DWA, Carazo P, Whiting MJ (2012) Learning outdoors: male lizards show flexible spatial learning under semi-natural conditions. Biol Lett 8:946–948
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2019) nlme: linear and nonlinear mixed effects models. https://CRAN.R-project.org/package=nlme
Pough FH (1991) Recommendations for the care of amphibians and reptiles. ILAR News 33:S1–S21
Pritchard DJ, Hurly TA, Tello-Ramos MC, Healy SD (2016) Why study cognition in the wild (and how to test it)? J Exp Anal Behav 105:41–55
R Development Core Team (2008) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org
Santos LR, Ericson BN, Hauser MD (1999) Constraints on problem solving and inhibition: object retrieval in cotton-top tamarins (Saguinus oedipus oedipus). J Comp Psychol 113:186–193
Schiller PH (1949a) Analysis of detour behavior; learning of roundabout pathways in fish. J Comp Physiol Psychol 42:463–475
Schiller PH (1949b) Delayed detour response in the octopus. J Comp Physiol Psychol 42:220–225
Schoener TW, Schoener A (1982) Intraspecific variation in home-range size in some Anolis lizards. Ecology 63:809–823
Scholes NW (1965) Detour learning and development in the domestic chick. J Comp Physiol Psychol 60:114–116
Scholes NW, Wheaton LG (1966) Critical period for detour learning in developing chicks. Life Sci 5:1859–1865
Schwarz S, Mangan M, Zeil J, Webb B, Wystrach A (2017) How ants use vision when homing backward. Curr Biol 27:401–407
Shettleworth SJ (2010) Cognition, evolution, and behavior, 2nd edn. Oxford University Press, New York
Sol D, Duncan RP, Blackburn TM, Cassey P, Lefebvre L (2005) Big brains, enhanced cognition, and response of birds to novel environments. P Natl Acad Sci USA 102:5460–5465
Sol D, Lapiedra O, González-Lagos C (2013) Behavioural adjustments for a life in the city. Anim Behav 85:1101–1112
Sol D, Price TD (2008) Brain size and the diversification of body size in birds. Am Nat 172:170–177
Sol D, Timmermans S, Lefebvre L (2002) Behavioural flexibility and invasion success in birds. Anim Behav 63:495–502
Spigel IM (1964) Learning, retention, and disruption of detour behavior in the turtle. J Comp Physiol Psychol 57:108–112
Stamps JA (1977) The function of the survey posture in Anolis lizards. Copeia 1977:756–758
Steinberg DS, Leal M (2018) Wild vs lab - Cognition outside the box. In: Bueno-Guerra N, Amici F (eds) Field and laboratory methods in animal cognition: a comparative guide. Cambridge University Press, Cambridge, pp 279–285
Szabo B, Noble DWA, Whiting MJ (2019) Context-specific response inhibition and differential impact of a learning bias in a lizard. Anim Cogn 22:317–329
Tebbich S, Sterelny K, Teschke I (2010) The tale of the finch: adaptive radiation and behavioural flexibility. Philos Trans R Soc B 365:1099–1109
Vernouillet A, Anderson J, Clary D, Kelly DM (2016) Inhibition in Clark’s nutcrackers (Nucifraga columbiana): results of a detour-reaching test. Anim Cogn 19:661–665
Vlamings PHJM, Hare B, Call J (2010) Reaching around barriers: the performance of the great apes and 3-5-year-old children. Anim Cogn 13:273–285
von Bayern AMP, Heathcote RJP, Rutz C, Kacelnik A (2009) The role of experience in problem solving and innovative tool use in crows. Curr Biol 19:1965–1968
Wickham H (2009) ggplot2: Elegant graphics for data analysis. Springer-Verlag, New York
Wilkinson A, Kuenstner K, Mueller J, Huber L (2010) Social learning in a non-social reptile (Geochelone carbonaria). Biol Lett 6:614–616
Wright TF, Eberhard JR, Hobson EA, Avery ML, Russello MA (2010) Behavioral flexibility and species invasions: the adaptive flexibility hypothesis. Ethol Ecol Evol 22:393–404
Wynne CDL, Leguet B (2004) Detour behavior in the Quokka (Setonix brachyurus). Behav Process 67:281–286
Acknowledgments
We thank J. Jones and W. McHargue for assistance in the field; the members of the Chipojo lab, D. Steinberg, R. Cocroft, G. Burghardt; and two anonymous reviewers for helpful comments that greatly improved this manuscript; the National Science Foundation (DEB-0949357 and IOS-1051793); and the Bahamas Ministry of Agriculture and the Bahamas Environment, Science, and Technology Commission of the Ministry of the Environment for permission to conduct this research.
Ethics approval
This research adhered to the guidelines of the Institutional Animal Care and Use Committee at University of Missouri, Columbia protocol (#8244). We followed the Recommendations for the Care of Amphibians and Reptiles (Pough 1991) in the treatment of all animals used in this study. This is a field study, and as part of this study, animals were only individually marked and immediately released. The Bahamas Ministry of Agriculture and the Bahamas Environment, Science, and Technology Commission of the Ministry of the Environment provided permission to conduct this research.
Funding
This work was partially funded by the National Science Foundation (DEB-0949357 and IOS-1051793 to ML).
Author information
Authors and Affiliations
Contributions
LS carried out the experiment, participated in data analysis, participated in the design of the study, and helped in drafting the manuscript; ML conceived of the study, helped with the data analysis, and drafted the manuscript. All authors gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Communicated by T. Madsen
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Storks, L., Leal, M. Thinking outside the box: problem-solving in free-living lizards. Behav Ecol Sociobiol 74, 75 (2020). https://doi.org/10.1007/s00265-020-02852-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-020-02852-x