Abstract
The relationship between individual physiological traits and social behaviour is an important research area because it can examine how mechanisms of behaviour link to functional outcomes. It is hypothesised that correlative and causative links between physiology and individual behaviour may be altered by social interactions. Here, we assess how nutritional stress (20-h starved, 90-h starved) and routine metabolic rate (RMR) determine the movement and foraging behaviour of threespine sticklebacks (Gasterosteus aculeatus), both individually and in a social context. Results showed that there was no statistically significant relationship between RMR and behaviour. The nutritional stress treatment had significant opposite effects on voluntary swim speed, dependent on whether fish were assayed asocially (alone) or socially (in shoals of three). Greater nutritional stress caused voluntary swimming speeds to reduce in an asocial context but increase in a social context, although both relationships were not significant. Additional results exploring social behaviour parameters such as the frequency and duration of shoaling interactions suggests that alterations in fish swim speed between the two nutritional stress treatments may be due to competition effects. This study links state-dependent individual behaviour to social foraging performance and reinforces the theory that social context is an important modulator of the relationships between physiology and behaviour.
Significance statement
Recent research has highlighted that the social environment may shape how physiology and behaviour are linked. This area of research, however, requires data from empirical studies that measure and experimentally manipulate physiological traits of individually identifiable animals and tests them under asocial and social conditions. Using threespine sticklebacks foraging for bloodworms, we show that routine metabolic rate did not have a statistically significant effect on fish locomotion or risk-taking. Greater nutritional deprivation caused fish to decrease their swimming speed when they were alone (likely in an effort to reduce energy expenditure); however, when assayed in groups, competitive forces between shoal mates caused them to swim at faster voluntary speeds. Nutritional stress therefore had a significant socially dependent effect on fish locomotion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animal sociality is common across taxa; however, the science investigating the physiological mechanisms that determine both the composition of phenotypes within groups and the behaviour of individuals within groups is underdeveloped (Killen et al. 2017; Seebacher and Krause 2017), this is despite growing evidence that animal sociality has important ecological and evolutionary implications (Burton et al. 2011; Farine et al. 2015; Killen et al. 2017). Whilst individual benefits from grouping may include reduced predation risk, reduced energetic cost of locomotion, and increased access to mates and social information, individuals must weigh these benefits against costs, which may include increased competition for food or greater transmission rates of parasites and disease (Ward and Webster 2016). Critically, the trade-offs between these costs and benefits (which ultimately determine behaviour) are unlikely to be equal between individuals within a group or population because they depend on short-term (minutes, hours, days) and longer-term (months, years) variations in physiological state (Houston and McNamara 1999).
Despite evidence that many animal groups sort based on phenotypic similarity, there is also evidence of between-individual variation in physiological state (e.g. nutritional state, metabolic rate, hormone levels) in naturally occurring groups (Lihoreau et al. 2015; Ward and Webster 2016). Differences in physiology are known to relate to both inter- and intra-individual variation in behaviour (Careau et al. 2008; Huntingford et al. 2010; Metcalfe et al. 2016), with inter-individual variation in behaviour thought to arise and be maintained via the differential effect of the environment on an individual’s particular physiological state, as well as the subsequent positive feedbacks between physiological state and behavioural performance (Biro and Stamps 2010; Wolf and Weissing 2010; Dingemanse and Wolf 2013; Sih et al. 2015). Critically, the social environment will affect these interactions, and conspecifics should be viewed as an additional external stimulus—dynamically affecting energetic intake, predation risk, and reproductive opportunities of individual group members (Janson 1985; Stacey and Koenig 1990; Wolf and Moore 2010).
Facultatively shoaling fish species are an ideal model system to examine the costs and benefits of vertebrate group behaviour, primarily due to their tractability and an ability to experimentally manipulate both their internal and external stimuli (Killen et al. 2011; Berdahl et al. 2013; Schaerf et al. 2017; Jolles et al. 2018). They are also biologically meaningful models for determining whether individual relationships between physiology and behaviour are maintained in a social context as wild individuals can be found alone or within groups. Membership in these groups is often fluid, with group size and individual composition dependent on a range of interacting internal and external stimuli (Krause 1993; Croft et al. 2005). They offer researchers an excellent opportunity to assess physiology-behaviour correlates in individuals and whether these correlates are maintained within groups.
The effect of energetic or nutritional state will vary dynamically over time as animals feed and move around their environment, and this is known to affect individual and group movement behaviour of facultatively shoaling species. In general, extensive or chronic food deprivation is likely to cause animals to reduce activity in order to save energy; however, more acute or recent food deprivation may lead to temporary increases in activity and movement (Speigel et al. 2013). For example, voluntary swimming speed in mosquitofish (Gambusia holbrooki) was affected by acute alterations in nutritional state (Hansen et al. 2015a). Nutritionally stressed mosquitofish (starved for 24 h) had higher average swimming velocities and greater variances in velocity than recently fed mosquitofish. In rainbowfish (Melanotaenia duboulayi), nutritional stress (starved for 48 h) affected within-group positional differences, with nutritionally stressed fish forming smaller shoals and spending more time at the front of shoals where they attained more food compared with recently fed fish (Hansen et al. 2015b, 2016a; see also Krause 1993; McLean et al. 2018). A recent study on x-ray tetras (Pristella maxillaris) showed that shoals with a higher proportion of hungry individuals (starved for ~ 48 h) swam faster, increased their inter-individual distance, and had greater mean group transfer entropy, which suggests greater information flow between fish (Wilson et al. 2019). Both fish in isolation and fish within shoals take more risks if they are food deprived (Krause et al. 1992; Balaban-Feld et al. 2019). Balaban-Feld et al. (2019) found that under-fed (1/10th ration) fish took more foraging trips into a risky area than well-fed fish did in the presence of an avian predator. Nutritional stress, through its effect on speed, spatial positioning, and risk-taking, is therefore an important physiological determinant of group dynamics in fish.
Nutritional stress and its effect on energetic state are dynamic across short time scales; however, there is strong evidence for longer-term, consistent inter-individual differences in energetic state determined by metabolic rate. Resting metabolic rate, the energy an animal requires for basic maintenance, has been found to be repeatable in fish and related to many behaviours, including risk-taking (Finstad et al. 2007; Huntingford et al. 2010), dominance (Metcalfe et al. 1995; Huntingford et al. 2010), and shoaling position (Killen et al. 2012; Ward et al. 2018; McLean et al. 2018) (see Metcalfe et al. 2016 for a review). Critically, a correlation between metabolic rate and certain behaviours is likely context dependent (e.g. nutritional state: Killen et al. 2011; habitat complexity and predictability of food supply: Finstad et al. 2007; Reid et al. 2012). Nutritional stress has been proposed as a revealer or amplifier of correlations between behaviour and metabolic rate (Finstad et al. 2007; Killen et al. 2013). For example, under conditions of food deprivation, fish with higher metabolic rates should experience greater nutritional stress and motivation to forage than fish with lower metabolic rates, thereby revealing correlations between behaviour (e.g. risk-taking) and metabolic rate, which may not be evident under conditions of food surplus (Killen et al. 2011). Similarly, it has been proposed that any correlation between metabolic rate and behaviour of individual animals may strengthen when these animals are put into groups, which are competing for resources (Killen et al. 2013). In a recent study of common minnows (Phoxinus phoxinus) competing for food in a swim tunnel, McLean et al. (2018) showed that within-group positional shifts after feeding were explained by aerobic metabolic scope (after accounting for specific dynamic action)—fish faced a trade-off between the energetic costs of swimming at the front of the group and those of processing the recently consumed meal.
Threespine sticklebacks are a model species in behavioural ecology that form loose fission-fusion groups within which nutritional state is known to influence social behaviour (e.g. Krause 1993; Frommen et al. 2007; Riddell and Webster 2017). Here, we assess how nutritional stress (20-h starved, 90-h starved) determined the movement and foraging behaviour of threespine sticklebacks, both individually and in a social context. We tested individually identified fish repeatedly in a common risk-taking foraging assay, both by themselves and in groups of three, where fish had to leave a shelter before moving across an open arena to a food patch (Ioannou and Dall 2016; McDonald et al. 2016). We also assessed the relationship of these fish’s metabolic rate to their movement and foraging behaviour when tested asocially and socially in this foraging assay under the two nutritional state treatments. Hypotheses exist for the effect of short-term (e.g. nutritional stress) and longer-term (e.g. metabolic rate) physiological determinants of individual behaviour (Biro and Stamps 2010; Wolf and Weissing 2010; Dingemanse and Wolf 2013; Sih et al. 2015); however, how these long- and short-term determinants interact and how their effects on individual movement scale to group behaviour are less certain. For example, a need to stay cohesive in groups may weaken physiological determinants of individual behaviour, or, conversely, competition between group members may strengthen the correlations between individual physiology and behaviour (Killen et al. 2013). We predict that in our experiment, nutritional stress (a short-term determinant of behaviour) will relate positively to individual risk-taking movements, locomotion, and foraging performance, with hungrier fish (90-h starved) emerging from the shelter faster, swimming faster, and finding the foraging patch faster than less hungry fish (20-h starved). Within groups, we predict competition and social facilitation and conformity to amplify these results as hungrier fish have a greater energetic need to out-compete conspecifics than less hungry fish do. Similarly, we predict metabolic rate (a long-term determinant of behaviour) will correlate with risk-taking behaviour (latency to leave the shelter—fish with higher routine metabolic rate (RMR) will leave the shelter faster), locomotion (swimming velocity—fish with higher RMR will swim faster), and foraging performance (latency to ingest the food item—fish with higher RMR will eat the first bloodworm), and for greater nutritional stress to strengthen the correlation.
Methods
Species and holding
Threespine sticklebacks (n = 72) were collected using electrofishing from Putah Creek in December 2017, upstream from the town of Winters, CA, USA, above the Putah Creek Diversion Dam (38.489406°, − 122.011605°). Fish were kept in a 220-L flow through circular tank supplied with chilled well water at the UC Davis Centre for Aquatic Biology and Aquaculture. The tank was outside and experienced a natural day:night light cycle. Fish were held at 10 °C ± 0.5 and fed ad libitum frozen bloodworms (San Francisco Bay Brand, Inc.) for 3 months before the experiment began. We maintained and tested fish at this temperature to ensure fish were not in breeding condition (Ward et al. 2004). Fish used in the experiment had mean ± SE standard length of 41.3 ± 0.6 mm and a mean ± SE mass of 1.15 ± 0.05 g (n = 24). Fish used in the experiment were visually selected out of the larger sample of 72 wild caught fish if they were large enough for tagging (Webster and Laland 2009).
After a metabolic assay (see below for details), experimental fish (n = 24) were randomly separated amongst 8 holding tanks (flow through 85 L) into groups of 3 (social group I). Holding tanks were supplied with chilled well water held at 10 °C ± 0.5. Fish were kept at a 12:12 h day:night light cycle. Before being placed in their tank, each fish received a coloured tag placed over the first dorsal spine (black, green, or yellow, ACE® Economy Electrical Tape Vinyl) to allow for individual identification (Webster and Laland 2009). Fish were fed ad libitum frozen bloodworms for 1 h, once daily.
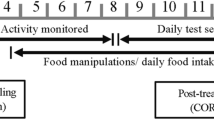
Overview of experimental period
Experimental fish underwent a metabolic assay and series of behavioural assays over a 28-day period. The metabolic rate assay occurred on day 0. A maximum of 6 fish (2 groups of 3) could be assayed each day. The order fish groups were assayed was randomised. After the metabolic assay on day 0 (see below for details), fish were placed into their holding tanks in groups of 3 (social group I) where they were allocated to a nutritional stress treatment (“Nut Stress”). Half of the tanks were allocated as 20-h starved and half as 90-h starved. Treatments were randomised in terms of tank position. The 20-h starved tanks were fed to satiation daily (they were presented with ad libitum frozen bloodworms for 1 h), receiving their last meal 20 h before each assay. The 90-h starved tanks were fed to satiation daily (as above) and received their last meal 90 h before each assay. These food restriction times are comparable with other behavioural studies using this species (Krause 1993; Riddell and Webster 2017).
As part of their first social group, fish then underwent two asocial behavioural assays (days 5 and 9), where each of the three fish was assayed separately, followed by a social behaviour assay (day 13), where all three fish in the social group were assayed together. After this, on day 15, all fish were rearranged amongst the tanks into new groups of 3 (social group II) such that no fish had any of the same shoal mates from social group I. Groups that were 20-h starved in social group I now underwent the 90-h starved treatment and vice versa. Fish then underwent two more asocial assays (days 20 and 24) and a social assay (day 28) as part of their second social group (social group II). Therefore, over the course of the entire experiment (28 days), fish were assayed a total of 4 times asocially (twice at 20-h starvation and twice at 90-h starvation) and twice socially (once at 20-h starvation and once at 90-h starvation). For both social groups (I and II), fish were housed with their conspecifics for 5 days before the first asocial behavioural assay.
Twenty-four hours after the end of the final social behavioural assay, fish were euthanised in MS-222 (Western Chemical Tricaine-S, 250 mg/L), and standard length (SL, mm) and mass (g) were recorded along with the visible presence of parasites (Schistocephalus solidus). Only 3 fish out of 24 had visible parasites. These 3 fish did not have statistically different metabolic rates or behavioural measurements, nor were they outliers, so the variable was excluded from all analysis.
Metabolic rate
For metabolic rate trials, seven custom-built 140-mL cylindrical glass respirometry chambers held on a plastic rack placed within a water bath filled with well water (0.4 ppt) were used. The water bath was continuously aerated with an air stone and temperature was maintained at 10 °C ± 0.5. Even dispersion of oxygenated water was attained by mixing the water with a pump. The entire water bath was enclosed in black plastic to reduce environmental disturbance. Oxygen consumption was measured using fibre optic-based dipping probes (Witrox 4-channel oxygen regulator systems, Loligo Systems, Tjele, Denmark) connected to a computer running AutoResp™ (v 2.3.0). Oxygen probes were calibrated prior to each trial using oxygen-free water (0.16 M sodium sulphate solution) and oxygen-saturated water (vigorous bubbling of sample with atmospheric air). To minimise background bacterial respiration, before and after every trial, 250 mL of bleach (Clorox® Regular Bleach) was added to the water bath and allowed to circulate through the system (pumps, hosing and respirometry chambers) for 2 h. Sodium metabisulphite was then added to the water bath and circulated through the equipment for 30 min before the water was drained and replaced with well water. The respirometry chambers were also cleaned with a 90% ethanol solution.
Six fish (2 of the 8 tanks) were run during a single trial with the 7th chamber left empty as a blank control. This was repeated 4 times on consecutive days (24 fish in total were assayed). Fish were fasted for 48 h to ensure they were in a post-absorptive state, before being moved from their holding tanks into the respirometry chambers at approximately 15:30 h. Oxygen concentrations were measured once per second. Oxygen consumption rates were collected overnight until approximately 11:00 h using intermittent flow respirometry. This time period was chosen as preliminary measures indicated that the lowest respiration rates were achieved overnight. Intermittent flow respirometry is a series of short, closed respirometry measurement periods (here 10 min), interrupted by flushing intervals where oxygen-depleted water is replaced (here 9 min). A wait period is needed to allow water to mix and for oxygen readings to decline linearly (Svendsen et al. 2016) (here 1 min). After the final measurement period, the fish were removed from the chambers, measured for standard length and mass, and placed back in their holding tanks, and the equipment was cleaned and prepared for the next set of 6 fish.
Mass-specific oxygen consumption (μmol g−1 min−1) was calculated using the slope of the decrease in O2 during each 10-min measurement period. Data was checked to ensure that oxygen consumption was consistent through time with an R-squared value above 0.98, indicating minimal spontaneous activity during the measure period. Any data that was below this criterion was not included for analysis. We then took the average of the 5 lowest measurement values for each fish as its routine metabolic rate (“RMR”). We were unable to attain RMR for one of the 24 fish due to equipment malfunction. Overall, the oxygen consumption rates per gram wet mass measured in this study were comparable with other studies of Gasterosteus aculeatus (Dalziel et al. 2012), which indicates that the techniques utilised provide a reasonable estimate of RMR.
Behavioural assay
Experimental arena
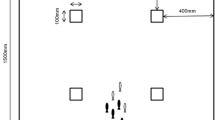
The design of the arena resembles those used with threespine sticklebacks in previous decision-making assays (see Ioannou and Dall 2016; McDonald et al. 2016). The arena (1350 mm long) was constructed out of 5-mm-thick white Perspex®, which created a shelter and open area separated by a remotely operated sliding door (see Fig. 1 for dimensions). At the end furthest from the door was a 20-mm red stimulus (red Vinyl tape wrapped around plastic air tubing). This is known to be highly conspicuous to sticklebacks (Ioannou and Krause 2009; Ioannou and Dall 2016; McDonald et al. 2016). The purpose of this stimulus was to attract the fish to the end of the arena where a foraging patch of 2 blood worms per fish was also placed. Halfway between the door and the stimulus was a 100 × 100 mm piece of Perspex®. The purpose of this piece was to delineate the positions within the arena from which fish were able to see the red stimulus (see Fig. 1). The arena was filled to a depth of 100 mm with well water (same source as holding tanks, 10 °C ± 0.5), and the arena was surrounded by white plastic sheeting to prevent external stimuli disturbing the fish, whilst allowing for evenly dispersed lighting for video recording and tracking.
Image and dimensions of the white Perspex® arena used for asocial and social behavioural assays. Visible on the left is the door to the shelter (100 × 100mm) in which fish started the assay. Green lines represent the start line, HW (halfway) line, and end line. Visible on the right is the red foraging stimulus and 2 food items (frozen bloodworms). The red lines indicate the regions of the arena where fish have a line of sight to the food items that is unobscured by the barrier (100 × 100mm) in the middle of the arena. Fish were given 5 min in the shelter before the door was raised remotely and were subsequently free to exit the shelter and forage for 510 s before being removed from the arena. Assays were filmed from above with a GoPro Hero 5 Session
Six asocial trials, or two social trials, were run each day (13:00–15:00 h). The order fish were assayed each day was randomised throughout the experiment. Fish were placed into the shelter for 5 min before the door was raised—allowing access to the open arena and the bloodworms. Prior to the door being raised, 2 bloodworms per fish (2 in the asocial and 6 in the social trials) were placed with forceps at the far end of the arena, directly underneath the red stimulus (see Fig. 1). Trials lasted 8.5 min and were filmed from above with a GoPro Hero 5 Session at 30 fps and 2.7 K in “Linear” field of view. Using EthoVision® XT (Noldus) automated tracking program, we determined each fish’s individual trajectory, recorded as a series of (x, y) coordinates (mid-body of fish) for all frames of the video, broken only by when the fish reentered the shelter and could not be visualised.
Behavioural parameters
Risk-taking movements
In each of the behavioural assays, using EthoVision® XT, we calculated each fish’s (a) latency to leave the shelter (defined by when the fish crossed a line that was one body-length distance (40 mm) from the shelter: “Exit”), (b) time to reach the halfway barrier (defined by when the fish crossed the halfway line of the arena: “HW”), and (c) time to reach the food patch at the end (defined by when the fish had crossed a line 80 mm from the end of the arena and 40 mm from the food items: “End”) (see Fig. 1, Table 1). If fish did not emerge from the shelter during the trial, they were given a max score of 510 s for exit and excluded from other analyses. From these parameters, we then calculated the time taken for the fish to move from the exit line to the halfway line (“Exit to HW”) and the time for the fish to move from the halfway line to the end line (“HW to End”) (Table 1). These last two measures were calculated because the design of the arena was such that fish were unable to see the food patch for the first of these two intervals (see Fig. 1) and we wanted to examine whether the effect of nutritional state affected fish differently depending on whether they could see this foraging stimulus. We also calculated each individual’s mean latency to leave the shelter across all four individual assay rounds, which we inversed and defined as their risk-taking score (“Risk Score”), such that the higher the value, the riskier the average behaviour of the fish was (Supplementary Table 1).
Locomotion and social grouping parameters
For the behavioural assays, using EthoVision® XT, we calculated the mean velocity (mm/s) of each fish during the entire trial, scaled by standard length (mm) (“Mean Velocity”) (Table 1). For the social assays, we also calculated the following social parameters: the inter-individual distance (defined as the mean distance in mm between a focal fish and each of its conspecifics at every time step over the course of the entire trial, one summary value for the entire trial) (“IID”), the frequency of 2 body-length interactions (defined as the number of times a focal fish moved to within 2 body lengths, 80 mm, of any conspecific) (“Soc Freq”), and the mean duration (s) of these 2 body-length interactions (defined as the mean time a focal fish stayed within 2 body lengths, 80 mm, of any conspecific before moving greater than 2 body lengths away from it) (“Soc Dur”). We used a 2 body-length criteria because it is used in this species to define when two individuals are shoaling (Riddell and Webster 2017), and swimming pairs of other facultative shoaling species, mosquitofish (Gambusia holbrooki), golden shiners (Notemigonus crysoleucas), and minnows (Phoxinus phoxinus), position themselves in front or behind their neighbours at ~ 1.5–2 body lengths (Partridge 1980; Herbert-Read et al. 2011; Katz et al. 2011) (Supplementary Table 1).
Foraging performance
To quantify foraging performance in the social trials, we determined which fish ate a bloodworm first (“BW First”) and the total number of bloodworms every fish ate during the trial (“BW Total”) (Supplementary Table 1).
To minimise observer bias, blinded methods were used when behavioural data were recorded.
Data analysis
All analysis was conducted in R, version 3.6.1 (R Core Team 2019), using RStudio (v1.2.1335). We present mixed-effects models fitted using the lme4 and glmer packages (Bates et al. 2014) with restricted maximum likelihood and Satterthwaite approximations for degrees of freedom to approximate p values of model parameter estimates. Full models were constructed with all variables of a priori interest. Subsequently, random effects were evaluated by assessing the degree of variance explained and whether estimated coefficients for fixed effects were influenced by the presence or absence of the random effects. If little variance was attributed to the random effects and the estimated fixed coefficients remained consistent with and without the random effects specified in the model, random effects were removed to increase parsimony. After random effects were selected, all possible nested models were fit using maximum likelihood, then compared by AICc (Burnham and Anderson 2002; Zuur et al. 2009) using the package MuMIn (Barton 2019). Criteria for the final model selection was the model with the fewest parameters that was also within 1 AIC of the lowest score. Assumptions of homogeneity of variance and normality were visually assessed with residual plots.
Nutritional stress and mass
To assess the efficacy of the nutritional stress treatments, we used a linear mixed model (LMM) evaluating the relationship between mass gained (“Mass Gain”) as a dependent variable and treatment (interacting categorical variables of “Nut Stress” and “Nut Order” (the order in which the fish underwent the nutritional stress treatments)) as an independent variable, whilst controlling for other relevant independent variables (“RMR” and “Initial Mass”). The body mass of the fish was recorded before and after each nutritional treatment. Fish ID was included in the full model as a random effect.
Relationship between social state, physiological variables, and behaviour
Movement behaviour
We conducted a series of LMMs to evaluate relationships between behavioural variables and both physiological and social states. Full models were constructed with “Nut Stress” (90 h or 20 h) and “Social State” (asocial or social) as one pair of interacting categorical independent variables and “RMR” and “Nut Stress” as another pair of interacting independent variables. Fish ID was considered a random effect. Using separate models, we tested the effect of these independent variables on five dependent variables, including locomotion (“Mean Velocity”) and risk-taking movements: latency to leave the shelter (“Exit”), time to reach the food patch (“End”), time to reach the halfway barrier (“Exit to HW”), and time from the halfway barrier to the food patch (“HW to End”). This last variable was included because fish could only see the foraging stimulus after they had passed the halfway point of the experimental arena. The movement variables were log transformed so the dependent variable would not be bounded. To evaluate risk-taking movement that occurred after exiting the shelter, we removed fish which never left the shelter and thus did not have measurements of transit time within the arena.
Social grouping
For the social assays, we assessed whether “Nut Stress” and “RMR” had an effect on social behaviours. We performed three LMMs with either “Soc Freq”, “Soc Dur”, or “IID” set as the dependent variable and “Nut Stress” and “RMR” as interacting independent variables. For these models, we considered fish ID and group ID as crossed random effects.
Foraging performance
To explore how physiology and individual behaviour affected foraging performance in a social context, we performed generalised linear mixed models (GLMMs). We ran two GLMMs to predict the dependent variables (a) “BW First” (using a binomial error structure and logit link function) and (b) “BW Total” (using a Poisson error structure with a log link function). Each model included the continuous independent variables of “Risk Score” and “Ind Mean Velocity”, two measures of an individual’s performance when assayed alone, as well as the interaction between “RMR” and “Nut Stress”. For both models, fish ID and group ID were considered crossed random effects.
Results
Nutritional stress and mass
“Nut Stress” (20- vs 90-h) and the individual RMR measurements (mean ± SE, 0.13 ± 0.003 μmol g−1 min−1, min = 0.097, max = 0.155, n = 23) were important in explaining the variance in mass gained. Fish starved 20 h gained more mass (mean ± SE, 0.115 ± 0.01 g) than 90-h starved fish which, on average, lost mass (mean ± SE, − 0.014 ± 0.008 g). The linear model estimated an effect size of an additional 0.131 g gained by fish in the 20-h treatment (SE = 0.013). Fish with lower RMR gained more mass, on average, with the linear model estimating an additional 0.862 g gained for each unit increase in RMR (SE = 0.489, p = 0.085).
Relationship between social state, physiological variables, and behaviour
Movement behaviour
Mean velocity of individual fish was best explained by the additive effects of “Nut Stress” (β = 0.214, SE = 0.140, p = 0.130) and “Social State” (β = − 0.168, SE = 0.125, p = 0.183), as well as their interaction (β = − 0.585, SE = 0.178, p = 0.001). The interaction term indicates that the effect of “Social State” on mean velocity is dependent upon “Nut Stress”. The 90-h starved fish swam faster than 20-h starved fish when in groups but slower than 20-h starved fish when assayed in isolation, whereas 20-h fish did not change speed in relation to social state (see Fig. 2, Supplementary Table 2). The model included a random effect for fish ID.
The mean velocity (mm/s) of individual fish in asocial (I) and social (G) assays. Colours represent nutritional stress treatments: blue indicates 20-h starved fish and red indicates 90-h starved fish. Boxes indicate the inter-quartile range (IQR), with the central line depicting the median and the whiskers extending to 1.5*IQR
“Social State” was an important predictor of risk-taking movements. Fish assayed in groups were faster to leave the shelter (“Exit”; β = 0.697, SE = 0.131, p < 0.0001), faster to reach the end of the arena (“End”; β = 0.319, SE = 0.12, p = 0.009), and faster to swim from the halfway line to the end of the arena (“HW to End”; β = 0.389, SE = 0.188, p = 0.041). “Nut Stress” was also an important predictor of latency to reach the end of the arena (“End”), with fish starved for 90 h reaching the end tendentially faster than fish starved for 20 h (β = − 0.217, SE = 0.117, p = 0.066). None of the predictors examined had strong relationships with time taken to swim from the shelter to halfway (“Exit to HW”) (Supplementary Table 2). All four models for risk-taking movements included a random effect for fish ID.
Social grouping
“Nut Stress” had an effect on social grouping behaviour. Fish starved for 90 h had more interactions (“Soc Freq”: β = 8.008, SE = 2.071, p < 0.001), although they were of shorter duration (“Soc Dur”: β = − 0.324, SE = 0.118, p = 0.009) compared with 20-h starved fish (Fig. 3, Supplementary Table 3). The duration of social interactions was also influenced by RMR, where fish with higher RMR had tendentially longer interaction durations (β = 8.661, SE = 4.606, p = 0.067). None of the predictors examined had strong relationships with inter-individual distance (“IID”) (Supplementary Table 3). The random effects included in these models varied, with model predictions for “Soc Freq” improving when random intercepts were included for both fish ID and group ID, whilst there was no improvement in model predictions with random intercepts for “Soc Dur”. The model predictions for “IID” only improved when group ID was included.
The effect of nutritional stress treatment (20-h and 90-h) on shoaling parameters. a The frequency of shoaling interactions within 2 body lengths. b The mean duration of these shoaling interactions (s). Colours represent nutritional stress treatments: blue indicates 20-h starved fish and red indicates 90-h starved fish. Boxes indicate the inter-quartile range (IQR), with the central line depicting the median and the whiskers extending to 1.5*IQR
Foraging performance
In terms of foraging performance, neither the behaviour of fish in the asocial assays nor their RMR predicted which fish ate the first bloodworm in the social assays (“BW First”), although the model was improved with the inclusion of fish ID as a random effect. However, to predict how many bloodworms an individual fish would eat in group assays (“BW Total”) (mean ± SE, 1.9 ± 0.14), “RMR” was included as an independent variable in the final model (estimate = − 14.904, SE = 8.100, p = 0.066), indicating that fish with a lower RMR ate more bloodworms. This final model did not include any random effects (Supplementary Table 4).
Discussion
Nutritional stress interacted with social context to affect locomotion
Nutritional stress treatment had significant different effects on the risk-taking movements and voluntary locomotory behaviour of threespine sticklebacks depending on whether they were foraging alone or within a small shoal. Most notably, fish that were under greater nutritional stress (90-h starved fish) swam more slowly than 20-h starved fish when foraging asocially; however, when they were assayed socially, shoals under greater nutritional stress swam comparatively faster. The 20-h starved fish did not change speed depending on social state. The single relationships were not significant (Fig. 2). We hypothesise that when foraging asocially, fish under greater nutritional stress were potentially trying to conserve energy by swimming slowly or were exploring their environment more thoroughly. For fish under greater nutritional stress, the relative benefit of a food item would have been greater compared with fish under less nutritional stress (McNamara and Houston 1989; Nonacs 2001). In social situations, increased competition between these particularly stressed individuals may have affected individual behaviour by intensifying individual foraging effort, resulting in greater movement velocities. Fish in this state may have been especially receptive to social information, and indeed recent research examining the influence of nutritional state on shoaling dynamics found that shoals composed of hungry individuals had greater potential information flow between group members (Wilson et al. 2019). In our experiment, fish under greater nutritional stress had more frequent shoaling interactions with conspecifics (within 2 body lengths), and these interactions were of a shorter duration than for fish under less nutritional stress (Fig. 3). This suggests individual fish under greater nutritional stress may reduce the costs of competition and simultaneously monitor conspecifics by routinely updating themselves with information on the state of conspecifics through short but frequent shoaling interactions. It is also plausible that the short but frequent shoaling interactions were the result of false positives of conspecific foraging activity; that is, they gathered false social information of conspecific foraging when in fact there was no foraging activity. Future work could investigate whether energetically deprived individuals are relatively more susceptible to inaccurate social information compared with satiated individuals (Clément et al. 2017). This may be due to differences in the cost of missing a foraging opportunity. With less to lose from ignoring a weak signal of potential food, satiated individuals may be slower and more accurate in their use of social information.
When tested socially, all fish within a group had the same level of nutritional deprivation. Further empirical work should investigate the more realistic scenario of individual fish within groups having different levels of satiation (see Balaban-Feld et al. 2019), as it is plausible for there to be as much within-group variation in satiation as there is between-group variation in satiation for facultatively shoaling species. McLean et al. (2018) showed how fish altered their spatial positions within a shoal dependent on the interaction between meal size, the energetic cost of digestion, and RMR. This study was undertaken in a swim tunnel where fish were forced to exercise at a specific velocity. It will be important to see how the impacts of within-group variation in satiation on foraging dynamics differ for free-swimming shoals, as there is evidence that the effects of metabolic rate on spatial positioning may differ when fish shoals are free-swimming rather than in a swim tunnel (Killen et al. 2012; Ward et al. 2018).
Our main result that individual velocity is dependent on the interaction between nutritional stress and social context is important in the broader context of the costs and benefits of grouping behaviour. Lower levels of nutritional stress had seemingly little effect on voluntary speeds of fish when they were placed into groups, which suggests conformity and cohesiveness will be more easily maintained in contexts where there is a low level of motivational conflict between individuals and when fish are moving slowly (Tunstrøm et al. 2013). However, when fish were starved for longer time periods and were subsequently assayed in groups, at least some individuals necessarily had to increase their velocities in order to maintain a level of shoal cohesiveness (see also Jolles et al. 2017) and retain the ability to compete for limited resources (which they potentially achieved by altering the nature of their social interactions (see Fig. 3)). Flexible inter-individual interaction rules are important if social animals are to respond adaptively to changes in internal and external stimuli. Maintaining shoal cohesiveness this way may be possible over the short term, and under certain environmental contexts, but if energetic costs become too great and heterogeneity in internal state increases, shoals will likely fission and individuals will be forced to swim asocially or form different shoals with fish in similar states to retain the benefits of grouping.
RMR did not affect locomotion or risk-taking behaviour
Fish with lower RMR gained more mass throughout the entire experiment, which may be expected as fish underwent prolonged periods of nutritional deprivation (Metcalfe et al. 2016). However, the predicted effects of RMR on risk-taking movement behaviour and locomotion in this study did not occur. This contrasts with previous studies that have shown fish with higher metabolic rates are more risk-prone (e.g. Huntingford et al. 2010; see Metcalfe et al. 2016 for a review) and that prolonged nutritional deprivation (7 days) revealed a positive correlation between RMR and risk-taking behaviour (Killen et al. 2011). The original model estimated for swimming speed did show a large but variable relationship between RMR and swimming speed; however, it was not positive as predicted, but negative, with fish with a lower RMR swimming faster. Whilst a positive correlation between RMR and risk-taking behaviour is commonly predicted (Killen et al. 2011), an alternative hypothesis to be considered is that a lower RMR may increase a fish’s energetic flexibility for mean swimming speed. When under nutritional stress and prioritising saving energy, fish with lower RMRs may have more energetic reserves to allocate to behaviour—allowing for faster swimming velocities that may result from increased activity and exploration (Careau et al. 2008). This relationship, it is important to note, was not included in the final model selected in our study. It is possible that important parameters might have been undetected in our models due to our small sample size and caution should be taken when interpreting the results. Also, the variation between individual RMR values was low in this study (e.g. more than 4 times smaller than that in McLean et al. 2018), which is an important consideration when interpreting results, as small variation between fish’s RMR could be easily masked by other factors.
The low temperatures in this study (10 °C) may help explain why there were no strong relationships between RMR and behaviour as, despite being starved for 90 h, the energetic requirements of these fish may not have been great enough to expose consistent correlations between RMR and behaviour (18 °C in Huntingford et al. 2010; 7 days food deprivation in Killen et al. 2011). In opposition to this, however, fish under the 90-h starved treatment did on average lose body mass. Certainly, future work should more rigorously manipulate the degree of nutritional deprivation alongside environmental temperature to see how these two factors interact to affect the relationship between RMR and behaviour.
The effect of nutritional stress and RMR on foraging
Nutritional stress may interact with foraging context to impact social foraging dynamics. Here, nutritional stress affected movement after the fish had left the shelter, as fish under greater nutritional stress reached the foraging patch faster than less nutritionally stressed fish (Supplementary Table 2c). Analysis showed that the estimated effect of nutritional state was stronger after the fish had passed the halfway line (and thus could see the foraging patch). These results contrast to a recent social foraging study using threespine sticklebacks, which found that nutritional state had no effect on the time taken for groups to find patches, and indeed, when a patch was found, it was well-fed fish that converged on them faster than food-deprived fish (72-h starvation, 8 °C) (Riddell and Webster 2017). The authors in this paper had evidence that convergence time was determined by the social organisation of the shoal, as well-fed fish organised themselves into fewer but larger subgroups who arrived at the patches together. It is likely that the differences in the results of the two studies relate to key differences in experimental design. In Riddell and Webster 2017, the visual food stimulus was hidden, only visible when a fish had entered the patch. Conspecifics were therefore reacting to the feeding behaviour of the first fish that arrived at the food patch, whereas in the current study, fish were repeatedly assayed under the same conditions and the food item was highlighted by an obvious foraging stimulus, visible to all fish from the halfway line of the arena. Foraging context (in this case distinguished by the type of food cue available and the predictability of the food items location) is therefore an important factor affecting social foraging dynamics. Research into how different types of resource information is obtained and spread between individuals should continue (Dall et al. 2005), especially between individuals under varying levels of motivation.
In terms of foraging performance, whilst individual characteristics were poor predictors of which fish ate the first bloodworm, we found that RMR was an important predictor for the total amount of bloodworms eaten during a social trial, with fish with lower RMR consuming more bloodworms (Supplementary Table 4b). Heavier fish had lower RMRs, and although there were no visible signs of aggression at the food patches, larger threespine sticklebacks foraging on bloodworms in patchy environments are known to outcompete conspecifics based on a morphological advantage (Hansen et al. 2016b), as they have an increased probability of successful food capture and can ingest it at a faster rate (Gill and Hart 1996).
Conclusion
This experiment explored how individual behaviour translates between asocial and social foraging contexts with consideration of the effects of RMR and manipulations of individual nutritional state. Including nutritional state and measures of metabolic rate in group foraging assays links the mechanisms behind individual behaviour to group-level responses. RMR did not predict risk-taking behaviour or locomotory characteristics. The main result in this study was that the effect of nutritional stress on locomotory behaviour was dependent on social context, with asocial velocities decreasing under greater nutritional stress, whilst social velocities increased. A socially dependent, differential effect of nutritional stress on locomotory parameters is an important consideration for researchers working in the field of collective movement and reinforces the theory that social context is an important modulator of the relationships between physiology and behaviour (Killen et al. 2013).
Data availability
Data will be made available upon reasonable request.
References
Balaban-Feld J, Mitchell WA, Kotler BP, Vijayan S, Tov Elem LT, Abramsky Z (2019) State-dependent foraging among social fish in a risky environment. Oecologia 190:37–45
Barton K (2019) MuMIn: multi-model inference. R package version 1(43):6. https://CRAN.R-project.org/package=MuMIn
Bates D, Mӓchler, Bolker B, Walker S (2014) Fitting linear mixed-effects models using lme4. arXiv1406.5823
Berdahl A, Torney CJ, Ioannou CC, Faria JJ, Couzin ID (2013) Emergent sensing of complex environments by mobile animal groups. Science 339:574–576
Biro PA, Stamps JA (2010) Do consistent individual differences in metabolic rate promote consistent individual differences in behaviour? Trends Ecol Evol 25:653–659
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer-Verlag, New York
Burton T, Killen SS, Armstrong JD, Metcalfe NB (2011) What causes intraspecific variation in metabolic rate and what are its ecological consequences? Proc R Soc Lond B 278:3465–3473
Careau V, Thomas D, Humphries MM, Réale D (2008) Energy metabolism and animal personality. Oikos 117:641–653
Clément RJG, Vicente-Page J, Mann RP, Ward AJW, Kurvers RHJM, Ramnarine IW, de Polavieja GG, Krause J (2017) Collective decision making in guppies: a cross-population comparison study in the wild. Behav Ecol 28:919–924
Croft DP, James R, Ward AJW, Botham MS, Mawdsley D, Krause J (2005) Assortative interactions and social networks in fish. Oecologia 143:211–219
Dall SR, Giraldeau LA, Olsson O, McNamara JM, Stephens DW (2005) Information and its use by animals in evolutionary ecology. Trends Ecol Evol 20:187–193
Dalziel AC, Ou M, Schulte PM (2012) Mechanisms underlying parallel reductions in aerobic capacity in non-migratory threespine stickleback (Gasterosteus aculeatus) populations. J Exp Biol 215:746–759
Dingemanse NJ, Wolf M (2013) Between-individual differences in behavioural plasticity within populations: causes and consequences. Anim Behav 85:1031–1039
Farine DR, Montiglio PO, Spiegel O (2015) From individuals to groups and back: the evolutionary implications of group phenotypic composition. Trends Ecol Evol 30:609–621
Finstad AG, Einum S, Forseth T, Ugedal O (2007) Shelter availability affects behaviour, size-dependent and mean growth of juvenile Atlantic salmon. Freshw Biol 52:1710–1718
Frommen JG, Luz C, Bakker TCM (2007) Nutritional state influences shoaling preference for familiars. Zoology 110:369–376
Gill AB, Hart PJB (1996) Unequal competition between three-spined stickleback, Gasterosteus aculeatus, L., encountering sequential prey. Anim Behav 51:689–698
Hansen MJ, Schaerf TM, Ward AJW (2015a) The effect of hunger on the exploratory behaviour of shoals of mosquitofish Gambusia holbrooki. Behaviour 152:1659–1677
Hansen MJ, Schaerf TM, Ward AJW (2015b) The influence of nutritional state on individual and group movement behaviour in shoals of crimson-spotted rainbowfish (Melanotaenia duboulayi). Behav Ecol Sociobiol 69:1713–1722
Hansen MJ, Schaerf TM, Krause J, Ward AJW (2016a) Crimson spotted rainbowfish (Melanotaenia duboulayi) change their spatial position according to nutritional requirement. PLoS One 11(2):e0148334
Hansen MJ, Ward AJW, Fürtbauer I, King AJ (2016b) Environmental quality determines finder-joiner dynamics in socially foraging three-spined sticklebacks (Gasterosteus aculeatus). Behav Ecol Sociobiol 70:889–899
Herbert-Read JE, Perna A, Mann R, Schaerf T, Sumpter DJT, Ward AJW (2011) Inferring the rules of interaction of shoaling fish. P Natl Acad Sci USA 108:18726–18731
Houston AI, McNamara JM (1999) Models of adaptive behaviour: an approach based on state. Cambridge University Press, Cambridge
Huntingford FA, Andrew G, Mackenzie S, Morera D, Coyle SM, Pilarczyk M, Kadri S (2010) Coping strategies in a strongly schooling fish, the common carp Cyprinus carpio. J Fish Biol 76:1576–1591
Ioannou CC, Dall SR (2016) Individuals that are consistent in risk-taking benefit during collective foraging. Sci Rep 6:33991
Ioannou CC, Krause J (2009) Interactions between background matching and motion during visual detection can explain why cryptic animals keep still. Biol Lett 5:191–193
Janson CH (1985) Aggressive competition and individual food consumption in wild brown capuchin monkeys (Cebus apella). Behav Ecol Sociobiol 18:125–138
Jolles JW, Boogert NJ, Sridhar VH, Couzin ID, Manica A (2017) Consistent individual differences drive collective behavior and group functioning of schooling fish. Curr Biol 27:2862–2868
Jolles JW, Laskowski KL, Boogert NJ, Manica A (2018) Repeatable group differences in the collective behaviour of stickleback shoals across ecological contexts. Proc R Soc B 285:20172629
Katz Y, Tunstrom K, Ioannou CC, Huepe C, Couzin ID (2011) Inferring the structure and dynamics of interactions in schooling fish. P Natl Acad Sci USA 108:18720–18725
Killen SS, Marras S, McKenzie DJ (2011) Fuel, fasting, fear: routing metabolic rate and food deprivation exert synergistic effects on risk-taking in individual juvenile European sea bass. J Anim Ecol 80:1024–1033
Killen SS, Marras S, Steffesnsen JF, McKenzie (2012) Aerobic capacity influences the spatial position of individuals within fish schools. Proc R Soc Lond B 279:357–364
Killen SS, Marras S, Metcalfe NB, McKenzie DJ, Domenici P (2013) Environmental stressors alter relationships between physiology and behaviour. Trends Ecol Evol 28:651–658
Killen SS, Marras S, Nadler L, Domenici P (2017) The role of physiological traits in the assortment among and within fish shoals. Philos Trans R Soc B 372:20160233
Krause J (1993) The influence of hunger on shoal size choice by three-spined sticklebacks, Gasterosteus aculeatus. J Fish Biol 43:775–780
Krause J, Bumann D, Todt D (1992) Relationship between the position preference and nutritional state of individuals in schools of juvenile roach (Rutilus rutilus). Behav Ecol Sociobiol 30:177–180
Lihoreau M, Buhl J, Charleston MA, Sword GA, Raubenheimer D, Simpson SJ (2015) Nutritional ecology beyond the individual: a conceptual framework for integrating nutrition and social interactions. Ecol Lett 18:273–286
McDonald ND, Rands SA, Hill F, Elder C, Ioannou CC (2016) Consensus and experience trump leadership, suppressing individual personality during social foraging. Sci Adv 2:e1600892
McLean S, Persson A, Norin T, Killen SS (2018) Metabolic costs of feeding predictively alter the spatial distribution of individuals in fish shoals. Curr Biol 28:1144–1149
McNamara JM, Houston AI (1989) State-dependent contests for food. J Theor Biol 137:457–479
Metcalfe NB, Taylor AC, Thorpe JE (1995) Metabolic rate, social status and life-history strategies in Atlantic salmon. Anim Behav 49:431–436
Metcalfe NB, Van Leeuwen TE, Killen SS (2016) Does individual variation in metabolic phenotype predict fish behaviour and performance? J Fish Biol 88:298–321
Nonacs P (2001) State dependent behavior and the marginal value theorem. Behav Ecol 12:71–83
Partridge BL (1980) The effect of school size on the structure and dynamics of minnow schools. Anim Behav 28:68–77
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org
Reid D, Armstrong JD, Metcalfe NB (2012) The performance advantage of a high resting metabolic rate in juvenile salmon is habitat dependent. J Anim Ecol 81:868–875
Riddell J, Webster MM (2017) How does hunger affect convergence on prey patches in a social forager? Ethology 123:811–817
Schaerf TM, Dillingham PW, Ward AJW (2017) The effects of external cues on individual and collective behavior of shoaling fish. Sci Adv 3:e1603201
Seebacher F, Krause J (2017) Physiological mechanisms underlying animal social behaviour. Philos Trans R Soc B 372:20160231
Sih A, Mathot KJ, Moirόn M, Montiglio PO, Wolf M, Dingemanse NJ (2015) Animal personality and state-behaviour feedbacks: a review and guide for empiricists. Trends Ecol Evol 30:50–60
Speigel O, Harel R, Getz WM, Nathan R (2013) Mixed strategies of griffon vultures’ (Gyps fulvus) response to food deprivation lead to a hump-shaped movement pattern. Mov Ecol 1:5
Stacey PB, Koenig WD (1990) Cooperative breeding in birds: long term studies of ecology and behaviour. Cambridge University Press, Cambridge
Svendsen MBS, Bushnell PG, Steffensen JF (2016) Design and setup of intermittent-flow respirometry system for aquatic organisms. J Fish Biol 88:26–50
Tunstrøm K, Katz Y, Ioannou CC, Huepe C, Lutz MJ, Couzin ID (2013) Collective states, multistability and transitional behavior in schooling fish. PLoS Comput Biol 28:e1002915
Ward AJW, Webster MM (2016) Sociality: the behaviour of group-living animals. Springer International Publishing, Geneva
Ward AJW, Hart PJB, Krause J (2004) The effects of habitat- and diet-based cues on association preferences in three-spined sticklebacks. Behav Ecol 15:925–929
Ward AJW, Herbert-Read JE, Schaerf TM, Seebacher F (2018) The physiology of leadership in fish shoals: leader have lower maximal metabolic rates and lower aerobic scope. J Zool 305:73–81
Webster MM, Laland KN (2009) Evaluation of a non-invasive tagging system for laboratory studies using three-spined sticklebacks Gasterosteus aculeatus. J Fish Biol 75:1868–1873
Wilson ADM, Burns ALJ, Crosato E, Lizier J, Prokopenko M, Schaerf TM, Ward AJW (2019) Conformity in the collective: differences in hunger affect individual and group behavior in a shoaling fish. Behav Ecol 30:968–974
Wolf JB, Moore AJ (2010) Interacting phenotypes and indirect genetic effects: a genetic perspective on the evolution of social behavior. In: Westneat DF, Fox CW (eds) Evolutionary behavioral ecology. Oxford University Press, New York, pp 225–245
Wolf M, Weissing FJ (2010) An explanatory framework for adaptive personality differences. Philos Trans R Soc B 365:3959–3968
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
We thank Teejay O’Rear and Tommy Agosta for assistance in catching fish and the Thomas Young Lab and Marie Stillway (UC Davis) for sharing their laboratory resources. We would also like to thank the members of the Fangue Laboratory for assistance in fish rearing and maintenance. We would also like to acknowledge the input from two anonymous reviewers whose comments greatly enhanced the manuscript.
Funding
This work was supported by the California Agricultural Experimental Station of the University of California Davis (grant numbers CA-D-ASC-2252-H and CA-D-ASC-2253-RR to AET and CA-D-ASC-2091-H to NAF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the University California, Davis, and approval from the Institutional Animal Care and Use Committee (IACUC) was granted (no. 20178).
Additional information
Communicated by J. Frommen
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 24 kb)
Rights and permissions
About this article
Cite this article
Hansen, M.J., Ligocki, I.Y., Zillig, K.E. et al. Risk-taking and locomotion in foraging threespine sticklebacks (Gasterosteus aculeatus): the effect of nutritional stress is dependent on social context. Behav Ecol Sociobiol 74, 12 (2020). https://doi.org/10.1007/s00265-019-2795-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-019-2795-4