Abstract
Nest quality is an important aspect of courtship and mate choice, offering females direct benefits through offspring survival and, if it reflects male genetic quality, also indirect ones. Nest characteristics may thus affect both male mating success and reproductive success. Using the sand goby, where males build nests by covering mussel shells or stones in sand, we tested the role of nest material in male nest site choice, nest construction, and female mate choice. We examined the effect of sand texture (coarse or fine, depending on grain size) in two different settings: (A) when the male was free to choose between nest sites in different sand textures and other males were absent, and (B) when the male was denied a choice of sand texture and another male was present behind a partition. In (B), we also examined the effects of sand texture on female preference. In (A), males took up nest sites equally often in coarse and fine sand, but nests built in fine sand had greater sand cover. In (B), there was no difference in nest sand cover, but a greater number of males, and in particular males that weighed less and had been assigned coarse sand, refrained from building a nest at all. This suggests that sand texture does affect nest building in sand gobies, manifesting itself directly through nest sand cover, or indirectly through failure to build a nest. Moreover, we found that females preferred to spawn in well-covered nests regardless of sand texture.
Significance statement
Nests offer eggs and offspring protection from predators and inclement weather, but building material may affect both the properties of the nest and the quality of the construction. Here, we presented male sand gobies with nest sites in either fine-grained or coarse-grained sand, assessed the sand cover of the nest, and allowed females to spawn. We found that grain size influenced the amount of sand cover on the nest and affected the fraction of males that refrained from building a nest. Female spawning decision depended on the amount of sand cover, but neither males nor females expressed a preference for sand texture. Our results show that nest material is an important but indirect aspect of mating success, which may influence habitat utilization in the wild.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To animals that rely on a nest to house eggs or offspring, aspects of the nest can be expected to affect offspring survival. If so, these aspects reflect nest quality and may play a part not only in reproductive success but also in mating success. While much attention has been devoted to the effect of male competition on nest site occupancy, male traits on nest building, and the role of nest quality on female mate choice, less attention has been given to how nest materials affect nest quality, and how this in turn affects mate choice.
If the male builds a nest, female assessment of available nests may benefit the female both directly in terms of ensuring offspring protection and indirectly if it is linked to the genetic quality of the male. For example, both nest site and quality may affect how well offspring are protected from predators and adverse environmental conditions. Thus, female baya weaverbirds (Ploceus philippinus) prefer nests on high and slender branches which may protect against predators (Quader 2005). Similarly, nests sheltered from the waves increase the nesting success, in terms of successful nest building and the eventual production of fry, in five-spotted wrasse (Symphodus roissali) (Raventos 2006). Nest quality may also be indicative of the quality of the nest-building male, and even act as an extended phenotype. For example, in extreme cases where females do not use the nest for egg laying, such as the satin bowerbird (Ptilonorhynchus violaceus), nest quality is nonetheless a key aspect of mate choice (Borgia 1985). Furthermore, if nest quality contributes to reproductive success and there is variation in nest-building ability in one sex, nest building itself may come under sexual selection through a preference by the opposite sex for high-quality nests. In both three-spined (Gasterosteus aculeatus) and fifteen-spined (Spinachia spinachia) sticklebacks, males build nests from plant matter held together with secretional threads of glycoproteins, such that the quality of the nest reflects the condition and stress level of the male (Barber et al. 2001; Östlund-Nilsson 2001); likewise, in black wheatears (Oenanthe leucura), males with larger wing area can carry heavier stones to the nest, which results in earlier and higher frequency of egg laying (Møller et al. 1995). On the other hand, in barn swallow (Hirundo rustica), attractive males contributed less to the nest building, suggesting that in situations where female reproductive success is affected both by the quality of the male and the quality of the nest, nest building can also represent a compensatory tactic for less attractive males (Soler et al. 1998).
Even though nest quality may be an important factor in female mate choice, the link between nest material and nest quality has only occasionally been examined, and then often with a focus on parasite load. For example, nest parasite load was reduced by the inclusion of aromatic plants in spotless starlings (Sturnus unicolor; Soler et al. 2017) and old nest material in pied flycatchers (Ficedula hypoleuca; Mappes et al. 1994). It was also reduced in the presence of smoked cigarette butts in urban house finches (Carpodacus mexicanus) and house sparrows (Passer domesticus; Suárez-Rodríguez et al. 2013), although at the price of elevated genotoxicity in the blood cells of chicks (Suárez-Rodríguez and Macías Garcia 2014). The scarcity of studies examining how nest material influences the physical properties of nests is notable, but the few existing studies have revealed important effects of material on nest architecture. Generally, both the size and the lining material can affect the thermal properties of a nest (Hilton et al. 2004). For example, heat loss and water absorption in nests of the thorn-tailed rayadito (Aphrastura spinicauda) were influenced by the surface-to-volume ratio as well as the inclusion of plant materials and feathers (Botero-Delgadillo et al. 2017).
In this study, we address the link between nest material, nest appearance, and their effect on male and female nest preferences in the sand goby (Pomatoschistus minutus). Male sand gobies build nests by excavating a burrow underneath a mussel shell or stone and covering it in sand, leaving only a small opening. Sand gobies inhabit shallow bays with a range of sandy substrates, and we focus on the importance of sand texture as defined by grain size. Previous work on nest building in sand gobies has shown that nest properties, such as degree of sand cover and nest opening size, vary between males and that nest appearance (among other cues) affects female spawning decision with females preferring nests that are well covered by sand (Svensson and Kvarnemo 2005; Lehtonen et al. 2007; Lehtonen and Wong 2009). Furthermore, males adjust nest appearance in response to the environment. In the presence of potential sneaker males (Svensson and Kvarnemo 2003, 2005) and egg predators (Lissåker and Kvarnemo 2006; Olsson et al. 2016), the opening is made smaller, while it is enlarged under lower levels of dissolved oxygen (Lissåker et al. 2003; Lissåker and Kvarnemo 2006; Olsson et al. 2016). Finally, nest coverage has also been shown to be important in avoiding nest predation (Lindström and Ranta 1992; Jones and Reynolds 1999; Lissåker and Kvarnemo 2006).
Previous studies have shown that nest building is a costly investment to male sand gobies (Olsson et al. 2009) and that males choose nests in sandy habitats over rocky habitats in the field, unless the rock nest is larger (Lehtonen and Lindström 2004). This suggests that choice of nest site is an important decision and that males may express preferences based on sand texture. We thus hypothesize that sand grain size may affect nest appearance and properties, and that this in turn may influence male nest material preferences and female nest choice. Using two experimental settings, with either a solitary male given a free choice of sand texture, or a male being assigned sand texture in the presence of another male (behind a partition) and a female being allowed to choose a mate and spawn, we address the following questions: (1) Which sand texture (fine vs. coarse) do males prefer? (2) Does sand texture affect nest appearance? (3) Are there differences in nest building and nest appearance in the different settings, e.g., if the male does or does not have a choice of sand texture? (4) Do females show a preference for nests built from fine or coarse sand?
Material and methods
Study species
The sand goby inhabits near-shore marine and brackish waters in northern Europe (Miller 1986) and during the breeding season, which typically lasts from April to June, adult fish migrate to shallow, sandy bays (Hesthagen 1977). Males build nests by excavating a burrow underneath a mussel shell or stone and covering it with sand. Both males and females are polygamous and spawn repeatedly, with territorial nest-holding males courting females by fin displays and “lead swims” towards the nest (reviewed in Forsgren 1999). In addition to nest characteristics, females have been found to use male size, coloration, courtship display, presence of eggs in the nest, and fanning rates as cues in mate choice (Forsgren 1992, 1997a; Forsgren et al. 1996; Pampoulie et al. 2004). The male guards and ventilates the clutch until hatching, which happens up to 3 weeks after spawning, depending on temperature (Kvarnemo 1994). Sand gobies are common in a range of sandy habitats, while on muddier substrates, it is often replaced by the phenotypically similar common goby (Pomatoschistus microps; Tallmark and Evans 1986).
Experimental design
The study was carried out at the Swedish west coast (The Sven Lovén Centre Kristineberg, University of Gothenburg; lat 58.24, long 11.44), in May and June 2007. Sand gobies were caught in a nearby bay (Bökevik) using a hand trawl. The fish were brought to the lab, separated by sex, and placed in 115-L storage aquaria furnished with approximately 2 cm of sand to burrow in. Fish numbers in storage tanks varied due to field collections and use in experiments but did not exceed 40 fish. All tanks (storage and experimental tanks) were continuously supplied with seawater delivered by the laboratory surface water pumps. Consequently, experiments were run at natural seawater temperature and we obtained recordings of sea surface temperature, logged each hour at Väderöarna WR buoy (lat 58.48, long 10.93), from the open database provided by the Swedish Meteorological and Hydrological Institute (SMHI 2017). A large window together with timer-controlled lamps ensured that natural light conditions were maintained. Fish in storage tanks were fed daily with chopped mussel meat (Mytilus edulis).
Sand texture
We defined two classes of sand texture, coarse and fine, depending on grain size. We obtained these by taking sand from a beach where sand gobies build nests, and sifting it through sieves (mesh sizes of 0.5 mm and 1 mm) such that coarse sand was composed of grains with diameters between 0.5 and 1.0 mm and fine sand of grains with a diameter < 0.5 mm (mostly > 0.25 mm but also some fraction smaller than that). Sand in the field comprises a mixture of grain sizes, and this method produced sand consistent with finer and coarser sand of local sand goby habitats.
Experiment A: One male, choice of nest site
In the first experiment, individual males were introduced to tanks measuring 50 × 36 cm and 30 cm deep (50 L); eight tanks were used simultaneously. Each tank was partially divided by an opaque partition that created two nesting compartments, both of which connected to an open foreground area (Fig. 1a). Each nesting compartment was furnished with a layer (about 3 cm deep) of either fine or coarse sand and an empty nest site (a halved clay flower pot). In the foreground area, where inflow and outflow of water were located, sand was a 50:50 mixture of fine and coarse sand. The relative position (left/right) of the coarse and fine sand compartments was randomized for each tank, but once a tank was furnished, the sand texture in the compartments was not changed. To stimulate nest building, two ripe females, assigned at random to each tank, were confined inside a plastic container placed in the foreground area, visible from both nest compartments. The male was released into the tank in the middle of the foreground area and allowed to freely choose a nest site. The male was given a maximum of 3 days to initiate nest building, and another 24 h to complete it once it had started (i.e., cover the pot with sand and excavate underneath). At this point, nests were photographed (as described below), the chosen sand texture was noted, the male was captured, and his total length was measured. If no nest-building activity was detected within 3 days, the replicate was excluded from analyses. After the trial ended, the sand was smoothed and the pots replaced, before a new replicate was started.
Experimental setup in experiment A (a) and experiment B (b) investigating sand texture choice in the sand goby. The aquarium was divided into two adjacent nest compartments with either coarse or fine sand (dark and light gray, respectively) which both bordered a female area with a 50:50% mix of coarse and fine sand (medium gray). The nest compartments were separated by an opaque partition, while the female area was accessible in experiment A but closed off during the first phase of experiment B by a transparent partition (dashed line)
A total of 31 trials were successfully conducted and only three males did not build; however, in one case, the male built nests in both compartments. This trial was retained for nest quality analysis but excluded from the male preference analysis.
Experiment B: Two males, no nest site choice, female choice
In the same tanks used in experiment A, a female compartment was created by adding a clear Plexiglas partition that separated the foreground area from the two nesting compartments (Fig. 1b). The tank was also replumbed to have an inflow of water in each nest compartment and outflow in the female area; small perforations in the clear partition allowed for water flow. In the first phase of the experiment, two males were size-matched to within 1 mm and weighed before they were assigned to the two nest compartments of the aquarium. The opaque divider prevented males from visual interaction, but did not necessarily prevent knowledge of a second male via auditory, vibrational, or olfactory means. To stimulate male behavior, two ripe females, again chosen at random and confined inside a plastic container, were placed in the foreground compartment, visible to both male compartments. Any male that failed to build a nest within 2 days was replaced with another size-matched male. On the morning that both nests had been built, the stimulus females were removed and the nests were photographed, as described below. In the second phase of the experiment, a ripe female was introduced to the female compartment and allowed to move freely inside it. The position and behavior of the males and the female were recorded every 15–20 min until 15 observations had been made. Males were recorded as being inside the nest, displaying by the nest (including any display behavior such as fin flaring, tail-lifting, or leading display; i.e., approaching the female and then swimming towards the nest), showing other behavior by the nest (lying still, swimming around, or burrowed in sand), displaying at the partition, or showing other behavior at the partition (also as detailed above). Female display of dark eyes, indicating readiness to spawn, was also recorded. All fish were observed on 15 occasions; all behavior and position information was recorded for each male at each observation point. In some cases, more than one behavior or position would be observed (e.g., if a male was moving to interact with a female and then back to his nest). In case the fish had completely burrowed in the sand and could not be sighted, no behavior was recorded at that observation point. After the final observation, the transparent partition separating the foreground from the nest compartments was removed and the fish were observed for 15 min to determine if the female would immediately spawn. At this point all fish could freely interact. The female was allowed two nights to spawn, although most had spawned after the first night. Spawning latency was categorized as “immediate” if it occurred within the observed 15 min, else “overnight” or “second night,” depending on when eggs were discovered in a nest. After spawning, the sand texture of the chosen nest was noted. After the trial ended, the sand was smoothed and the pots and fish were replaced. If the female did not spawn, the second phase of the experiment was repeated with another female. The males were not reused if the female spawned or if two successive females failed to spawn.
Of the 47 trials conducted, females spawned in 32, although in one trial, one of the males died, and in another, the female spawned in both nests. These replicates were excluded from the female preference analyses.
Quantification of nest appearance
Halved clay flowerpots with an outer diameter of 7 cm were used as standardized nest sites. All completed nests were photographed from above, from the front, and from an angle facing the nest opening, to allow measurement of three aspects of nest appearance: sand height on top of the nest, area of the nest opening, and exposed area of the pot. The rim of each pot was marked at 10 mm intervals to provide a scale in the images. ImageJ (Schindelin et al. 2012; Schneider et al. 2012) was used to quantify the height of the nest cover, nest opening area, and exposed pot area. In some cases (54 images), the scale was obscured and other aspects of those nests were used to set a scale, usually the thickness of the pot. In one case, the pot was so completely covered that the rim was obscured and the sand height could not be accurately estimated. In this case, sand height was set to 10 mm, which was judged to be the lowest possible value when compared with other nests. The relationship between the three nest appearance measurements was examined by performing a principal component analysis (rda, package vegan, Oksanen et al. 2017). The first component of the PCA explained 67.13% of the total variance (loadings: sand height = − 0.36, nest opening area = 0.43, pot exposure = 0.43) and was used to create a single nest score parameter. The second and third components had eigenvalues < 1 and were thus not considered further. It should be noted from the signs of the loadings that a higher nest score means that the nest had a larger opening, less sand on top, and a more exposed pot, i.e., less sand cover. Therefore, to make the nest score parameter more intuitive, it was multiplied with − 1 so that a higher score denotes a nest with more sand cover and a smaller opening.
Quantification of male size
Male total length was measured to the nearest millimeter on a measuring board. We measured male weight by carefully wiping excess water off the fish before gently placing it in a tared cup of water. Male weight was recorded on a digital balance (Mettler PM600) to the nearest 0.01 g. We calculated a male condition index as 100 × male weight/(male length)3.
Quantification of behavior
In the second phase of experiment B, we calculated apparent female sand texture preference while the transparent partition was in place as the difference between the number of times she was observed on the coarse sand side and the fine sand side. We calculated a dark eye score for females as the sum of the number of instances she was recorded displaying dark eyes. We summarized male behavior based on the frequency of a given behavior relative to the total behavioral observations from that male, e.g., display score was the total number of display behaviors noted divided by the total number of behaviors observed for that male (typically 15 but on occasion slightly more than 15 or slightly less, as detailed above). Approximately 48% of all observations consisted of males being in the nest, while courtship display at the nest or at the partition was observed only on 8.7% and 10.1% of observations, respectively. These patterns of behavior are not atypical for this species, especially for observations made in person rather than via video (Kvarnemo et al. 1995).
Statistical analyses
Which sand texture (fine vs. coarse) do males prefer?
Male choice of sand texture (experiment A) was tested using a binomial test with an assumed null hypothesis probability of 0.5. We examined factors affecting male preference by fitting a logistic regression with sand texture at the chosen nest site as the response variable and male length and temperature as predictors (model specification: sand texture of the chosen nest site ~ male length + temperature, fine sand arbitrarily assigned a value of 0 and coarse sand a value of 1) and obtained the minimal adequate model by stepwise removal of terms (beginning with the least significant term) as long as the difference between the full and reduced model was not significant (p > 0.05, assessed by likelihood ratio test), and checked it for overdispersion. Since model coefficients are affected by other variables included in the model specification, a stepwise selection process allows us to examine whether terms close to significance remain non-significant during model reduction.
Does sand texture affect nest appearance?
In both experiments A and B, we examined how sand, temperature, and male length affected nest score. In experiment A, we fitted a linear model with nest score as response variable and sand texture, temperature, and male length as predictors (model specification: nest score ~ sand texture + temperature + male length). In experiment B, we fitted a mixed effects model, with nest score as response variables; sand texture, temperature, and male length as fixed effects; and replicate as random effect, to account for the two nest builders per replicate (model specification: nest score ~ sand texture + temperature + male length + (1|replicate)). Again, we obtained the minimal adequate model through stepwise removal of non-significant terms and inspected the residuals of the minimal model for deviance from normality. We used restricted likelihood ratio test (RLRT; exactLRT, package RLRsim, Scheipl et al. 2008), to determine the significance of the random factor (RLRT = 5.41, p = 0.008).
Are there differences in nest building and nest appearance in the different settings?
To compare nest building performance between the two experimental setups, we performed a mixed effects ANOVA with nest score as response variable, experiment as fixed effect, and replicate as random effect (model specification: nest score ~ experiment + (1|replicate)), as there were two nests per replicate in experiment B. Again, we used restricted likelihood ratio test to determine the significance of the random factor (RLRT = 5.60, p = 0.0085).
Males that did not build a nest within the allowed time were replaced. We tested the fraction of males replaced in experiment A, compared with experiment B, using Fisher’s exact test. We examined the effect of sand texture on the fraction of males that were replaced in experiment B using a binomial test with a null hypothesis of 0.5. The effect of male size, measured as total length, weight, and condition index, was analyzed in separate Mann-Whitney tests, after Shapiro-Wilk tests showed that the size variables deviated from normality. We investigated the relationship between the display score of individual males to their nest score using Spearman’s rank correlation.
Do females show a preference for nests built in fine or coarse sand?
We tested apparent female preference for sand texture (partition down) using a t test against μ = 0. We tested female choice of sand texture (based on where females spawned) using binomial tests with an assumed null hypothesis probability of 0.5. Because each female in experiment B was offered a choice between two males and nests, and to allow us to analyze the effect of nest score on female choice, we created a variable to reflect nest score difference—the difference between the nest scores of the nest in coarse sand and the nest in fine sand. We did the same with male weight difference and display score difference. A similar variable for the difference in length would have been redundant, since the males were matched for body length. We examined factors affecting preference by fitting a logistic regression with sand texture of the chosen nest site as dependent variable and male length and temperature as predictors (model specification: sand texture of the chosen nest site ~ nest score difference + display score difference + weight difference + temperature, sand texture scored as described above), and again obtained the minimal adequate model by stepwise removal of non-significant terms, and checked it for overdispersion. The frequency of dark eyes relative to spawning latency was tested using a Conover-Iman test, which performs a Kruskal-Wallis test and, if this is significant, post hoc pairwise comparisons with Bonferroni correction between the three spawning groups (immediately, overnight, and second night; conover.test, package conover.test, Dinno 2017).
It was not possible to record data blind because our study involved focal animals in the laboratory. All statistical tests were performed in R version 3.5.0 (R Core Team 2018).
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Results
Which sand texture (fine vs. coarse) do males prefer?
Males showed no preference for either fine or coarse sand in experiment A (binomial test: ncoarse sand = 14, nfine sand = 16, p = 0.86), and sand texture choice was also unaffected by male length and temperature (Table 1).
Does sand texture affect nest appearance?
In experiment A, nests built in fine sand had higher nest scores, i.e., more sand cover, than nests in coarse sand, but there was no effect of temperature or male length (Table 2; Fig. 2). In experiment B, there was no effect of sand texture, temperature, or male length on nest score (Table 3).
Are there differences in nest building and nest appearance in the different settings?
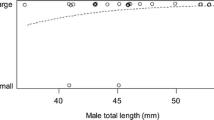
There was a non-significant trend towards higher nest scores, i.e., more sand cover, in experiment A (mean ± SE 0.28 ± 0.13) compared with experiment B (mean ± SE − 0.10 ± 0.11; mixed effects ANOVA, F1,95.96 = 3.26, p = 0.074). There was no difference between the fraction of males that were replaced (i.e., did not build a nest) in experiment A compared with experiment B (A: 3 males replaced, 32 males retained; B: 21 males replaced, 94 males retained; Fisher’s Exact test: p = 0.20). However, of the replaced males in experiment B, most (n = 16) had been assigned coarse sand (binomial test: p = 0.027). Comparing all the males in experiment B that built nests to those that were replaced, the replaced males weighed less, and while the difference in length was close to being significant, there was no difference in condition index (Mann-Whitney test: weight: W = 707.5, p = 0.043; length: W = 740.5, p = 0.074; condition index: W = 793.5 p = 0.16; Fig. 3). Males with higher display scores had higher nest scores, although the correlation was weak (Spearman’s test: n = 94, adj.rho2 = 0.06, p = 0.01).
Male characteristics and nest building in different sand textures in the sand goby. Boxplots (horizontal line: median; box hinges: first and third quartiles; whiskers: largest value maximum 1.5*IQR from the hinge; dots: outliers; N: sample size) of condition factor, length, and weight of males that built a nest (dark gray) and males that did not (light gray), and were thus replaced, for coarse and fine sand
Do females show a preference for nests built in fine or coarse sand?
Prior to the removal of the partition in experiment B, females did not differ in the amount of time spent near the coarse and fine sand compartments; thus, females showed no apparent preference for either sand texture (mean ± SE number of times − 0.06 ± 1.26, t test against μ = 0: t46 = − 0.05, p = 0.96). Of the 30 replicates in which females spawned in only one nest and both males survived, 8 resulted in immediate (i.e., within the observed 15 min) spawning, 17 in overnight spawning, and 5 in spawning the second night. Females that spawned immediately had higher dark eye scores than females that spawned overnight or second night (Conover-Imam test: n = 30, Kruskal-Wallis χ2df = 2 = 7.70, p = 0.02, pairwise comparisons: immediate-overnight z = 3.10, p = 0.007, immediate-second night z = 1.89, p = 0.11, overnight-second night z = − 0.50, p = 0.93; Fig. 4). Female spawning decision was not affected by sand texture (coarse sand spawning: n = 13, fine sand spawning: n = 17; binomial test: p = 0.58), but was influenced by of the difference in nest scores (Table 4). For identical nest scores (i.e., nest score difference = 0), the minimum adequate model thus predicted that the females were equally likely to spawn in either fine sand or coarse sand (predicted probability (95% CI) = 0.5 (0.38–0.62); Fig. 5).
The effect of nest score difference on female spawning decision in the sand goby. Higher nest scores indicate nests with more sand cover and a nest score difference > 0 shows that the chosen nest had a higher score than the rejected nest. The black line shows the predicted probability of spawning occurring in coarse sand nests (according to the minimum adequate model; Table 4), while black and gray points show the nest score difference of the coarse and fine sand nests in which females spawned.
Discussion
We found that female spawning decision was affected by nest appearance, with females preferentially choosing nests with more sand cover. Indeed, we found no preference for sand texture per se among either males or females. This seems somewhat surprising since males that were offered a choice between sand textures built nests with higher nest score, i.e., more sand cover, in fine sand than in coarse sand, and males that were only offered coarse sand were more likely to refrain from building a nest at all.
We found that nests in fine sand had higher nest scores than nests in coarse sand, but this difference was only significant when males were given a choice between nest sites and no other male was present. Conversely, when males were denied a choice and another male was present, a significant number of males that had been assigned a nest site in coarse sand, and especially males of lower weight, did not build a nest at all. These results suggest that coarse sand is more difficult to build in, especially for lighter males. Furthermore, if male-male competition extends to nest building and the perceived presence of another male is interpreted as greater competition, small males in coarse sand may be at a prohibitive disadvantage and therefore refrain from nest building. In other animals, type and availability of nest material can affect both the structure of the nest and the number of nesting individuals. For example, the ability of laboratory mice to build complex nests, similar to nests found in the wild, depended on available nest material (Hess et al. 2008). Moreover, depletion of nest material reduced the total number of nests but not average nest quality in rooks (Corvus frugilegus), suggesting that the abundance of material constituted a threshold for building rather than a predictor of quality (Rutnagur 1990, as cited in Hansell 2000). Another possibility is that choosing a nest site itself affects nest score. When male sand gobies in another study were allowed to choose between nests of different sizes, successive nests had consistent degrees of sand cover, while males that were denied a choice built nests of variable appearance (Japoshvili et al. 2012).
Despite the effect of sand texture on nest score and nest building, we did not find a male preference for either sand texture. Sand goby nests are built by swirling up sand at the nest site, which may explain the male preference for a sandy habitat (Lehtonen and Lindström 2004), but the difference between sand textures in our experiment was much smaller than the difference between the sand and cobbles found in the natural habitats studied by Lehtonen and Lindström. Our study was also limited to the initial building of the nest, whereas a male that acquires a clutch must guard it until hatching, which requires nest maintenance. If different sand textures carry different maintenance costs, for instance because smaller sand grains are more easily transported by wave action or currents (McLaren and Bowles 1985), this may affect the successful rearing of offspring and the total cost of the brood cycle to the male.
Surprisingly, there was no effect of temperature on nest score, even though water temperature rose as the season progressed. Metabolic rates increase as temperature rises (Clarke and Johnston 1999), which may leave less energy for nest building. In addition, since warmer water holds less dissolved oxygen, males could have responded by increasing the nest opening to ensure adequate oxygenation (Lissåker et al. 2003; Lissåker and Kvarnemo 2006; Olsson et al. 2016). Nevertheless, no effect of temperature on nest building was found in this study.
We also found no effect of the time the female spent on the coarse and fine sand sides, prior to removing the partition, on female spawning decision, but females displaying dark eyes spawned more quickly. That dark eyes indicate readiness to spawn is consistent with previous work (Olsson et al. 2017), but previous studies carried out under laboratory conditions similar to ours suggest that the time allowed should be sufficient for females to arrive at a spawning decision (Forsgren 1997b), which is often made even more quickly in the field (Forsgren 1997a).
Female spawning decision was influenced by nest score, but not by sand texture, male weight, or courtship display. If the purpose of the nest is to protect offspring from predation or harsh conditions, choosiness may produce direct benefits. In penduline tits (Remiz pendulinus), nest quality affects sheltering capacity during brooding (Hoi et al. 1994). Similarly, both sand gobies and common gobies have been observed to increase nest sand cover in the presence of a predator (Jones and Reynolds 1999; Lehtonen et al. 2013), and small nest entrances offer better protection against egg predators (Olsson et al. 2016). If females also gain indirect benefits, a link between some aspect of male genetic quality and nest quality is expected. For example, it has been suggested that male three-spined sticklebacks may advertise their paternal skills through decorated nests openings, which would explain why females prefer to spawn in such nests (Östlund-Nilsson and Holmlund 2003). It is therefore not uncommon that female mate choice is influenced by multiple signals (e.g., Wagner and Reiser 2000; Candolin 2003; Berson and Simmons 2018; Mowles et al. 2018). Previous work has shown that female sand gobies prefer larger (Forsgren 1992) and intensely courting males (Forsgren 1997a; but see Lehtonen 2012), and also that there is a link between males preferred by females and hatching success (Forsgren 1997b). However, previous evidence on whether nest appearance is associated with male attractiveness or offers less attractive males an alternative means to attract females has been ambiguous (Svensson and Kvarnemo 2005; Lehtonen and Wong 2009). In our study, we found no effect of male length on nest score and only a weak correlation between male courtship display and nest score, and also no effect of male courtship or weight on female spawning choice, although our observations did not capture all occasions when males may have engaged in courtship and the size-matching of males may have obscured the effect of size. We therefore suggest that female preference for nest appearance is consistent with seeking direct benefits.
In conclusion, we found that in the sand goby, females preferentially spawn in nests with substantial sand cover, making nest appearance a key factor in mate choice. Moreover, nest appearance is influenced by sand texture, and it appears that coarser sand hampers nest building. Finally, our results imply that the decision on whether to build a nest or not is complex, and affected by sand texture, male size, freedom of choice, and perhaps also the presence of other males.
References
Barber I, Nairn D, Huntingford FA (2001) Nests as ornaments: revealing construction by male sticklebacks. Behav Ecol 12:390–396
Berson JD, Simmons LW (2018) Sexual selection across sensory modalities: female choice of male behavioral and gustatory displays. Behav Ecol 29:1096–1104
Borgia G (1985) Bower quality, number of decorations and mating success of male satin bowerbirds (Ptilonorhynchus violaceus): an experimental analysis. Anim Behav 33:266–271
Botero-Delgadillo E, Serrano D, Orellana N, Poblete Y, Vásquez RA (2017) Effects of temperature and time constraints on the seasonal variation in nest morphology of the thorn-tailed rayadito (Aphrastura spinicauda). Emu 117:181–187
Candolin U (2003) The use of multiple cues in mate choice. Biol Rev 78:575–595
Clarke A, Johnston NM (1999) Scaling of metabolic rate with body mass and temperature in teleost fish. J Anim Ecol 68:893–905
Dinno A (2017) conover.test: Conover-Iman test of multiple comparisons using rank sums. https://cran.r-project.org/package=conover.test
Forsgren E (1992) Predation risk affects mate choice in a gobiid fish. Am Nat 140:1041–1049
Forsgren E (1997a) Mate sampling in a population of sand gobies. Anim Behav 53:267–276
Forsgren E (1997b) Female sand gobies prefer good fathers over dominant males. Proc R Soc Lond B 264:1283–1286
Forsgren E (1999) Sexual selection and sex roles in the sand goby. In: Almada V, Oliveira R, Gonçalves E (eds) Behaviour and conservation of Littoral fishes. ISPA, Lisboa, pp 249–274
Forsgren E, Karlsson A, Kvarnemo C (1996) Female sand gobies gain direct benefits by choosing males with eggs in their nest. Behav Ecol Sociobiol 39:91–96
Hansell M (2000) Bird nests and construction behaviour. Cambridge University Press, Cambridge
Hess SE, Rohr S, Dufour BD, Gaskill BN, Pajor EA, Garner JP (2008) Home improvement: C57BL/6J mice given more naturalistic nesting materials build better nests. J Am Assoc Lab Anim Sci 47:25–31
Hesthagen I (1977) Migrations, breeding, and growth in Pomatoschistus minutus (Pallas) (Pisces, Gobiidae) in Oslofjorden, Norway. Sarsia 63:17–26
Hilton GM, Hansell MH, Ruxton GD, Reid JM, Monaghan P (2004) Using artificial nests to test importance of nesting material and nest shelter for incubation energetics. Auk 121:777–787
Hoi H, Schleicher B, Valera F (1994) Female mate choice and nest desertion in penduline tits, Remiz pendulinus: the importance of nest quality. Anim Behav 48:743–746
Japoshvili B, Lehtonen TK, Wong BBM, Lindström K (2012) Repeatability of nest size choice and nest building in sand gobies. Anim Behav 84:913–917
Jones JC, Reynolds JD (1999) Oxygen and the trade-off between egg ventilation and brood protection in the common goby. Behaviour 136:819–832
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmerTest package: tests in linear mixed effects models. J Stat Softw 82:1–26
Kvarnemo C (1994) Temperature differentially affects male and female reproductive rates in the sand goby: consequences for operational sex ratio. Proc R Soc Lond B 256:151–156
Kvarnemo C, Forsgren E, Magnhagen C (1995) Effects of sex ratio on intra-and inter-sexual behaviour in sand gobies. Anim Behav 50:1455–1461
Lehtonen TK (2012) Signal value of male courtship effort in a fish with paternal care. Anim Behav 83:1153–1161
Lehtonen T, Lindström K (2004) Changes in sexual selection resulting from novel habitat use in the sand goby. Oikos 104:327–335
Lehtonen TK, Wong BB (2009) Should females prefer males with elaborate nests? Behav Ecol 20:1015–1019
Lehtonen TK, Rintakoski S, Lindström K (2007) Mate preference for multiple cues: interplay between male and nest size in the sand goby, Pomatoschistus minutus. Behav Ecol 18:696–700
Lehtonen TK, Lindström K, Wong BBM (2013) Effect of egg predator on nest choice and nest construction in sand gobies. Anim Behav 86:867–871
Lindström K, Ranta E (1992) Predation by birds affects population structure in breeding sand goby, Pomatoschistus minutus, males. Oikos 64:527–532
Lissåker M, Kvarnemo C (2006) Ventilation or nest defense - parental care trade-offs in a fish with male care. Behav Ecol Sociobiol 60:864–873
Lissåker M, Kvarnemo C, Svensson O (2003) Effects of a low oxygen environment on parental effort and filial cannibalism in the male sand goby, Pomatoschistus minutus. Behav Ecol 14:374–381
Mappes T, Mappes J, Kotiaho J (1994) Ectoparasites, nest site choice and breeding success in the pied flycatcher. Oecologia 98:147–149
McLaren P, Bowles D (1985) The effects of sediment transport on grain-size distributions. J Sediment Res 55:457–470
Miller PJ (1986) Fishes of the North-Eastern Atlantic and the Mediterranean. UNESCO, Paris
Møller AP, Linden M, Soler JJ, Soler M, Moreno J (1995) Morphological adaptations to an extreme sexual display, stone-carrying in the black wheatear, Oenanthe leucura. Behav Ecol 6:368–375
Mowles SL, Jennions MD, Backwell PR (2018) Robotic crabs reveal that female fiddler crabs are sensitive to changes in male display rate. Biol Lett 14:20170695
Oksanen J, Blanchet FG, Friendly M et al (2017) vegan: community ecology package. https://cran.r-project.org/package=vegan
Olsson KH, Kvarnemo C, Svensson O (2009) Relative costs of courtship behaviours in nest-building sand gobies. Anim Behav 77:541–546
Olsson KH, Kvarnemo C, Andrén MN, Larsson T (2016) Hypoxia increases the risk of egg predation in a nest-guarding fish. R Soc Open Sci 3:160326
Olsson KH, Johansson S, Blom E-L, Lindström K, Svensson O, Nilsson Sköld H,Kvarnemo C (2017) Dark eyes in female sand gobies indicate readiness to spawn. PLoS ONE 12:e0177714
Östlund-Nilsson S (2001) Fifteen-spined stickleback (Spinachia spinachia) females prefer males with more secretional threads in their nests: an honest-condition display by males. Behav Ecol Sociobiol 50:263–269
Östlund-Nilsson S, Holmlund M (2003) The artistic three-spined stickleback (Gasterosteous aculeatus). Behav Ecol Sociobiol 53:214–220
Pampoulie C, Lindström K, St. Mary CM (2004) Have your cake and eat it too: male sand gobies show more parental care in the presence of female partners. Behav Ecol 15:199–204
Quader S (2005) Elaborate nests in a weaverbird: a role for female choice? Ethology 111:1073–1088
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna http://www.R-project.org
Raventos N (2006) Nest site characteristics and nesting success of the five-spotted wrasse Symphodus roissali in the north-western Mediterranean Sea. J Fish Biol 68:305–309
Rutnagur R (1990) Nest structure and related building behaviour in the rook Corvus frugilegus. Unpublished PhD thesis, Glasgow University
Scheipl F, Greven S, Kuechenhoff H (2008) Size and power of tests for a zero random effect variance or polynomial regression in additive and linear mixed models. Comput Stat Data Anal 52:3283–3299
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
SMHI (2017) Väderöarna WR Boj, https://www.smhi.se/klimatdata/oceanografi/ladda-ner-oceanografiska-observationer/#param=seatemperature,stations=all,stationid=33015. Accessed 28 Sep 2017
Soler JJ, Cuervo JJ, Møller AP, de Lope F (1998) Nest building is a sexually selected behaviour in the barn swallow. Anim Behav 56:1435–1442
Soler JJ, Ruiz-Castellano C, Figuerola J, Martín-Vivaldi M, Martínez-de la Puente J, Ruiz-Rodríguez M, Tomás G (2017) Telomere length and dynamics of spotless starling nestlings depend on nest-building materials used by parents. Anim Behav 126:89–100
Suárez-Rodríguez M, Macías Garcia C (2014) There is no such a thing as a free cigarette; lining nests with discarded butts brings short-term benefits, but causes toxic damage. J Evol Biol 27:2719–2726
Suárez-Rodríguez M, López-Rull I, Garcia CMÍ (2013) Incorporation of cigarette butts into nests reduces nest ectoparasite load in urban birds: new ingredients for an old recipe? Biol Lett 9:20120931
Svensson O, Kvarnemo C (2003) Sexually selected nest building - Pomatoschistus minutus males build smaller nest openings in the presence of sneaker males. J Evol Biol 16:896–902
Svensson O, Kvarnemo C (2005) The importance of sperm competition risk and nest appearance for male behavior and female choice in the sand goby, Pomatoschistus minutus. Behav Ecol 16:1042–1048
Tallmark B, Evans S (1986) Substrate-related differences in antipredator behaviour of two gobiid fish species and the brown shrimp, and their adaptive value. Mar Ecol Prog Ser 29:217–222
Wagner WE Jr, Reiser MG (2000) The importance of calling song and courtship song in female mate choice in the variable field cricket. Anim Behav 59:1219–1226
Acknowledgments
The authors thank Sofie Schöld at the Swedish Meteorological and Hydrological Institute for helping to locate relevant temperature data from institute databases. We also thank staff and colleagues at Sven Lovén Centre Kristineberg for use of facilities and support during the study and two anonymous reviewers for providing valuable comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Ethical permission for the experimental procedures was obtained from the Swedish Animal Welfare Agency (dnr 211-2007) and University of Florida (UF IACUC #E644).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Communicated by J. Lindström
Rights and permissions
About this article
Cite this article
Olsson, K.H., Forsgren, E., Merilaita, S. et al. Effect of sand texture on nest quality and mating success in a fish with parental care. Behav Ecol Sociobiol 73, 96 (2019). https://doi.org/10.1007/s00265-019-2711-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-019-2711-y