Abstract
Prey species use pursuit-deterrent signals to discourage an attack, by informing a predator either that the latter has been detected, or that the prey is capable of escaping if attacked. These signals tend to be conspicuous behaviors, such as bobbing, stotting, and predator inspection. Dark-eyed juncos (Junco hyemalis) show prominent tail-flashing in social displays during the breeding season, but they continue to tail-flash in winter. We examined whether such tail-flashing functions as a pursuit-deterrent signal by measuring tail-flashing rates in the presence and absence of a taxidermically mounted hawk predator. Our results showed that juncos tail-flash more in the presence of the predator and at higher rates when in direct view of the predator. This suggests that tail-flashing is directed to the predator as a pursuit-deterrent signal. Additionally, juncos reduced tail-flashing when feeding far from cover (low escape probability), suggesting that tail-flashing likely has an attraction cost. We also found a marked group size effect with solitary juncos tail-flashing more than those in large groups, indicating an additional cost to tail-flashing that need not be paid in larger groups. This additional cost is not related to food intake, since we found no negative association between food intake and tail-flashing. We observed tail-flashing in other sparrow species co-occurring with juncos at the study site, suggesting that tail-flashing as a pursuit-deterrent behavior may be widespread in this taxonomic group of birds.
Significance statement
Many animals, and especially birds, use tail movements as a signal in various contexts. Dark-eyed juncos make extensive use of tail-flashing as a breeding signal, but they continue to tail-flash during the winter. Our experiment provides evidence that such tail-flashing serves as a pursuit-deterrent signal, a function previously not examined in these birds. Juncos tail-flashed more in the presence of a visible predator, suggesting that the signal is directed towards the predator. Tail-flashing was maximal when close to cover, indicating that tail-flashing incurs a cost of attracting predator attention. A negative relationship between flock size and tail-flashing suggests an additional cost to this behavior, which we demonstrate is not related to food intake rate. We suspect that tail-flashing functions more widely as a pursuit-deterrent signal in the broad phylogenetic group of emberizid sparrows.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prey animals often use signals to actively communicate information about risk in the environment (Caro 2005). These signals occur in various forms and serve different adaptive functions. For instance, kangaroo rats (Dipodomys spectabilis) use footdrumming to communicate with predators (Randall and Matocq 1997), and several birds emit vocal alarm calls to both conspecifics and predators (Yorzinski and Patricelli 2010). There are at least two general categories of prey signals based on the intended signal recipient. One type of signal is directed to conspecifics or heterospecifics with information about potential risk (Townsend et al. 2012; Shah et al. 2015). A second type, pursuit-deterrent signaling, is directed to predators (Woodland et al. 1980). Pursuit-deterrent signals are assumed to inform the predator either of prey awareness (“perception advertisement”), or prey escape ability (“quality advertisement”; Caro 1995), in an attempt to discourage the predator from initiating an attack (Hasson 1991).
Pursuit-deterrent signals tend to be conspicuous behaviors such as aerial singing in skylarks (Alauda arvensis; Cresswell 1994), push-up displays by lizards (Anolis cristatellus; Leal and Rodriquez-Robles 1997; Leal 1999), and predator inspection by guppies (Poecilia reticulata; Godin and Davis 1995). These signals may carry risk to prey because the signal can draw attention or delay the prey’s escape (Hasson 1991; Fitzgibbon 1994). However, signaling theory suggests that if the signal is an “honest” indicator of the prey’s abilities, then signaling also provides a benefit that is higher than associated costs (Grafen 1990; Vega-Redondo and Hasson 1993). In this case, a successful pursuit-deterrent signal would encourage a predator to avoid attacking unprofitable prey and hunt elsewhere, while the prey can resume activities such as feeding (Tilson and Norton 1981; Hasson 1991). Game-theoretical models on pursuit-deterrence predict that signaling evolves when there is a cost to signaling, and when prey have a high chance of detecting the predator (Ramesh and Mitchell 2018). Pursuit-deterrence has been empirically investigated in a few species (reviewed in Caro 2005; Randler 2016), but may be widespread among vertebrates (Caro 1995; Randler 2006).
Several predictions have been put forth in order to determine whether a signal functions in pursuit-deterrence, and studies have used a combination of approaches to suit a particular species or question of interest. It is generally expected that a pursuit-deterrent signal (1) will occur at higher rates when a predator is present (Randler 2016); (2) is most likely to occur at intermediate distances to the predator or intermediate predation risk levels (Cooper 2010); (3) need not be restricted to a direct encounter with an approaching predator (Spitznagel 1996; Murphy 2007); and (4) will vary with the extant risk of predation (e.g., Cooper 2010). If the signal is associated with vigilance levels, then the signal may be negatively correlated with group size (Randler 2007), although evidence for this effect is mixed (e.g., Ryan et al. 1996). Experimental studies have used different types of surrogate predators to elicit prey response, including the use of approaching humans (Ryan et al. 1996) and artificial predator models (Deppe et al. 2003). These approaches provide insight about the signal from the prey’s perspective (Caro 1995), but they cannot measure the influence of the signal on a predator (Leal and Rodriquez-Robles 1997; Barbour and Clark 2012). A few studies have measured predator response to demonstrate effective pursuit-deterrence (Cresswell 1994; Godin and Davis 1995; Barbour and Clark 2012), but for most systems, predator behavioral data is difficult to obtain (Lima 2002).
A pursuit-deterrent signal seen across terrestrial vertebrate taxa includes various tail movements (summarized in Randler 2016). For instance, curly-tailed lizards (Leiocephalus carinatus) show vertical curling of their tails with greater intensity when approached by a predator (Cooper 2001), eastern swamphen (Porphyrio porphyrio) tail-flick at greater frequency towards intruders to reveal a white rump patch (Woodland et al. 1980), eastern phoebes (Sayornis phoebe) pump their tails at higher rates in the presence of the predator (Carder and Ritchison 2009), and ground squirrels (Otospermophilus beecheyi) tail-flag towards snakes eventually leading the snakes to abandon attack (Barbour and Clark 2012). Tail movements also occur in other adaptive contexts, including intra-specific sexual signaling and dominance displays (Hill et al. 1999; Randler 2016). Recent work suggests the possible multifunctionality of these tail-movement signals across contexts (Spitznagel, 1996; Alvarez et al. 2010; Bitton and Doucet 2013).
We focused our study on tail-flashing by dark-eyed juncos (Junco hyemalis), which are known to tail-flash as part of intra- and inter-sexual displays during the breeding season (Balph et al. 1979; Hill et al. 1999), but potential anti-predator functions of this signal are unknown. We define tail-flashing as rapid horizontal spreading of tail feathers, following Randler (2016). The stark contrast in the color of their tail feathers, with dark inner and white outer feathers, presumably makes tail-flashing highly conspicuous in juncos (Randler 2016). This tail-flashing behavior occurs in courtship and territorial displays of juncos, as well as in signaling social status and dominance, along with other behaviors such as tail-fanning (Balph et al. 1979; Hill et al. 1999). In winter, however, juncos continue to tail-flash, albeit in a manner not obviously associated with breeding displays. These birds are also well south of their breeding range, and thus not territorial. Small wintering birds focus mainly on survival (avoiding predation and starvation, Lima 2002), and thus tail-flashing during the harsh conditions of winter is likely related to survival rather than breeding per se.
In this study, we examined whether tail-flashing by wintering juncos is an anti-predator pursuit-deterrent signal in a series of field experiments. We measured tail-flashing rates in free-ranging juncos in the presence and absence of a taxidermic hawk model predator, with an additional treatment level of whether or not the predator was visible to foraging juncos. Higher tail-flashing rates in the general presence of the predator would support a pursuit-deterrent function (Randler 2016). Additionally, increased tail-flashing by juncos in direct view of the predator would further support the pursuit-deterrence hypothesis (Hasson 1991; Alvarez 1993). We also varied predation risk by varying distance to cover; birds foraging far from cover perceive increased predation risk (Randler 2007, 2016; Camp et al. 2012). Higher tail-flashing farther from cover would indicate tail-flashing is perhaps an honest signal of prey quality (e.g., escape ability) (Hasson 1991). In contrast, if signaling is reduced when far from cover, then tail-flashing may instead impose an “attraction” cost of drawing the attention of predators (Murphy 2007).
Given that juncos forage in mixed flocks during winter, we also examined the effects of group size on tail-flashing. Several empirical studies support the general idea that birds are safer in groups by means of enhanced predator detection or numerical dilution of risk (Krause and Ruxton 2001; Beauchamp 2015). A negative association between tail-flashing and group size would suggest that there is some cost to tail-flashing that need not be paid in larger groups. Finally, we measured feeding rates to assess a potential foraging-related cost of tail-flashing. Here again, a negative association between feeding rate and tail-flashing would indicate that the latter interferes with the former, and thus imposes an energetic cost of limiting food intake.
Methods
Study site and species
The study was conducted in western Vigo County, Indiana, USA, between December 2016 and mid-March 2017. We observed free-ranging birds on a ground-level concrete pad (6 × 4 m) surrounded by a mature forest to the east and early successional fields on the other sides (Fig. 1). The pad was divided into six distinct feeding bays of approximately equal area using wooden partitions 10 cm tall which prevented birds from seeing each other across bays. Birds respond only to those in their own bay (Lima and Zollner 1996); thus, the bays facilitated the formation of a range of smaller group sizes, allowing to more easily examine the effect of group size on tail-flashing. Leafless brush cover was provided in two 2.5 × 1.2 × 1.2 m wooden frames placed adjacent to the eastern side of the pad. A blind (1.5 × 1.2 × 1.2 m) was placed 1 m from the western edge of the feeding pad. Finely ground cornmeal was spread evenly on the surface of the pad at a density that prevented rapid food depletion, and minimized competition and associated aggressive interactions. We videotaped feeding birds through a two-way mirror from inside the blind using a Panasonic® high-definition video camera (model: HC-W570). Each focal bird was recorded for a minimum of 10 s and up to 60 s. Standard focal sampling methods were used in the study, such that if group size changed during a focal observation, then that observation was concluded and another individual was selected for focal observation. Observations began 1 h after sunrise and ended after videotaping 40–50 focal birds or 2 h, whichever occurred earlier.
Dark-eyed juncos dominated the study site and comprised 70% of the birds recorded at the site. About 120 juncos visited the site, as judged by the maximum number present during periods of snow cover. Juncos were observed feeding in mixed species flocks with other emberizid sparrows—American tree sparrows (Spizelloides arborea), white-throated sparrows (Zonotrichia albicollis), and song sparrows (Melospiza melodia)—in decreasing order of abundance. Occasional visitors to the feeding pad included fox sparrows (Passerella iliaca) and swamp sparrows (Melospiza georgiana). All focal observations were conducted on juncos, although the group size variable included numbers of conspecifics and heterospecifics in a focal bay. Previous work at the site suggests that juncos respond similarly to the presence of conspecifics and heterospecifics (Lima and Zollner 1996).
Statistical considerations
As described below, we assessed the behavior of focal juncos during each daily observational session, taking precautions to minimize the re-sampling of individual birds during a given session. We were, however, constrained to work with unmarked birds, since wintering juncos routinely use heat-saving postures that obstruct individual identification using leg bands (Carr and Lima 2012). It is likely that a given bird was sampled multiple times over the course of the study, hence treating individual focal birds as statistically independent would be problematic.
To minimize the effects of pseudoreplication in our analysis, we characterized the behavior of the juncos sampled during a given observational session by a single value (depending on the behavior in question, see the “Data analysis” section). We assume that, over several such sessions, these values represent independent estimates of the daily behavior of the group of birds visiting our study site. Such a metric will vary across sessions for a variety of reasons, even when drawn from the same (large) pool of birds. For instance, the juncos visiting the site experience frequent changes in the prevailing social situation (the number and identity of birds present, and their distribution across the study site), variation in recent experience with predators, etc. Our approach assumes that, with enough daily sessions, it is possible to distinguish this day-to-day variation in our metric from an overall response by the group of juncos to changes in the experimental setup. We believe that this approach is statistically conservative, but nevertheless some degree of pseudoreplication is likely present in our data. For the current study, we see this approach and the benefits of working with free-ranging birds as preferable to working in a more artificial environment with perhaps greater statistical control of our subjects. We also note that our analysis of repeated observations from a single (albeit large) group of juncos means that our results technically apply only to the population of juncos at our site, but our subject population was not unusual in any way.
Experiments
We conducted two experiments in succession. The first was the “predator experiment” which ran from 19 December 2016 to 8 February 2017. Here, we used a taxidermically mounted immature female Cooper’s hawk (Accipiter cooperii), a common predator of dark-eyed juncos in the study area (Roth et al. 2008). The hawk specimen (henceforth predator) was mounted in an upright, alert posture, perched on a branch looking straight ahead. Perched hawks proffer an intermediate threat to prey species (Mathot et al. 2009), but perhaps not an extremely imminent threat of attack. Birds are also known to be capable of distinguishing head and body orientation of predators and perceive increased predation risk when the head and gaze of a predator face them directly (Cantwell et al. 2016). The taxidermically mounted hawk used in this study is also a more realistic model predator than plastic models, which evoke a lesser response due to the absence of natural cues such as texture and feathers (Němec et al. 2015).
The “predator experiment” consisted of two treatment levels—predator absent and predator present. For the predator-present setup, the predator was placed facing the pad atop a 1.6-m-tall pedestal, 3 m from the western edge of the feeding pad. To prevent habituation of the juncos to the predator, the predator was present every third observation session, while the pedestal remained at the site throughout the study period. To assess the intended recipient of tail-flashing, and whether tail-flashing was a signal of risk perception, the predator-present sessions were further divided into two sublevels—blocked and visible—where the predator was placed on one side of the observation blind so that it was blocked from the view of foraging juncos in two bays and visible to juncos in four bays. The location of the predator was alternated every predator-present session such that blocked bays during one session were visible the next, and vice versa. There were a total of 24 predator-absent and 12 predator-present observation sessions. Note that while the predator-absent and predator-present conditions occurred on separate sessions, blocked and visible conditions occurred during each predator-present session. Cover was placed adjacent to the pad throughout the entire predator experiment, as described in the study site setup.
The “cover experiment” ran from 9 February to 15 March 2017. This experiment consisted of two treatment levels—cover near and cover far. The setup for cover near is as described in the study site setup, with cover placed adjacent to the eastern side of the feeding pad. For cover far, we moved the cover frames 10 m away from the feeding pad, thus forcing juncos to feed well away from immediate safety. The distance from cover was alternated every observation session for a total of 12 cover-near and 13 cover-far observation sessions. The predator model was never present during this experiment.
Data analysis
All video recordings were scored by DR, at half the original recording speed using the Observer XT video analysis software (NOLDUS Observer XT 8.0, Noldus Information Technology, Netherlands). We recorded individual tail-flashes and food-pecks for each focal bird, and then summarized these data by tail-flashing rate (TFR) in flashes per second and food-pecking rate (PR) in pecks per second. For each focal bird, we also recorded the group size in its foraging bay, which generally ranged from one to six birds. Observations of groups of six and larger were relatively few, and thus pooled to six or more birds for analysis. All statistical analyses were performed in R 3.1.3 (R Core Team 2015). We analyzed data blind to treatment in the predator experiment, where video recordings did not reveal which birds were in view of a predator, or whether a predator was present. However, blinded analyses were not possible in the cover experiment, since the location of cover was inevitably apparent in the video recordings.
We summarized the results from each observation session by a single value, using one of two methods. First, we determined the relationship between tail-flashing rate and group size, which was characterized by the slope of tail-flashing rate vs. group size regressions for each observation session. This allowed us to examine the effect of group size on tail-flashing, and provided a method to compare the group size effect across treatments. A negative slope indicates that TFR decreases with increasing group size. We conducted a t test (assuming unequal variances, R Core Team 2015) to examine whether slopes obtained over an experiment were significantly different from zero. To examine differences in means between treatment levels, we performed an ANOVA for the predator experiment and a t test for the cover experiment. The second metric used to characterize each daily session was the mean tail-flashing rate (MTFR), pooling all data for a session, which allowed direct comparison of general tail-flashing rates across treatments. We present the raw TFR data, but most statistical analyses were performed using the above session-specific metrics.
To compare tail-flashing between predator-present and absent conditions, we calculated the average of “blocked” and “visible” MTFR values for a given predator-present session, and compared those averaged values to the MTFR values from predator-absent sessions, using a two-sample t test. To further address whether tail-flashing was higher in birds that could see the predator, we determined whether session-specific differences between visible and blocked MTFR values were significantly different from zero, using a one-sample t test. To examine whether juncos perceived increased risk when feeding farther from cover, we compared the daily MTFR values from cover near sessions with those of cover far, using a two-sample t test.
To identify potential foraging costs of tail-flashing, we examined associations between individual TFR and PR values for each group size in both predator and cover experiments. Each relationship was characterized by the Pearson’s correlation between TFR and PR in the raw data. A negative correlation indicates a foraging cost of tail-flashing. We then statistically examined the resulting correlations to determine whether the means of correlation coefficients from each experiment were significantly different from zero, using a one-sample t test.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Results
We found that individual tail-flashing rate (TFR) decreases with increase in group size, a result consistent across treatments in both predator and cover experiments. This general group size effect is apparent in a visual inspection of the raw data from the predator experiment (Fig. S1) and cover experiment (Fig. S2). Individual birds showed much variation in TFR, from 0 to 1 tail-flash per second (occasionally higher), with higher values becoming less frequent as group size increases.
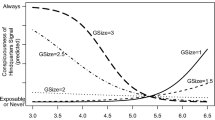
To statistically assess the effect of group size, as outlined earlier, we characterized each daily observation session by a single metric—in this case, the slope of TFR vs. group size regressions for each session. An example from a typical session (Fig. 2) shows considerable variation and a generally negative slope. Within the predator experiment, the group size slopes so obtained were consistently negative across treatment levels and sublevels (Fig. 3a). This is readily apparent from predator-absent sessions, with nearly all data points below zero and a mean slope of − 0.024 that is significantly less than zero (t = − 4.4212, df = 23, p = 0.0002). The mean slope when the predator was present and visible (− 0.041) was also significantly less than zero (t = − 2.8414, df = 11, p = 0.016). When the predator was present but blocked from the view of juncos, the mean slope (− 0.025) was less than zero but not statistically different from zero (t = − 1.5504, df = 11, p = 0.15). There was also a tendency for greater variation in slopes with the predator present, with a few cases of slopes well above zero (Fig. 3a). Nevertheless, the central tendencies across these three conditions within the predator experiment did not differ significantly (ANOVA, F = 0.6193, df = 2, p = 0.55, Fig. 3a). Thus, all treatment levels showed similar group size effects, with a decrease in tail-flashing as group size increased.
For the cover experiment, the distributions of daily slopes (Fig. 3b) did not differ significantly between the near and far conditions (t = − 0.1811, df = 22.14, p = 0.858), and were similar to those obtained from the predator absent treatment in the predator experiment (Fig. 3a). The mean slopes were also significantly less than zero for both treatments (Near: t = − 3.5721, df = 11, p = 0.0044; Far: t = − 2.5655, df = 12, p = 0.025). So, across treatments in both predator and cover experiments, we found consistent decreases in TFR as group size increases. Thus, differences in our mean-based behavioral metric for characterizing daily results (see below) do not reflect changes in the relationship between TFR and group size, but rather a general increase or decrease in TFR over all group sizes.
Tail-flashing rates across experiments
As outlined earlier, we characterized each daily predator experiment session by its mean tail-flashing rate (MTFR). During this experiment, we further partitioned tail-flashing data for a given predator-present session by taking the average of MTFRs of birds that could see the predator (visible) and those blocked from viewing the predator (blocked). Tail-flashing in the presence of the predator was generally higher than that observed in the absence of the predator, as indicated by a comparison of predator-absent and average predator-present data (Fig. 4, t = − 4.1041, df = 16.433, p = 0.0008). Further, higher tail-flashing with the predator in view is evident in the largely positive slopes of lines connecting each visible and blocked MTFR pair in Fig. 4. Here, the average difference (0.097) between MTFR pairs was significantly greater than zero (t = 4.848, df = 11, p = 0.0005), indicating a significant tendency for greater tail-flashing when the predator was in view.
Tail-flashing rates as influenced by predator presence or absence. Each point represents a session-specific mean of tail-flashing rates. Data from predator-absent sessions are on the left, with those from the predator-present sessions on the right. Predator-present sublevels (blocked and visible) from each session are connected pairwise by straight lines to show sign of difference between them. Average values of each blocked and visible pair of mean tail-flashing rates during predator-present sessions are shown as filled circles
During the cover experiment, juncos tail-flashed at generally higher rates when feeding near cover rather than farther away (Fig. 5). This trend is apparent in the raw data (Fig. S2). To statistically examine the differences between cover near and far, we again used daily MTFR values. These session-specific MTFR values were typically greater for birds feeding near to cover (Fig. 5), and the means of the two cover treatments differed significantly (t = 3.6406, df = 18.141, p = 0.0018).
Tail-flashing vs. feeding
Here, we address the question of whether tail-flashing interferes in some way with feeding, thus providing one possible explanation for why TFR drops with increasing group size. We do so by examining correlations between TFR and pecking rate (PR) in the raw data across various experimental conditions.
In the predator experiment, plots of TFR and PR for each combination of group size and treatment level did not show any strong relationships between tail-flashing and pecking rates (Fig. S3). Most trends were neutral or slightly positive; negative trends were not apparent. To examine this statistically, we proceeded as before, by characterizing each of the relationships in Fig. S3 by its correlation coefficient. The mean of these correlation coefficients was 0.115, which differed significantly from 0 (t = 3.7591, df = 17, p = 0.0016), suggesting an overall positive correlation between tail-flashing and pecking rates. We found similar support for a weakly positive trend in correlations between TFR and PR for the cover experiment (Fig. S4), using the same statistical approach, with a mean overall correlation coefficient (0.14) that was significantly different from zero (t = 3.7513, df = 11, p = 0.0032). Our analysis thus provides no evidence for a negative relationship between TFR and PR in both predator and cover experiments.
Discussion
Our results suggest that tail-flashing in wintering juncos is a signal directed towards predators and has a pursuit-deterrent function. The average rate of junco tail-flashing when the predator was present was higher than when the predator was absent. This suggests that juncos were aware of the presence of the predator and responded by increasing tail-flashing rates. Second, birds that were able to maintain visual contact with the predator while feeding tail-flashed more than those that could not. These results are consistent with the idea that the predator is a direct and intended recipient of the tail-flashing signal. Behaviors currently identified as pursuit-deterrent signals occur at higher rates in the presence of a predator (Woodland et al. 1980; Hasson 1991; Randler 2016), and often involve approach or facing the predator and continuing to signal at high rates (Fitzgibbon 1994; Godin and Davis 1995). Studies on moorhens (Gallinula sp.; Ryan et al. 1996; Randler 2007) and eastern phoebes (S. phoebe; Carder and Ritchison 2009) show similar patterns of increase in frequency of tail movement in the presence of the predator. In addition to known functions of tail-flashing by juncos during the breeding season (Balph et al. 1979; Hill et al. 1999), our study provides evidence for a novel pursuit-deterrent function, suggesting that tail-flashing is a multifunctional signal in this species. Among bird species, examples of a signal performed across both social and anti-predator contexts include singing in skylarks (Cresswell 1994) and tail wagging in turquoise-browed motmots (Eumomota superciliosa; Murphy 2007).
A notable aspect of our results is that tail-flashing in juncos occurred in the absence of the predator, albeit at a reduced rate. While such signaling is not unusual for pursuit-deterrent signals (Spitznagel 1996; Randler 2007), it may indicate possible dishonest signaling (Murphy 2007). If so, as in the case of tail wagging in motmots, tail-flashing in dark-eyed juncos could be maintained by selection since it also occurs at other times of the year as a component of the breeding display, generally given in an honest context (Spitznagel 1996). Alternatively, signaling in the absence of predators may suggest tail-flashing is a “vigilance” signal, i.e., a signal that increases when the predator approaches and that communicates alertness to the predator (Randler 2006). We also found that tail-flashing in juncos has a negative group size effect, which is a key characteristic of vigilance behaviors (Beauchamp 2015). However, contrary to expectations of vigilance signals, we found that tail-flashing decreases far from cover, which we attribute to the cost of drawing a predator’s attention. Additionally, we did not find a negative correlation between tail-flashing and pecking, as would be expected under the vigilance signaling hypothesis (Randler 2006). These results together provide mixed support for vigilance signaling. We note that vigilance and pursuit-deterrence need not be mutually exclusive. Vigilance signals may also function as pursuit-deterrent signals, especially with ambush predators such as the Cooper’s hawk used in this study, where predators could choose whether or not to attack a prey based on visible cues such as tail movements (Randler 2006).
It is possible that tail-flashing may be directed to conspecifics instead of predators, where tail-flashing could function in flock formation and maintenance (Balph 1977; Elgar 1986). Birds are known to be safer in groups and often aggregate in flocks to reduce individual predation risk (Beauchamp 2015). If tail-flashing signals conspecifics to join a flock, then we would expect a reduction in tail-flashing as the flock is formed, i.e., lower tail-flashing in larger group sizes. We did find a drop in tail-flashing rate as group size increases. However, this conspecific signaling hypothesis fails to explain the results of our cover experiment. If tail-flashing is indeed directed to conspecifics for the goal of flock formation, then we would expect more tail-flashing when juncos are far from cover and thus at greater risk (Elgar 1986; Randler 2007). Instead, we found less tail-flashing by juncos far from cover. Alternately, if juncos signal conspecifics about presence of the predator, then we expect little tail-flashing in solitary birds, and little tail-flashing in the absence of the predator (Randler 2007). Again, we did not see this in our study; solitary juncos showed the highest tail-flashing rates, and we observed tail-flashing in the absence of the predator. Furthermore, because juncos tail-flashed significantly less often in larger groups, it seems unlikely that juncos were signaling each other with aggressive intent, especially since aggression (though minimal) increased with group size. Tail-flashing could signal “nervousness”, but this idea also fails to explain why tail-flashing occurs at lower rates far from cover.
The reduction in tail-flashing when far from cover suggests that juncos perceive greater risk in the open (Cooper 2001), and that tail-flashing likely bears an attraction cost. That is to say, they draw less attention to themselves from potential predators when they are forced to feed in the open. In the “cover experiment”, cover was removed 10 m away from the feeding pad, a clearly high predation risk scenario for these birds (Roth et al. 2006). Furthermore, it is likely that tail-flashing in juncos is a signal of “perception” or awareness of the predator, rather than prey ability to escape (“prey quality”, sensu Caro, 1995). Signals of perception need not be as costly as those that indicate prey quality (Randler 2016). For instance, foot shakes in lizards are shown to have a pursuit-deterrent function and not be energetically costly (Font et al. 2012), and tail movements may not impose significant cost as they often occur without break from foraging (Randler 2016). In these instances, the cost lies in increased conspicuousness to hidden predators (Randler 2016).
There may be other costs to tail-flashing, as indicated by the negative effect of group size on tail-flashing rate, which is a prominent feature of our results. While few studies on pursuit-deterrence have quantified and discussed an effect of group size, a similar negative relationship between pursuit-deterrent signaling and group size has been observed in the purple swamphen (Porphyrio porphyrio; Woodland et al. 1980), two species of moorhen (Alvarez 1993; Ryan et al. 1996), and Thomson’s gazelles (Eudorcas thomsonii; Fitzgibbon 1994). It is generally accepted that the benefit from signaling drops in larger groups (Fitzgibbon 1994), but not much is understood about the cost of signaling that would explain the group size effect. If tail-flashing has a foraging cost, we would expect a negative relationship between tail-flashing rate and pecking (feeding) rate (Randler 2006). In fact, we found a weakly positive association between tail-flashing and pecking in juncos, indicating that tail-flashing does not interfere with feeding. An energetic cost to tail-flashing could also be expressed via thermoregulatory responses to ambient temperature. On extremely cold days, juncos have been observed to rest on the ground or use their tails as a prop when standing on one foot (Carr and Lima 2012), both postures in which tail-flashing is physically difficult and does not occur. We did not have many cold days during the study period, which had much record warmth, so we are unable to sufficiently address this energetic cost. There may also be a movement cost to tail-flashing that interferes with quick take-off from the feeding pad in case of a sudden attack (Carr and Lima 2012).
The rarer emberizid sparrows visiting our study site also exhibited tail-flashing and tail-flicking. American tree sparrows tail-flashed in a manner visually similar to juncos, but perhaps more vigorously. The tail movement of white-throated sparrows may be better characterized as a tail-flick (Randler 2016), which they also performed frequently. The color pattern of tail feathers on these species also differs from the junco, with the American tree sparrow having a less distinct contrast, and the white-throated sparrow having little if any. We suspect that such tail movements are a widespread pursuit-deterrent signal in the emberizid family (Spitznagel 1996), and warrant further study.
References
Alvarez F (1993) Alertness signalling in two rail species. Anim Behav 46:1229–1231. https://doi.org/10.1006/anbe.1993.1315
Alvarez F, Sánchez C, Angulo S (2010) Relationships between tail-flicking, morphology, and body condition in moorhens. J Field Ornithol 77:1–6. https://doi.org/10.1111/j.1557-9263.2006.00001.x
Balph M (1977) Winter social behaviour of dark-eyed juncos: communication, social organization, and ecological implications. Anim Behav 25:859–884. https://doi.org/10.1016/0003-3472(77)90038-0
Balph M, Balph D, Romesburg H (1979) Social status signaling in winter flocking birds: an examination of a current hypothesis. Auk 96:78–93
Barbour MA, Clark RW (2012) Ground squirrel tail-flag displays alter both predatory strike and ambush site selection behaviours of rattlesnakes. Proc R Soc Lond B 279:3827–3833. https://doi.org/10.1098/rspb.2012.1112
Beauchamp G (2015) Animal vigilance: monitoring predators and competitors. Academic Press, Cambridge
Bitton P-P, Doucet SM (2013) A multifunctional visual display in elegant trogons targets conspecifics and heterospecifics. Behav Ecol 25:27–34. https://doi.org/10.1093/beheco/art065
Camp MJ, Rachlow JL, Woods BA, Johnson TR, Shipley LA (2012) When to run and when to hide: the influence of concealment, visibility, and proximity to refugia on perceptions of risk. Ethology 118:1010–1017. https://doi.org/10.1111/eth.12000
Cantwell LR, Johnson WT, Kaschel RE, Love DJ, Freeberg TM (2016) Predator-risk-sensitive foraging behavior of Carolina chickadees (Poecile carolinensis) and tufted titmice (Baeolophus bicolor) in response to the head orientation of snake predator models. Behav Ecol Sociobiol 70:533–539. https://doi.org/10.1007/s00265-016-2070-x
Carder ML, Ritchison G (2009) Tail pumping by eastern phoebes: an honest, persistent predator-deterrent signal? J Field Ornithol 80:163–170. https://doi.org/10.1111/j.1557-9263.2009.00218.x
Caro TM (1995) Pursuit-deterrence revisited. Trends Ecol Evol 10:500–503. https://doi.org/10.1016/S0169-5347(00)89207-1
Caro TM (2005) Antipredator defenses in birds and mammals. University of Chicago Press, Chicago
Carr JM, Lima SL (2012) Heat-conserving postures hinder escape: a thermoregulation-predation trade-off in wintering birds. Behav Ecol 23:434–441. https://doi.org/10.1093/beheco/arr208
Cooper WEJ (2001) Multiple roles of tail display by the curly-tailed lizard Leiocephalus carinatus: pursuit deterrent and deflective roles of a social signal. Ethology 107:1137–1149. https://doi.org/10.1046/j.1439-0310.2001.00754.x
Cooper WEJ (2010) Pursuit deterrence varies with predation risks affecting escape behaviour in the lizard Callisaurus draconoides. Anim Behav 80:249–256. https://doi.org/10.1016/j.anbehav.2010.04.025
Cresswell W (1994) Song as a pursuit-deterrent signal, and its occurrence relative to other anti-predation behaviours of skylark (Alauda arvensis) on attack by merlins (Falco columbarius). Behav Ecol Sociobiol 34:217–223. https://doi.org/10.1007/BF00167747
Deppe C, Holt D, Tewksbury J, Broberg L, Petersen J, Wood K (2003) Effect of northern pygmy-owl (Glaucidium gnoma) eyespots on avian mobbing. Auk 120:765–771. https://doi.org/10.1642/0004-8038(2003)120[0765:EONPGG]2.0.CO;2
Elgar MA (1986) The establishment of foraging flocks in house sparrows: risk of predation and daily temperature. Behav Ecol Sociobiol 19:433–438. https://doi.org/10.1007/BF00300546
Fitzgibbon CD (1994) The costs and benefits of predator inspection behaviour in Thomson’s gazelles. Behav Ecol Sociobiol 34:139–148. https://doi.org/10.1007/BF00164184
Font E, Carazo P, Pérez i de Lanuza G, Kramer M (2012) Predator-elicited foot shakes in wall lizards (Podarcis muralis): evidence for a pursuit-deterrent function. J Comp Psychol 126:87–96. https://doi.org/10.1037/a0028568
Godin J-GJ, Davis SA (1995) Who dares, benefits: predator approach behaviour in the guppy (Poecilia reticulata) deters predator pursuit. Proc R Soc Lond B 259:193–200. https://doi.org/10.1098/rspb.1995.0028
Grafen A (1990) Biological signals as handicaps. J Theor Biol 144:517–546. https://doi.org/10.1016/S0022-5193(05)80088-8
Hasson O (1991) Pursuit-deterrent signals: communication between prey and predator. Trends Ecol Evol 6:325–329. https://doi.org/10.1016/0169-5347(91)90040-5
Hill JA, Enstrom DA, Ketterson ED, Nolan V Jr, Ziegenfus C (1999) Mate choice based on static versus dynamic secondary sexual traits in the dark-eyed junco. Behav Ecol 10:91–96. https://doi.org/10.1093/beheco/10.1.91
Krause J, Ruxton GD (2001) Living in groups. Oxford University Press, New York
Leal M (1999) Honest signalling during prey-predator interactions in the lizard Anolis cristatellus. Anim Behav 58:521–526. https://doi.org/10.1006/anbe.1999.1181
Leal M, Rodriquez-Robles JA (1997) Signalling displays during predator-prey interactions in a Puerto Rican anole, Anolis cristatellus. Anim Behav 54:1147–1154. https://doi.org/10.1006/anbe.1997.0572
Lima SL (2002) Putting predators back into behavioral predator–prey interactions. Trends Ecol Evol 17:70–75. https://doi.org/10.1016/S0169-5347(01)02393-X
Lima SL, Zollner PA (1996) Anti-predatory vigilance and the limits to collective detection: visual and spatial separation between foragers. Behav Ecol Sociobiol 38:355–363. https://doi.org/10.1007/s002650050252
Mathot KJ, van den Hout PJ, Piersma T (2009) Differential responses of red knots, Calidris canutus, to perching and flying sparrowhawk, Accipiter nisus, models. Anim Behav 77:1179–1185. https://doi.org/10.1016/j.anbehav.2009.01.024
Murphy TG (2007) Dishonest ‘preemptive’ pursuit-deterrent signal? Why the turquoise-browed motmot wags its tail before feeding nestlings. Anim Behav 73:965–970. https://doi.org/10.1016/j.anbehav.2006.10.020
Němec M, Syrová M, Dokoupilová L, Veselý P, Šmilauer P, Landová E, Lišková S, Fuchs R (2015) Surface texture and priming play important roles in predator recognition by the red-backed shrike in field experiments. Anim Cogn 18:259–268. https://doi.org/10.1007/s10071-014-0796-2
R CoreTeam (2015) R: a language and environment for statistical computing. R Foundation for statistical computing, Vienna http://www.R-project.org
Ramesh D, Mitchell WA (2018) Evolution of signalling through pursuit deterrence in a two-prey model using game theory. Anim Behav. https://doi.org/10.1016/j.anbehav.2018.10.012
Randall JA, Matocq M (1997) Why do kangaroo rats (Dipodomys spectabilis) footdrum at snakes? Behav Ecol 8:404–413. https://doi.org/10.1093/beheco/8.4.404
Randler C (2006) Is tail wagging in white wagtails, Motacilla alba, an honest signal of vigilance? Anim Behav 71:1089–1093. https://doi.org/10.1016/j.anbehav.2005.07.026
Randler C (2007) Observational and experimental evidence for the function of tail flicking in Eurasian moorhen Gallinula chloropus. Ethology 113:629–639. https://doi.org/10.1111/j.1439-0310.2007.01369.x
Randler C (2016) Tail movements in birds — current evidence and new concepts. Ornithol Sci 15:1–14. https://doi.org/10.2326/osj.15.1
Roth TC, Lima SL, Vetter WE (2006) Determinants of predation risk in small wintering birds: the hawk’s perspective. Behav Ecol Sociobiol 60:195–204. https://doi.org/10.1007/s00265-005-0156-y
Roth TC, Vetter WE, Lima SL (2008) Spatial ecology of wintering Accipiter hawks: home range, habitat use, and the influence of bird feeders. Condor 110:260–268. https://doi.org/10.1525/cond.2008.8489
Ryan DA, Bawden KM, Bermingham KT, Elgar MA (1996) Scanning and tail-flicking in the Australian dusky moorhen (Gallinula tenebrosa). Auk 113:499–501
Shah SS, Greig EI, MacLean SA, Bonter DN (2015) Risk-based alarm calling in a nonpasserine bird. Anim Behav 106:129–136. https://doi.org/10.1016/j.anbehav.2015.05.011
Spitznagel A (1996) Why dippers dip - on the adaptive significance of fitness-signalling and predator-pursuit deterring movements in birds. Zool Anz 235:89–99
Tilson R, Norton P (1981) Alarm duetting and pursuit deterrence in an African antelope. Am Nat 118:455–462
Townsend SW, Rasmussen M, Clutton-Brock T, Manser MB (2012) Flexible alarm calling in meerkats: the role of the social environment and predation urgency. Behav Ecol 23:1360–1364. https://doi.org/10.1093/beheco/ars129
Vega-Redondo F, Hasson O (1993) A game-theoretic model of predator-prey signaling. J Theor Biol 162:309–319. https://doi.org/10.1006/jtbi.1993.1089
Woodland D, Jaafar Z, Knight M (1980) The “pursuit deterrent” function of alarm signals. Am Nat 115:748–753
Yorzinski JL, Patricelli GL (2010) Birds adjust acoustic directionality to beam their antipredator calls to predators and conspecifics. Proc R Soc Lond B 277:923–932. https://doi.org/10.1098/rspb.2009.1519
Acknowledgments
Bill Mitchell and Ken Schmidt provided valuable assistance and comments on an earlier version of the manuscript. Two anonymous reviewers provided several useful comments and perspectives on our work.
Funding
This study was funded by the Department of Biology and the College of Graduate and Professional Studies at Indiana State University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was conducted under a protocol approval by the Indiana State University Institutional Animal Care and Use Committee (Protocol no. 688775-2). “Guidelines to the use of wild birds in research”, as published and updated by the Ornithological Council, 2010, were followed.
Additional information
Communicated by C. M. Garcia
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 554 kb)
Rights and permissions
About this article
Cite this article
Ramesh, D., Lima, S.L. Tail-flashing as an anti-predator signal in small wintering birds. Behav Ecol Sociobiol 73, 67 (2019). https://doi.org/10.1007/s00265-019-2678-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-019-2678-8