Abstract
Behavioural diversity is a basic component of biodiversity, with implications in ecological interactions at the intra- and interspecific levels. The reproductive behaviour of Odonata (dragonflies and damselflies) is unique among insects and conditioned by the anatomical separation between the male’s reproductive organs and the intromittent organ. Prior to mating, males must translocate sperm from the genital pore in the ninth abdominal segment to the seminal vesicle located ventrally in the second abdominal segment. This behaviour, exclusive to odonates, is known as intra-male sperm translocation (ST). Here, we review the literature on ST and use phylogenetic comparative analyses to investigate the evolution of ST within the Odonata. Information on ST was compiled for 176 species, with the commonest variant being ST once per mating, after tandem formation (66%). Other variants found were ST involving precopulatory genital touching (10%), ST by the male alone before tandem (16%) or after copulation (5%), and repetition of ST during the same copulation (3%). The precopulatory genital touching might have evolved to detect female receptivity. ST before tandem formation might be favoured when mating opportunities are scarce and copulations are brief. ST after mating might be favoured if males need to be ready to copulate fast. Finally, repeated ST could have evolved through postcopulatory sexual selection in males with limited sperm removal ability, as a means to improve their sperm competition. The most plausible scenario for the evolution of ST is that the ancestors of the Odonata produced a spermatophore and attached it to the body, leading towards the evolution of the secondary genitalia in males. Our study emphasises the role of behavioural diversity to understand behavioural evolution.
Significance statement
Unique behaviours are exclusive of a few individuals, populations and/or species. The intra-male sperm translocation (ST) of dragonflies and damselflies is a unique behaviour in animals: before mating, males need to transfer sperm from the primary to the secondary genitalia, which are anatomically separated. Thus, the viability and quality of sperm (i.e. fertility) will depend on the timing of ST relative to copulation. Our literature review found a variety of ST variants, being ST in tandem and before copulation the ancestral strategy. We discuss putative evolutionary routes for all the variants found and emphasise the importance of retrieving detailed observations of such unique behaviours in the field, which could help to better understand behavioural evolution in this insect group. Behavioural diversity is rarely addressed by conservation strategies, despite unique behaviours being at a higher risk of extinction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Behavioural diversity or ethodiversity (Cordero-Rivera 2017a) is a fundamental level of phenotypic variability that can promote population stability in changing environments (e.g. Dingemanse et al. 2004), increase adaptability at the intraspecific level (Berger-Tal and Saltz 2016) and inform about evolutionary processes. Ethodiversity can thus play an important role in species resilience and consequently in how we can manage species for conservation strategies. Unfortunately, while many conservation strategies address the extinction of species and its possible cascading effects across trophic levels (Pérez-Méndez et al. 2016), the disappearance of behaviours is rarely considered (Caro and Sherman 2012). Behaviours that are exclusive of a limited number of individuals, populations or species are relevant from a conservation perspective, because they are in higher risk of extinction (Caro and Sherman 2012). For instance, sexual conflict can be an engine of speciation, because it may trigger antagonistic coevolution between the sexes, potentially leading to rapid divergent evolution of the characters related to reproduction (e.g. Cordero-Rivera 2017b). Therefore, the study of reproductive behaviours is relevant for our understanding of diversification and speciation.
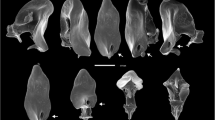
Insects from the order Odonata (dragonflies and damselflies) represent a clear example of rare behavioural diversity, regarding their copulatory behaviour. In most insects, copulation is performed with both partners oriented in opposite directions (Downes 1969; Rutowski 1982) (Fig. 1a). However, the reproductive behaviour of odonates is unique because during copulation, male and female remain attached by two points, forming the so-called “copulatory wheel” (Fig. 1b). In this position, the male anal appendages located at the tip of the abdomen grasp the female thorax (or head), and the female genitalia located at the distal part of the abdomen contacts with the male secondary genitalia, positioned in the proximal part of the abdomen. Such copulatory position occurs because the male intromittent organ is situated in the second and third abdominal segments (secondary genitalia), while the male primary genitalia are located in the ninth abdominal segment. Therefore, before insemination, the male must translocate sperm from the primary to the secondary genitalia, a behaviour called intra-male sperm translocation (hereafter, ST) (Fig. 1c–f). The ST behaviour is not found in any other insect (Shuker and Simmons 2014; Córdoba-Aguilar et al. 2018), although indirect insemination is also found in the cephalopods (octopuses, squids, cuttlefishes, nautilus and allonautilus; with the exception of some deep-sea species) and some arachnids from the order Araneae (spiders), subclass Acari (mites) and the order Solifugae (sunspiders). The ST is thus a clear example of diversity at the behavioural level with important evolutionary implications, because the viability and quality of the sperm, and hence fertility, will depend on the timing of ST relative to copulation (Rivas-Torres et al. unpublished).
Insects usually mate with both sexes facing opposite directions, like in butterflies (a Hipparchia sp. Fabricius, 1807; Satyridae). However, in odonates (b Orthetrum brunneun (Fonscolombe, 1837), Libellulidae), the sexes are orientated to the same direction, allowing flying in copula. The intra-male sperm translocation can take place after the male has grasped the female in tandem, like in c Neurobasis chinensis (Linnaeus, 1758) (Calopterygidae), with or without precopulatory genital touching. In some species, this behaviour is repeated up to seven times in the same copulation, like in d Coenagrion scitulum (Rambur, 1842) (Coenagrionidae). Finally, in some species, males translocate sperm alone before copulation, like in e Diphlebia lestoides (Selys, 1853), or after copulation, very close to the female as in f Euphaea masoni Selys, 1879 (Euphaeidae). Pictures were from A. Cordero-Rivera, except panel e, courtesy of Reiner Richter
However, little is known about its evolution, its relation with the evolution of the copulatory wheel, and particularly its diversity within the order. Our main aim was therefore to explore the variation of ST behaviour from an evolutionary perspective and discuss its possible connection with the copulatory wheel. We performed a literature review on the diversity of ST across Odonata, and we used phylogenetic comparative analyses to investigate the evolution of the ST behaviour within this insect order. Our literature review revealed differences in ST behaviour within and between species, and the comparative phylogenetic analyses suggested ST in tandem and before copulation, as the ancestral behaviour in Odonata. We discuss potential evolutionary routes for all the ST behaviours found, which could help to better understand behavioural evolution in this insect group, as well as certain aspects of the evolution of sexual behaviours and divergence in other animals.
Material and methods
Literature review of ST behaviour
Published data on ST in odonates were searched by querying Google scholar (http://scholar.google.com) for “sperm translocation” and “odonat*” or “intra-male sperm transfer” and “odonat*”. Searches were carried out in December 2016. Additional searchers were completed in November 2018 looking for ST behaviour in papers written in Spanish, French, German and Italian and manually looking in old English references, where the behaviour could be described in different terms (e.g. “filling of the sperm vesicle”). Japanese references were also included thanks to the help of colleagues. We also screened manually the papers published in Martinia (from 1991 to 2016), and the Journal of the British Dragonfly Society (from 1995 to 2016), which are non-digitised odonatological journals, and furthermore, we manually searched non-digitised issues of Odonatologica and the International Journal of Odonatology. We collated all available observations of ST, including how this behaviour was performed, and its duration (with sample size and standard error). We compiled information for a total of 176 species (see “Results”). Full details of the literature reviewed, variables considered and species included in the analyses are given in Supplementary Materials 1 and 2. For the purposes of our study, we have categorised ST in four characters: (i) before or after copula, (ii) alone or in tandem, (iii) non-repeated or repeated, and (iv) with or without precopulatory genital touching (see Table 1 and “Results”). Since the description of the precopulatory genital touching might not be stated explicitly in the revised papers, we established that when something similar to this behaviour was described just before the ST, we considered it as precopulatory genital touching.
Phylogenetic tree reconstruction
We aimed at understanding the evolution of ST using phylogenetic comparative analyses (Harvey and Pagel 1991). Therefore, we first constructed a phylogenetic tree including the odonate species for which data on ST were available in the literature. We searched GenBank (Clark et al. 2016, https://www.ncbi.nlm.nih.gov/genbank/) for sequences of the mitochondrial genes COI and 16S, and the nuclear genes 18S and 28S. In total, we retrieved sequence data for 129 species out of the 176 for which we had data on ST. We also retrieved sequences of the same genes from 31 species without information on ST, to increase the resolution of the tree, and for six species of Ephemeroptera, to be used as outgroups in the analysis. The final dataset included a total of 160 Odonata species (including 31 without data for ST), which represented 14 families of Zygoptera and 11 families of Anisoptera (see Supplementary Material Table S2).
All sequences were imported into Geneious version 9.1.7 (http://www.geneious.com, Kearse et al. 2012) for visual inspection before alignment. Sequences were aligned using the ClustalW algorithm (Thompson et al. 1994), as implemented in Geneious. We used BEAST version 2.4.8.0 (Bouckaert et al. 2014) to reconstruct the phylogenetic relationships among the study species. The phylogenetic reconstruction was performed with the nucleotide substitution models selected for each gene by the bModelTest package version 1.0.4 (Bouckaert and Drummond 2017), and a strict clock model and the Yule speciation model as priors. The analysis was run for 10 million generations and sampled every 1000 generations. The output was examined with Tracer version 1.6 (Rambaut and Drummond 2014) to assess convergence of the Markov-chain Monte Carlo onto a stationary distribution through the analysis of trace plots and effective sample sizes (ESS) of the model parameters (ESS > 200 was considered acceptable). TreeAnnotator version 2.4.8, included in the BEAST package, was used to build a maximum clade credibility tree after discarding 10% of sampled trees as burn-in. The consensus tree was visualised and edited with FigTree version 1.4.2 (available at: http://tree.bio.ed.ac.uk/software/figtree/). This tree was pruned to contain only those species with available ST information using the R package ape (Paradis et al. 2004), and the pruned tree was subsequently used for the phylogenetic comparative analyses.
Phylogenetic comparative analyses
We estimated the ancestral states of our study discrete characters related to sperm transfer using the function ace in the R package ape (Paradis et al. 2004). Our character states were ST performed (i) before vs. after copula, (ii) in tandem vs. alone vs. both in tandem and alone, (iii) with repetition vs. with no repetition vs. with and without repetition, and (iv) with precopulatory genital touching vs. without precopulatory genital touching vs. with and without precopulatory genital touching (Robertson and Tennessen 1984). We note that the combined states (i.e. both in tandem or alone) correspond to intra- or interpopulation variation of the ST behaviour. We used maximum likelihood estimation with equal rates of transition (Pagel 1994), and the likelihood of the ancestral states was computed using a joint estimation procedure (see Pupko et al. 2000). Finally, we also investigated possible correlated evolution between pairs of characters. For this analyses, we first recoded the study characters to binary. We coded the combined states to the least common variant of ST for each character. Our rationale behind this decision is that we wanted to give more weight to the least common behaviours, which are already under-represented in our dataset. Even though species with more than one type of ST have not stably evolved only one ST behaviour, they could offer hints on the evolution of ST across odonates. We used Pagel’s (1994) function to detect correlated evolution as implemented in the R package phytools (Revell 2012), with the function fitDiscrete from the package geiger (Harmon et al. 2008).
Results
Variability of ST behaviour
We found a total of 123 papers that provided information on ST from 176 species of odonates, belonging to 22 families (Table 1, Supplementary Materials 1 and 2). The ST is a behaviour that usually lasts for some seconds but shows high variability (mean ± SE 16.7 ± 2.7 s, range 0.2 to 150 s, N = 82 species) (Table 1).
At least five different variants of ST were described across species: (i) male alone, before the formation of precopulatory tandem; (ii) in precopulatory tandem, without genital touching, only once per copula; (iii) in precopulatory tandem after genital touching, only once per copula; (iv) in tandem, repeated during copulation; and (v) male alone, after copula (see Table 1). However, in some species [e.g. Libellula quadrimaculata (L., 1758) or Erythemis simplicicollis (Say, 1839)], we found a combination of the ST variants due to intra- or interpopulation variability (Fig. 2).
The variants of intra-male sperm translocation behaviour, mapped on a phylogeny of the studied Odonata. The ancestral behaviour of sperm translocation was estimated to be before copula, in precopulatory tandem, non-repeated and without genital touching. Family names have been added to the main branches of the tree
Overall, the majority of species (66%) perform ST in precopulatory tandem (Table 1), hence before copulation and only once per copula. Anisoptera males perform ST alone, before tandem formation in a higher proportion (31%) compared with Zygoptera (9%). The ST in tandem and repeated was found for 4% of species of Zygoptera, but no in Anisoptera (Table 1). Finally, ST alone, after copula, was found for 6% of the species of Zygoptera and 3% of Anisoptera (Table 1).
The families Coenagrionidae and Libellulidae are the most speciose and also display more variants of ST behaviour, including all five in the first family. The majority of coenagrionids studied performed ST in precopulatory tandem, and only once per copula (58%; Table 1). The second most common variant was the ST in tandem, but only after precopulatory genital touch (32%; Table 1). In libellulids, ST in tandem, only once per copula, was also the commonest variant (61%; Table 1), and the second commonest ST was male alone, but in this case, before tandem formation (33%; Table 1). ST by the male alone, after copulation, is a rare behaviour but is the dominant behaviour in the Euphaeidae (57%) and Polythoridae (67%), and it is also performed by the only living representative of Pseudolestidae.
Evolution of ST behaviour
DNA sequences were available for 129 out of the 176 species for which information on ST behaviour was available in the literature (Supplementary Material Table S2). The consensus phylogenetic tree was congruent with the currently accepted and relatively well-established phylogeny of the order (e.g. Lorenzo-Carballa and Cordero-Rivera 2014, but see Dijkstra et al. 2013), and the support for the main clades was high in most cases (posterior probability support higher than 0.6 and in many cases close or equal to 1; see Supplementary Material Fig. S1).
In our dataset, the majority of species (95%) perform ST before copula (Fig. 2). Six species perform ST after copula (four Zygoptera and two Anisoptera). According to the ancestral reconstruction for this trait, the variant of ST after copula has evolved independently in all cases except for the group Euphaea/Anisophaea (Zygoptera). Regarding ST in tandem and/or alone, most species perform it in tandem (76%), while 22 species do it alone (17%; seven Zygoptera and 15 Anisoptera), and nine species use both strategies (7%; three Zygoptera and six Anisoptera). These variants have independently evolved in all cases except for the group Euphaea/Anisophaea (Zygoptera), and the clade Nannothemis/Pachydiplax/Erythemis and for the species of Leucorrhinia (Anisoptera). In the case of ST performed repeatedly or not, we found that the majority of species (96%) perform it just once. Only within Zygoptera, we found three species that repeat sperm transfer (2%) and two that perform both variants (2%; corresponding to five independent origins). Regarding ST performed with or without genital touching, only five species have ST with genital touching (4%) and three perform both variants (2%; all of them within Zygoptera). These cases correspond to independent origins except for the group of Ischnura ramburii, I. graellsii and I. elegans. In summary, the ancestral state of ST would be before copula, in precopulatory tandem, non-repeated and without genital touching.
Finally, we did not find any significant correlated evolution between the different variants of ST (P > 0.05).
Discussion
Our review of the literature indicates that there are at least five variants of ST behaviour among Odonata. Our work also highlights the lack of basic information on the ST behaviour for the vast majority of species within this insect group: The total number of odonate species is estimated around 6000 (Lorenzo-Carballa and Cordero-Rivera 2014); however, we only found data on ST behaviour for 176 species.
Variation of ST behaviours
In most of the odonate species studied, males perform ST after having grasped the female in precopulatory tandem and once per copula (variant ii). This variant of ST is the ancestral state, according to our comparative phylogenetic analyses. Variant (i), the completion of ST by the male alone, before finding a female (Table 1), could be advantageous in species where females are rarely encountered and copulations are brief. Males that performed ST alone would be ready to copulate as soon as they grasp a female in tandem.
Variant (iii) involves performing ST only after precopulatory genital touching (Table 1), a behaviour that presumably signals female receptivity to the male (Robertson and Tennessen 1984). This behaviour might derive from the basal behaviour (i.e. variant ii), if males commonly encounter unreceptive females. High densities of unreceptive females would be predicted in populations/species whose females remain near the reproductive site in the maturation phase, like many Ischnura (Cordero et al. 1998). When females give no refusal signs, males can remain in tandem for very long periods, even for a full day in the laboratory (Cordero et al. 1992). Genital touching would therefore be adaptive for males, avoiding wasting sperm with unreceptive females, but also for females, because they would be released faster if signalling their unreceptiveness. In odonates, it has been assumed that females cannot be forced to copulate (e.g. Fincke 1997), although forced matings are possible in populations with high male densities and females ovipositing unguarded (Cordero-Rivera and Andrés 2002).
Only two species, Coenagrion scitulum (Rambur, 1842) and Megaloprepus caerulatus (Drury, 1782), are known to routinely perform variant (iv), i.e. repeated ST in one copulation (Table 1, Fig. 1d). This behaviour has been also recorded occasionally in three other zygopteran species: Lestes barbarus (Fabricius, 1798) (Lestidae), Ischnura aurora (Brauer, 1865) (Coenagrionidae) and Perissolestes remotus (Williamson & Williamson, 1924) (Perilestidae) (see Supplementary Material 1). In the case of C. scitulum, the repetition of ST during the copulation has been interpreted as a mechanism evolved in the context of sperm competition (Córdoba-Aguilar and Cordero-Rivera 2008). In this species, males are apparently unable to remove a significant portion of sperm from rivals using their genital ligula (Cordero et al. 1995). Therefore, by repeating ST and insemination, they might over-compete rival sperm. However, other species of odonates [e.g. Hypolestes trinitatis (Gundlach, 1888)] are also known to have limited ability to remove sperm, but ST is not repeated (Torres-Cambas and Cordero-Rivera 2011), and hence the link between both phenomena is not straightforward.
The most intriguing variant for ST is variant (v), i.e. the translocation of sperm by the male alone, after copulation (Fig. 1f, Table 1). This behaviour was first reported for the coenagrionid Mortonagrion hirosei Asahina, 1972, from Japan (Naraoka 2014), and then observed in nine additional species, including four Euphaeidae, and Pseudolestes mirabilis Kirby, 1900 (Cordero-Rivera and Zhang 2018). Translocating sperm after copulation could be explained if these species have evolved physiological mechanisms to maintain sperm alive for long periods of time (until next mating). However, at least for the first copula, males have to fill their sperm vesicle before copulation. Males could routinely perform ST each morning to be prepared for the next mating, but this has never been observed in P. mirabilis, the only species studied in detail (Cordero-Rivera and Zhang 2018).
In some species, we found several of the ST variants due to intra- or interpopulation variability. For example, males of Hetaerina americana Fabricius, 1798 were reported performing ST alone before copula in one population, in tandem without precopulatory touch in another population and in tandem with precopulatory touch at a third locality (Supplementary material Table 1). Two ST variants were also reported for different populations of I. aurora and Libellula quadrimaculata Linnaeus, 1758, and between males of a single population of Aeshna cyanea Muller, 1764 (Supplementary material Table 1). Intraspecific variability seems rare, and unlikely to be detected in short-term studies. For instance, only 5% of the 137 ST observed in two Enallagma species occurred before tandem formation (Logan 1971; cited by Corbet 1999). The reasons behind this intraspecific diversity of ST are unknown, but these species are excellent candidates for further investigation of the diversification of ST strategies within odonates.
Why has ST evolved?
The evolution of ST behaviour is likely related to the atypical copulation position in the Odonata. One plausible scenario for the evolution of ST behaviour is that ancestors of modern odonates produced a spermatophore, and deposited it on the substrate, a behaviour currently observed in arachnids, myriapods and wingless hexapods (Proctor 1998). The thick cerci of Namurotypus Brauckmann and Zessin, 1989 males (ancestor of the carboniferous Odonata) could have been used to firmly grasp the female behind her compound eyes. The male could then have directed the female over the spermatophore (Bechly et al. 2001), in a way similar to the “drag off” behaviour observed in whipspiders (Amblypygi) (Weygoldt 1969). Attaching the spermatophore to the male body is clearly more efficient and could be the selective pressure required for the evolution of secondary genitalia in odonates (reviewed in Cordero-Rivera and Córdoba-Aguilar 2010), and hence, the ST behaviour.
An alternative, yet more speculative hypothesis for the evolution of ST, could be related to sexual cannibalism, since females of several zygopterans are known to sometimes attack and eat mature conspecific males (Cordero 1992). We are aware of only one case of sexual cannibalism described during copulation: Robertson (1985) observed a female of Ischnura ramburi (Selys, 1850) that was repeatedly chased and grasped by a male until she finally attacked him and ate out his thorax, but the male succeeded initiating copulation before dying in copula. Other predatory animals, like some arachnids or cephalopods, have a specialised appendix used as an intromittent organ to introduce the spermatophore in the female reproductive organs. These two groups also show sexual cannibalism (Ibáñez and Keyl 2010; Li et al. 2012). Therefore, it could be hypothesised that the tandem and wheel position of odonates during copulation, along with the intra-male ST, might allow males to avoid sexual cannibalism (Chapman et al. 2003; Schneider 2014). From this idea, we propose the hypothesis that when the risk of sexual cannibalism is high, selection favours the evolution of secondary mechanisms to inseminate females safely for the males. This could be tested with a review of the insemination behaviours across different animal taxa and within a phylogenetic context.
Conclusions
Some of the ST variants seem to have evolved several times, but this behaviour needs further investigation in a larger number of species. No other insect group shows a behaviour equivalent to the ST of odonates (although some similarities can be found with a special appendage of some arachnids and the hectocotylus of cephalopods), and consequently we lack comparative evidence to understand the evolution of this enigmatic behaviour. ST is sometimes performed very fast, and therefore careful video recording of this behaviour, particularly in Anisoptera, is needed to avoid confusing movements to clean the abdomen with true ST. We currently lack information for a number of families of both Anisoptera (e.g. Austropetaliidae, Neopetallidae and Macromiidae) and Zygoptera (e.g. Chlorocyphidae, Amphipterygidae and Lestoideidae), and most of the species with information on ST are from temperate zones and fewer from tropical areas. This bias can be explained by the scarcity of field studies in tropical regions, and also due to the low sexual activity observed in tropical species (e.g. Sanmartín-Villar and Cordero-Rivera 2016). Although, tropical odonata usually appear at very low densities, they are priority for future research, because we expect a higher diversity of behaviours in tropical areas (Cordero-Rivera 2017a), and rare alternatives might be more common in these tropical families.
Our study emphasises also the relevance (and scarcity) of detailed natural history observations for most species. We expect that this review will encourage the scientific community towards more research in diversity of reproductive behaviours, with a special focus on tropical species. This applies not only to odonates, but to other animal taxa. This information, combined with modern molecular techniques and phylogenetic hypotheses, is fundamental to understand relevant questions about behavioural evolution as well as behavioural diversity (ethodiversity). This is a necessary step to increase awareness on the importance of conserving not only species but also behaviours (Cordero-Rivera 2017a).
References
Bechly G, Brauckmann C, Zessin W, Gröning E (2001) New results concerning the morphology of the most ancient dragonflies (Insecta: Odonatoptera) from the Namurian of Hagen-Vorhalle (Germany). J Zool Syst Evol Res 39:209–226
Berger-Tal O, Saltz D (2016) Conservation behavior: applying behavioral ecology to wildlife conservation and management. Cambridge University Press, New York
Bouckaert RR, Drummond AJ (2017) bModelTest: Bayesian phylogenetic site model averaging and model comparison. BMC Evol Biol 17:42. https://doi.org/10.1186/s12862-017-0890-6
Bouckaert R, Heled J, Kühnert D, Vaughan T, Wu CH, Xie D, Suchard MA, Rambaut A, Drummond AJ (2014) BEAST 2: a software platform for Bayesian evolutionary analysis. PLOS Comp Biol 10:1–6
Caro T, Sherman PW (2012) Vanishing behaviors. Cons Lett 5:159–166
Chapman T, Arnqvist G, Bangham J, Rowe L (2003) Sexual conflict. Trends Ecol Evol 18:41–47
Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell E, Sayers W (2016) GenBank. Nucleic Acids Res 44:D67–D72
Corbet PS (1999) Dragonflies. Behaviour and ecology of Odonata. Harley Books, Essex
Cordero A (1992) Sexual cannibalism in the damselfly species Ischnura graellsii (Odonata: Coenagrionidae). Ent Gen 17:17–20
Cordero A, Santolamazza-Carbone S, Utzeri C (1992) A twenty-four-hours-lasting tandem in Coenagrion scitulum (Ramb.) in the laboratory (Zygoptera: Coenagrionidae). Notul Odonatol 3:166–167
Cordero A, Santolamazza-Carbone S, Utzeri C (1995) Male disturbance, repeated insemination and sperm competition in the damselfly Coenagrion scitulum (Zygoptera: Coenagrionidae). Anim Behav 49:437–449
Cordero A, Santolamazza-Carbone S, Utzeri C (1998) Mating opportunities and mating costs are reduced in androchrome female damselflies, Ischnura elegans (Odonata). Anim Behav 55:185–197
Cordero-Rivera A (2016) Sperm removal during copulation confirmed in the oldest extant damselfly, Hemiphlebia mirabilis. PeerJ 4:e2077
Cordero-Rivera A (2017a) Behavioral diversity (Ethodiversity): a neglected level in the study of biodiversity. Front Ecol Evol 5:1–7
Cordero-Rivera A (2017b) Sexual conflict and the evolution of genitalia: male damselflies remove more sperm when mating with a heterospecific female. Sci Rep 7:7844. https://doi.org/10.1038/s41598-017-08390-3
Cordero-Rivera A, Andrés JA (2002) Male coercion and convenience polyandry in a Calopterygid damselfly (Odonata). J Insect Sci 2:14
Cordero-Rivera A, Córdoba-Aguilar A (2010) Selective forces propelling genitalic evolution in Odonata. In: Leonard J, Córdoba-Aguilar A (eds) The evolution of primary sexual characters in animals. Oxford University Press, Oxford, pp 332–352
Cordero-Rivera A, Zhang H (2018) Ethological uniqueness of a damselfly with no near relatives: the relevance of behaviour as part of biodiversity. Anim Biodivers Conserv 41:161–174. https://doi.org/10.32800/abc.2018.41.0161
Córdoba-Aguilar A, Cordero-Rivera A (2008) Cryptic female choice and sexual conflict. In dragonflies and damselflies. In: Córdoba-Aguilar A (ed) Model organisms for ecological and evolutionary research. Oxford University Press, Oxford, pp 189–202
Córdoba-Aguilar A, González-Tokman D, González-Santoyo I (2018) Insect behaviour. Oxford University Press, Oxford
Dijkstra KDB, Bechly G, Bybee SM, Dow RA, Dumont HJ, Fleck G, Garrison RW, Hämäläinen M, Kalkman VJ, Karube H, May ML, Orr AG, Paulson D, Rehn AC, Theischinger G, Trueman JWH, van Tol J, von Ellenrieder N, Ware J (2013) The classification and diversity of dragonflies and damselflies (Odonata). In: Zhang ZQ (ed) Animal biodiversity: an outline of higher-level classification and survey of taxonomic richness, Zootaxa, vol 3730, p 36-45
Dingemanse NJ, Both C, Drent PJ, Tinbergen JM (2004) Fitness consequences of avian personalities in a fluctuating environment. Proc R Soc B Biol Sci 271:847–852
Downes JA (1969) The swarming and mating flight of Diptera. Annu Rev Entomol 14:271–298
Fincke OM (1984) Giant damselflies in a tropical forest: reproductive biology of Megaloprepus caerulatus with notes on Mecistogaster (Zygoptera: Pseudostigmatidae). Adv Odonatol 2:13–27
Fincke OM (1997) Conflict resolution in the Odonata: implications for understanding female mating patterns and female choice. Biol J Linn Soc 60:201–220
Harmon LJ, Weir JT, Brock CD, Glor RE, Challenger W (2008) GEIGER: investigating evolutionary radiations. Bioinformatics 24:129–131
Harvey PH, Pagel MD (1991) The comparative method in evolutionary biology. Oxford University Press, New York
Ibáñez CM, Keyl F (2010) Cannibalism in cephalopods. Rev Fish Biol Fish 20:123–136
Kearse M, Moir R, Wilson A, Stone-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Dummond A (2012) Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649
Li D, Oh J, Kralj-Fiser S, Kuntner M (2012) Remote copulation: male adaptation to female cannibalism. Biol Lett 8:512–515
Logan ER (1971) A comparative ecological and behavioral study of two species of damselflies, Enallagma boreale (Selys) and Enallagma carunculatum (Morse) (Odonata: Coenagrionidae). PhD Thesis, Washington State University, Pullman, Washington
Lorenzo-Carballa MO, Cordero-Rivera A (2014) Odonates. In: Vargas P, Zardoya R (eds) The tree of life. Sinauer, Sunderland, pp 352–363
Miller PL, Miller CA (1981) Field observations on copulatory behaviour in Zygoptera, with an examination of the structure and activity of male genitalia. Odonatologica 10:201–218
Naraoka H (2014) Reproductive behavior of Mortonagrion hirosei Asahina, 1972 in Miyagi prefecture, with special reference to intra-male sperm translocation and copulatory process. Tombo 54:46–50
Pagel M (1994) Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. Philos Trans R Soc Lond Ser B Biol Sci 255:37–45
Paradis E, Claude J, Strimmer K (2004) APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20:289–290
Pérez-Méndez N, Jordano P, García C, Valido A (2016) The signatures of Anthropocene defaunation: cascading effects of the seed dispersal collapse. Sci Rep 6:24820
Proctor HC (1998) Indirect sperm transfer in arthropods: behavioral and evolutionary trends. J Appl Entomol 43:153–174
Pupko T, Pe I, Shamir R, Graur D (2000) A fast algorithm for joint reconstruction of ancestral amino acid sequences. Mol Biol Evol 17:890–896
Rambaut A, Drummond AJ (2014) Tracer v 1.64. Available from: http://beast.bio.ed.ac.uk/Tracer
Revell LJ (2012) Phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol Evol 3:217–223
Robertson HM (1985) Female dimorphisms and mating behaviour in a damselfly, Ischnura ramburii: females mimicking males. Anim Behav 33:805–809
Robertson HM, Tennessen KJ (1984) Precopulatory genital contact in some Zygoptera. Odonatologica 13:591–595
Robey C (1975) Observations on breeding behavior of Pachydiplax longipennis (Odonata: Libellulidae). Psyche 82:89–96
Sanmartín-Villar I, Cordero-Rivera A (2016) Female colour polymorphism and unique reproductive behaviour in Polythore damselflies (Zygoptera: Polythoridae). Neotrop Entomol 45:658–664
Schneider JM (2014) Sexual cannibalism as a manifestation of sexual conflict. Cold Spring Harb Perspect Biol 6:1–16
Shuker DM, Simmons LW (2014) The evolution of insect mating systems. Oxford University Press, Oxford
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Torres-Cambas Y, Cordero-Rivera A (2011) Limited spermathecal sperm removal ability in the damselfly Hypolestes trinitatis (Gundlach) (Odonata: Megapodagrionidae). Int J Odonatol 14:321–328
Utzeri C, Ottolenghi C (1992) Further observations on intra-male sperm translocation behaviour in Anisoptera. Notul Odonatol 3:145–149
Weygoldt P (1969) The biology of Pseudoscorpions. Harvard University Press, Cambridge
Acknowledgements
We thank Reiner Ritcher for providing the picture in Fig. 1e and for sharing with us his unpublished observations, Andreas Martens for his help with German literature and Naoya Ishizawa for providing us with pdfs and translations of very relevant Japanese papers. Two anonymous reviewers provided comments that helped us improved our manuscript.
Funding
ART is supported by an FPI grant of the Spanish Ministry of Economy and Competitiveness (MINECO, BES-2015-071965). Funding was provided by a grant from MINECO, including FEDER funds to ACR (CGL2014-53140-P).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by N. Wedell
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rivas-Torres, A., Outomuro, D., Lorenzo-Carballa, M.O. et al. The evolution and diversity of intra-male sperm translocation in Odonata: a unique behaviour in animals. Behav Ecol Sociobiol 73, 54 (2019). https://doi.org/10.1007/s00265-019-2660-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-019-2660-5