Abstract
Multiple and multimodal signals can evolve because they convey different information to different receivers or in different contexts. From the perspective of display receivers, however, multimodal signals may pose a challenge since evolutionary changes in any one aspect of the signal may require shifts in other aspects of receiver physiology and behavior. Here, we use field experiments with four species of Sceloporus lizards to test whether evolutionary loss of one element of a complex signal (a colorful belly patch) has led to a change in the behavioral response to a live conspecific. Instead, we found that males of three species (S. merriami, cozumelae, and siniferus) responded to the live conspecific with increased visual and decreased chemical behavior, supporting a Sensory Isolation hypothesis in which animals minimize interference by isolating a single sensory modality, for example, closing eyes to pay closer attention to a sound or smell. In an exception that offers additional support, males of the fourth species, S. parvus, also showed a trade-off in their response, but responded to the live stimulus with more chemical and less visual behavior. We found little evidence that lizards that have lost production of one signal element (belly color) have also altered their response behavior as a consequence. These results emphasize the potentially important role of receiver response in maintaining complex and multimodal signals.
Significance statement
Animals use all of their sensory systems to communicate with each other, but using more than one sense at a time can be a challenge. Here, we presented male lizards in the field to a tethered intruder to ask whether lizards that have lost one element of the signal (a color patch) over evolutionary time have also evolved their response to communicative signals. Instead, we found that males of three species responded primarily with visual behavior and decreased their use of chemical behavior, as if focusing their attention entirely on the visual sensory modality. Males of the fourth species responded primarily with chemical behavior, and decreased their use of visual behavior. These results suggest that there may be mechanical constraints limiting communication signals that make use of more than one sensory system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multimodal or multicomponent signals are common, and likely evolved because different signal components convey information to different receivers or in different contexts (see reviews by Partan and Marler 1999; Hebets and Papaj 2005; Higham and Hebets 2013; Halfwerk and Slabbekoorn 2015; Hebets et al. 2016). Although studied primarily from the perspective of display producers, complex and multimodal communication may also benefit signal receivers, for example, if redundant signals and signal components help receivers to perceive an important message. On the other hand, perceiving complex and multimodal signals may be costly to signal receivers, for example, if perception of one signal or signal component interferes with response to another or if simultaneous perception requires special tuning or maintenance of sensory systems. In this case, evolutionary loss of one signal or signal component may lead to changes in other aspects. Here, we make use of natural differences among lizard species to ask whether evolutionary loss of a colorful signal patch is associated with a change also in signal receivers and the ways in which they respond to conspecifics.
From the perspective of senders, many communicative signals have multiple parts because those parts serve different functions. Some elements, for example, may grab the receiver’s attention whereas others convey detailed information (e.g., Preininger et al. 2013; Endler et al. 2014). In other cases, signal components may work most effectively in different contexts, increasing the space and distance across which signals can carry information (e.g., Stafstrom and Hebets 2013; Uetz et al. 2013; Uy and Safran 2013). There are also signal components that carry different aspects of information (e.g., Ruppli et al. 2013) or are directed towards different receivers (e.g., Zanollo et al. 2013). When signal components make use of multiple sensory modalities, they are generally more effective in terms of ensuring that messages are received (Partan 2013). Although producing more than one signal or signal component can be costly (e.g., Wilson et al. 2013), sexual selection may also act directly on complexity, favoring individuals that show potential mates or competitors that they are able to overcome those limitations (e.g., Ord et al. 2001; Ord and Martins 2006; Ward et al. 2013).
From the perspective of signal receivers, perception and response to complex or multimodal signals may be costly and can depend on individual variation in sensory ability (Ronald et al. 2012, 2018) or on the context in which the signals are perceived (e.g., Halfwerk and Slabbekoorn 2015). Sometimes, complex signals enhance or expand the information that receivers gather, whether those be intended conspecific receivers, eavesdroppers, or predators exploiting signals to locate their prey. For example, female wolf spiders detect multimodal cues more quickly than they do visual or vibratory cues alone (Uetz et al. 2009). Male Túngara frogs integrate information from multiple cues to estimate the distance from the sender (Halfwerk et al. 2014), and aphids combine input from different sensory systems to obtain more accurate and reliable cues about predator presence (Ben-Ari and Inbar 2014). Integrated perception of multimodal stimuli can also influence subsequent behavior, as in humans where a combination of visual and somatosensory stimuli may enhance motor learning (Wenderoth 2015), or in zebrafish in which experience in a visually-deprived context affects subsequent chemical behavior (Suriyampola et al. 2018). However, if signals or signal components are integrated, evolutionary loss of a signal component may lead to extended consequences for signal receivers. Receivers may need to compensate for loss of information by sharpening their sensory systems or by engaging in behavior that enhances their perception of the signal components that remain.
There is considerable indirect evidence of an interaction between visual and chemical senses in lizard signal receivers. Lizards in the Iguania use both chemical deposits and motion displays at territorial boundaries to attract potential mates or to deter potential intruders (Carpenter and Ferguson 1977). In addition, lizards sometimes respond to chemical cues with visual displays (Duvall 1979), and their chemical deposits exhibit ultraviolet colors that the lizards may use to identify territorial markers visually (Alberts 1989). Playback experiments designed for other purposes have also found connections between visual and chemical behavior. For example, Hews and Benard (2001) found that male Sceloporus virgatus, a species that has evolutionarily lost a colorful belly patch that is used by other Sceloporus species as a signal in male–male interactions, also used more chemosensory behavior in response to a live conspecific than did male Urosaurus ornatus, a closely related species that retains the color patch. Thompson et al. (2008) found that S. graciosus lizards presented with a combined visual and chemical stimulus responded with less chemosensory behavior than they did to a chemical stimulus alone. Further, Pruett et al. (2016) found that Sceloporus lizard species which increased chemosensory behavior in response to a chemical stimulus also produced fewer visual displays in response to that stimulus. Others have found a negative association between evolutionary changes in visual and chemical signals when looking across species and over longer periods of time (Martins et al. 2004; Ossip-Klein et al. 2013).
Here, we ask about the behavioral mechanisms involved in the behavioral response to multimodal signals, and test whether evolutionary losses of one element of Sceloporus lizard signals (a colorful belly patch) are consistently associated with shifts in response behavior. Most male Sceloporus lizards have blue belly patches that they display most prominently during aggressive male–male interactions (Carpenter and Ferguson 1977). However, males of a few of the 80–100 species in this genus have lost the conspicuous belly patch, such that ancestral reconstructions along a phylogeny find seven independent evolutionary losses (Ossip-Drahos et al. 2016). In an earlier study (Martins et al. 2015), we found that males of two species with plain white bellies use more aggressive headbob displays than do males of sister taxa that retain the belly patch, suggesting that evolutionary compensation has twice shifted the aggressive content of a static color patch to the more dynamic motion of the headbob display. Here, we use the same comparison of four species, but focus on response behavior, asking whether evolutionary loss of the color patch has influenced also the ways in which male lizards respond to a live conspecific.
Since white-bellied male Sceloporus use more aggressive headbob displays (Martins et al. 2015), we might expect them also to rely more heavily on motion displays in their behavioral response to conspecifics. For example, they may use more motion displays themselves, engaging conspecific intruders in an exchange of visual cues. Given the links between visual and chemical signals, we might also expect that males of species that have lost the colorful visual signal will be more attentive to chemical cues and to use more chemical investigation behavior in response to a potential intruder. Males of white-bellied species may also respond to intruders by engaging in more general behavior that maximizes multimodal sensory access. For example, animals may freeze to pay closer attention or move their heads towards the intruder, simultaneously moving the eyes, ears, and nose in ways that facilitate perception in more than one sensory modality at a time. Finally, the evolutionary loss of the blue belly patch may not have had any direct impact on response behavior. Here, we conduct field experiments to test among these possibilities, using males of four species of Sceloporus lizards that represent two independent evolutionary losses of the colorful blue belly patch.
Methods
Subject species
We conducted field experiments with four Sceloporus lizard species, chosen as part of a larger comparative study (Hews and Martins 2013) to represent two evolutionary losses of the colorful belly patches typical of this genus. The first evolutionary loss is represented by two species in clade A: S. cozumelae and S. parvus. We collected data during the peak mating period for each species, when males were actively defending territories and courting females. Specifically, we collected data from male S. cozumelae on sandy beaches near Cancún, México, in May 2013, and from male S. parvus in June 2013 in a desert scrub habitat near Querétaro, México. Male S. parvus have blue belly patches, whereas female S. parvus and both male and female S. cozumelae do not. Although male S. parvus exhibit two throat color morphs (blue-yellow and blue-white), we did not distinguish between these in our analyses since they do not differ substantively in their behavior (Hews et al. 2015). The second evolutionary loss is represented by two species in clade B (phylogenetic relationships described by Leaché (2010)) and Wiens et al. (2010)). For the second clade, we recorded the behavior of male S. merriami on the reddish-gray walls of a slot canyon in the Chihuahua desert Big Bend TX in May 2011 and 2012, and we studied male S. siniferus in the thick vegetation of a semi-deciduous tropical rainforest at the Huatulco National Park, MX, in June and July 2012. Male S. merriami have colorful belly patches with blue and green components, whereas female S. merriami and both male and female S. siniferus bellies are white to the human eye. These are the same four species that were studied in Martins et al. (2015).
Procedure
To measure the behavioral response of males to live conspecifics, we conducted field experiments, recording lizards in the field during undisturbed behavior (“Baseline” trials) and when presented with a live conspecific (“STI” trials). For each species, we first recorded the undisturbed behavior of each male in baseline trials, finding an individual male and videorecording for up to 10 min with a Canon Elura 100 camcorder from a distance of 2–10 m. The results of these baseline trials are reported also in Martins et al. (2015). For the current study, we also conducted staged territorial intrusions (STIs), following Ruby (1978) and Moore (1987) by tying a stimulus lizard to a fishing pole with a 10-cm string and placing that tethered animal on the substrate at a distance of 2 m from the focal subject. Stimulus lizards were always males captured in advance from another location to minimize familiarity, but were not size-matched or otherwise controlled. We used most stimulus lizards repeatedly in two to three STI trials, but used a few animals in four or five trials. If the focal subject did not appear to have seen the tethered stimulus after 5 min, we moved the tethered animal closer, to within 1 m of the focal subject. In all four species, the tethered stimulus animals did very little during these trials, usually freezing or attempting to flee. Trials were up to 30 min long, but durations were highly variable because the subject often ended the trial by moving out of sight. For this reason also, most baseline and STI trials involve different focal animals. We prematurely stopped any trial in which the subject physically attacked the stimulus lizard, and attempted to catch the subject lizard immediately after each trial to weigh and measure them.
In total, we conducted 18 baseline trials and 25 STIs with male S. parvus (samples ranged from 3 to 20 min in duration; median = 12 min). We conducted 23 baseline and 14 STIs with male S. cozumelae (samples ranged from 1 to 24 min in duration; median = 10 min). We conducted 51 baseline and 34 STIs with male S. merriami (samples ranged from 1 to 30 min in duration; median = 10 min). We conducted 34 baseline and 41 STIs with male S. siniferus (samples ranged from 1 to 16 min in duration; median = 11 min).
Scoring
As in Martins et al. (2015), we scored the frequencies of all behavior from the videotapes, but focused our attention on visual (e.g., shudderbobs, headbob displays, display-specific postures) and chemical (e.g., tongue flicks, gapes, jaw rubs) behavior. Although these behavior patterns are a mix of signal production and perception and may also serve other behavioral functions, they highlight a single sensory modality and were produced in response to visual and chemical cues (respectively) in other playback studies (e.g., Thompson et al. 2008; Pruett et al. 2016). To get a measure of total activity, we summed all behavioral acts including head movements, locomotion, attacks, tail wags, chemical behavior, and visual displays. Although these types of behavior can vary considerably in intensity, high-intensity behavior (e.g., attacks) is rare. Also, Sceloporus lizards typically do not combine behavior patterns into longer sequences, so our measure of total activity roughly reflects the number of times the animals engaged in any behavior at all. We then divided each total behavioral count by the total duration of the trial. For each headbob “display” (a stereotyped series of up-and-down motions), we also recorded the number of individual up-and-down motions (i.e., headbobs), and whether the display was accompanied by “full-show” postural elements (e.g., arched back, lateral flattening, gular extension). More headbobs in a display and the use of full shows have both been associated with aggression in Sceloporus lizards (Carpenter and Ferguson 1977; Martins 1993). To minimize unintentional biases, blinded methods were used in that those who scored the recordings had not observed the lizards in the wild and were not aware of our specific hypotheses and experimental predictions.
Statistical analyses
We used three-way ANOVAs to compare differences between behavioral responses during baseline and STI trials (factor 1: trial type). To test our primary hypotheses, we included a factor (factor 2: color) to test for the difference between species with colorful belly patches (S. parvus and S. merriami) and those with white bellies (S. cozumelae and S. siniferus), a third factor (factor 3: clade) comparing lizards in one clade (S. parvus and S. cozumelae) with those in the second clade (S. merriami and S. siniferus), and interaction terms. For each model, we examined the residuals to confirm that the data conformed to the usual assumptions (homoscedasticity and normality) of ANOVA. When assumptions were violated, we confirmed results using non-parametric Wilcoxon tests testing a single factor at a time. All models were fit using the aov and wilcox commands of the base package of R (R Development Core Team 2013).
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Results
Visual–chemical trade-offs
When presented with a live male stimulus, males of all four species showed opposing shifts in visual and chemical behavior. Although males of both species that have lost the colorful belly patches produced more headbob displays in the presence of a live conspecific than during baseline trials (right panels of Fig. 1), males of the two species that retain colorful belly patches responded differently in these two contexts (left panels of Fig. 1) leading to a significant three-way interaction between trial type, belly color, and clade (ANOVA: F1,232 = 5.0, p = 0.03). Males of three of the four species (all except S. parvus) produced more headbob displays in the presence of a live conspecific than they did during baseline trials (Fig. 1), leading to a significant effect of trial type on the number of headbob displays in the same model (ANOVA: F1,232 = 5.4, p = 0.02). Males of the two species with colorful belly patches (left side of Fig. 1) produced fewer total headbob displays in our trials (both baseline and STI) than did males of the two plain-bellied species (right side of Fig. 1), such that there was a significant main effect of belly color (ANOVA: F1,232 = 4.3, p = 0.04). Also, male S. parvus and S. cozumelae produced slightly more headbob displays in both types of trials than did male S. merriami and S. siniferus leading to a marginally significant effect of phylogenetic clade (F1,232 = 3.7, p = 0.05). Interactions between trial type and belly color (F1,232 = 0.1, p = 0.71), trial type, and phylogenetic clade (F1,232 = 0.7, p = 0.41) as well as between clade and color (F1,232 = 0.5, p = 0.48) were not statistically significant.
Male lizards of three of four species produced more headbob displays when confronted with a live conspecific (STI: Staged Territorial Intrusions) than in baseline trials. In contrast, male S. parvus produced fewer headbob displays in STIs than in baseline trials. Male lizards of the two species on the left (S. parvus and merriami) have colorful belly patches, whereas males of the two species on the right (S. cozumelae and S. siniferus) look white to the human eye. Error bars are + one standard error, and the number of trials is in parentheses
Using two-way ANOVAs to confirm results for each clade separately, we found that male lizards in clade B (S. merriami and S. siniferus) produced more headbob displays in STI than baseline trials (F1,156 = 6.6, p = 0.01), and that males of the species that has lost the colorful belly patch (S. siniferus) produced more headbob displays overall than did males of the species that retained the belly patch (S. merriami; F1,156 = 6.6, p = 0.01). There was no evidence for a significant interaction effect between belly color and trial type (F1,156 = 1.2, p = 0.27). For males of clade A (S. parvus and S. cozumelae), we found only a marginally significant interaction between belly color and trial type (F1,156 = 2.9, p = 0.09), with no significant effects of trial type (F1,156 = 0.2, p = 0.62) or belly color (F1,156 = 0.1, p = 0.78).
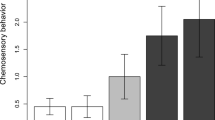
Males of the same three species that increased headbob display behavior in response to STIs also produced fewer chemical acts (summing tongue flicks, gapes, and jaw rubs) when presented with a live stimulus (Fig. 2), yielding a significant effect of trial type on chemical behavior (Wilcoxon test: W = 4677, p ≪ 0.01). Male S. merriami engaged in an especially high frequency of chemical behavior during baseline trials, but stopped almost entirely when confronted with a live stimulus (Fig. 2). Again, males of the fourth species (S. parvus) were different, tongue-flicking more rather than less when presented with a live conspecific.
Male lizards of three of four species engaged in less chemical behavior when confronted with a live conspecific (STI) than in baseline trials. In contrast, male S. parvus tongue-flicked more frequently in the presence of a tethered intruder. Male lizards of the species on the left (S. parvus and merriami) have colorful belly patches, whereas males of the species on the right (S. cozumelae and S. siniferus) look white to the human eye. Error bars are + one standard error, and the number of trials is in parentheses
White-bellied lizards increased activity in staged encounters
As shown also in Martins et al. (2015), male lizards with white bellies (S. cozumelae and S. siniferus) were less active than those with colorful belly patches (S. parvus and S. merriami) during baseline trials. However, they increased activity when presented with a conspecific stimulus (Fig. 3). The two species with colorful belly patches either decreased activity in the presence of a conspecific stimulus (S. merriami) or did not change activity substantively (S. parvus; Fig. 3). This led to a significant interaction between color and trial type in an ANOVA (F1,232 = 5.2, p = 0.02). None of the other interaction (clade × color: F1,232 = 2.5, p = 0.12; clade × trial type: F1,232 = 2.4, p = 0.12) or main effects of clade (F1,232 = 0.1, p = 0.92), color (F1,232 = 0.7, p = 0.40), or trial type (F1,232 = 0.1, p = 0.91) were statistically significant predictors of activity level.
Male lizards of species that have lost the colorful belly patches (S. cozumelae and siniferus) increase activity (i.e., summing bouts of locomotion, visual displays, etc.) when presented with a live stimulus (STI) as compared to that during baseline trials. Males of species with color patches on their bellies either decreased (S. merriami) or did not change (S. parvus) activity levels in STIs as compared to baseline trials. Error bars are + one standard error, and the number of trials is in parentheses
Response to tethered conspecifics was aggressive
Males of all four species produced more aggressive headbob displays during STIs than they did during baseline trials. Headbob displays during STIs included more full shows (postural displays with lateral flattening and gular extension; Fig. 4a). This effect of trial type was statistically significant whether tested in a non-parametric test (Wilcoxon test: W = 4677, p ≪ 0.01) or in an ANOVA including also clade and belly color as factors (F1,232 = 33.1, p ≪ 0.01). Because S. parvus produced very few headbob displays with full shows in either type of trial (Fig. 4a), ANOVA also detected a significant clade × color × trial type interaction effect (F1,232 = 6.1, p = 0.01), as well as significant clade (F1,232 = 15.1, p ≪ 0.01) and belly color (F1,232 = 10.0, p < 0.01) effects. Interactions between clade and color (F1,232 = 2.5, p = 0.12), clade and trial type (F1,232 = 2.0, p = 0.16), and color and trial type (F1,232 = 0.01, p = 0.94) were not statistically significant.
Male lizards of all four species produce more aggressive headbob displays when presented with a live stimulus (STI) than during baseline trials. a Number of headbob displays that include full-show body postures (arched back, lateral flattening, gular extension). b Average number of up-and-down motions per headbob display. Both measures are indicators of increased aggression. Error bars are + one standard error, and the number of trials is in parentheses
Headbob displays produced during STIs also included more total headbobs/display than did headbob displays produced during undisturbed baseline trials, especially in the species that have lost the colorful belly patch (S. cozumelae and S. siniferus: Fig. 4b). This resulted in a significant clade by color interaction effect (F1,232 = 11.6, p < 0.01) in our three-way ANOVA, as well as a significant effect of trial type (F1,232 = 8.6, p < 0.01). Because the two species in one clade (S. merriami and S. siniferus) used many more up-and-down motions in each headbob display than did the two species in the other clade (S. parvus and S. cozumelae), we also found in the same ANOVA model a significant main effect of clade (F1,232 = 273.6, p ≪ 0.01). Also, males of species that retained the color patch also used fewer headbobs in their displays than did males from species that have lost the color patch, leading to a significant main effect of color (F1,232 = 59.4, p ≪ 0.01). We found no evidence for a significant three-way interaction between clade, color, and trial type (F1,232 = 0.1, p = 0.80), or interactions between trial type and color (F1,232 = 1.3, p = 0.25), or clade (F1,232 = 1.4, p = 0.24).
Discussion
In this study, we found that evolutionary loss of a colorful belly patch was associated with little, if any, change in behavioral response to a live conspecific. Instead, we found an intriguing pattern of sensory trade-offs with male lizards of all four species responding to a live conspecific by increasing behavior involving one sensory modality and decreasing behavior that highlights other senses. Males of three species of Sceloporus lizards (S. merriami, S. cozumelae, and S. siniferus) responded to a live conspecific by increasing visual and decreasing chemical behavior, whereas males of the fourth species (S. parvus) responded with chemical behavior, while decreasing visual behavior. We also found no evidence that animals maximize use of more than one sensory modality at the same time, such as simultaneous increases in both visual and chemical behavior, in any of the four species.
Our finding that male Sceloporus lizards responded equally vigorously to simulated territorial intruders whether they were from species that have retained colorful belly patches or not suggests that there may be no evolutionary link between signal production and response behavior. The result is surprising because male Sceloporus lizards appear to compensate for the evolutionary loss of the color patch by producing more aggressive headbob displays when undisturbed (Martins et al. 2015). Also, plasma testosterone levels and numbers of androgen receptor-positive cells in key hypothalamic areas in males during peak breeding periods were lower in a white-bellied species than in a blue-bellied species (Hews et al. 2012), and white-bellied males were less active during baseline trials (Martins et al. 2015). Thus, our result that males of all four species behaved similarly indicates that white-bellied males also had a larger increase in activity levels between undisturbed baseline and simulated intruder trials, and is consistent with the earlier finding that white-bellied males responded more vigorously to field presentations of conspecific chemical secretions than to a blank control swab (Pruett et al. 2016). This greater increase in activity in the white-bellied species is in spite of having lower levels of circulating testosterone (Hews et al. 2016), and the strong relationships between hormones and motivated behavior in lizards (Wade 2011). Mechanistically, these results all suggest that androgens may not play a major role mediating these behavioral responses to chemical or visual presentations of intruders. Functionally, we need additional studies to determine whether the absolute amount of defensive behavior or the difference between baseline and intruder response behavior is more salient to territorial interactions and subsequent space-use decisions.
Our results also provide some support for a “Sensory Isolation” hypothesis, in which animals presented with a multimodal stimulus strive to isolate a single sensory modality. Ours are consistent with the results of other studies that found negative interactions between visual and chemical signals in lizards (e.g., Thompson et al. 2008; Ossip-Klein et al. 2013), and with the possibility of interference between sensory systems in signal receivers. Although lizards in our study did not close their eyes while tongue-flicking or close their nares while producing headbob displays, there may be mechanical constraints that limit visual attention during chemical behavior or chemo-perception during intent visual response behavior. During tongue flicks, for example, lizards usually directed their heads towards the substrate, perhaps limiting their visual fields and ability to see conspecific motion displays from a distance. Similarly, producing a visual headbob display involves lateral compression which can interfere with respiration (Brandt 2003) and may constrain the extent to which lizards can perceive chemically. The morphologies of brain regions associated with vision and chemical behavior differ between lizard species that communicate primarily using visual and chemical signals (Robinson et al. 2015). Also, the headbob displays observed in our study were highly stereotyped motions that fit the historical definition of a “fixed-action-pattern” in the sense that they were never interrupted (but see Stamps and Barlow 1973, for detailed discussion). To our knowledge, there are no reports of lizards tongue-flicking or turning their heads while producing headbob displays, and we have observed this only once ourselves in thousands of hours of behavioral observation (DKH and EPM, pers. obs.). Thus, producing a visual headbob display may constrain the extent to which lizards can perceive chemically.
Our results also emphasize that although species often show preferential use of one sensory modality over the others, these biases may not be consistent across behavioral contexts. For example, actively foraging lizard species (i.e., those that wander through the habitat searching for food) use chemical behavior (e.g., tongue-flicking) frequently and also use chemical cues to identify appropriate prey (e.g., Cooper 1995). We might thus expect actively foraging species also to rely heavily on chemical signals in communication. In the current study, however, we found that baseline frequencies of chemical behavior did not predict the use of chemical behavior during social interactions. In particular, of the four species in our study, male Sceloporus parvus produced the least chemosensory behavior during baseline trials (Martins et al. 2015), but responded chemically rather than visually to a live conspecific in the current experiments. In contrast, male S. merriami engaged in frequent chemosensory behavior during the baseline trials, yet responded primarily with visual behavior to a live conspecific. This result is similar to that found in a separate series of chemical playback trials conducted on the same four species (Pruett et al. 2016), in which S. parvus males also responded to experimental stimuli (whether a blank control or a conspecific chemical) with higher levels of chemosensory behavior than did S. merriami. This distinction between contexts may allow for separate phenotypic shaping by natural versus sexual selection. For example, animals that emphasize chemosensory mechanisms for foraging and exploratory behavior may have fewer evolutionary constraints on their vision and thus be better able to optimize visual displays for use in courtship or male–male aggressive encounters than would animals that use the same sensory modalities in both foraging and conspecific interactions.
Although most lizards in our study responded primarily to the visual aspects of the live conspecific, male Sceloporus parvus lizards responded chemically instead. Chemical cues may be especially important for S. parvus at this site where they are sympatric with an unusually large number of other Sceloporus species (three: S. grammicus, S. minor, and S. spinosus). In addition to the need to avoid the cost of unnecessary battles, two of the congeneric species at this site (S. minor and S. spinosus) are considerably larger than S. parvus and may be its occasional predators. Chemical signals may be less dangerous because they can be sampled at some temporal and spatial distance from the producer. At our study sites, the three other species in our current study were sympatric with only one other Sceloporus species (S. merriami with S. poinsettii, S. siniferus with S. melanorhinus, S. cozumelae with S. chrysostictus). Each of these three species also has a more complex headbob display pattern than does S. parvus (Carpenter 1978; Martins et al. 2015). S. parvus is also unique among the four species in that males exhibit two throat color morphs that do not differ detectably in behavior (Hews et al. 2015), although there may be differences in plasma testosterone levels and in the chemical composition of their femoral gland secretions (Pruett 2017). Additional studies of the importance of sympatric congeners as a selective pressure may be warranted given also the prominent examples of character displacement and adaptive radiation in lizards (e.g., in anoles: Losos 2011).
References
Alberts AC (1989) Ultraviolet visual sensitivity in desert iguanas: implications for pheromone detection. Anim Behav 38:129–137
Ben-Ari M, Inbar M (2014) Aphids link different sensory modalities to accurately interpret ambiguous cues. Behav Ecol 25:627–632
Brandt Y (2003) Lizard threat display handicaps endurance. Proc R Soc Lond 270:1061–1068
Carpenter CC (1978) Comparative display behavior in the genus Sceloporus (Iguanidae). Milwaukee Publ Mus Contr Biol Geol 18:1–71
Carpenter CC, Ferguson GW (1977) Variation and evolution of stereotyped behaviour in reptiles. In: Gans C, TInkle DW (eds) Biology of Reptilia: ecology and behaviour. Academic Press, New York, pp 335–554
Cooper WE (1995) Foraging mode, prey chemical discrimination, and phylogeny in lizards. Anim Behav 50:973–985
Duvall D (1979) Western fence lizard (Sceloporus occidentalis) chemical signals. I. Conspecific discriminations and release of a species-typical visual display. J Exp Zool 210:321–325
Endler JA, Gaburro J, Kelley LA (2014) Visual effects in great bowerbird sexual displays and their implications for signal design. Proc R Soc B 281:20140235
Halfwerk W, Slabbekoorn H (2015) Pollution going multimodal: the complex impact of the human-altered sensory environment on animal perception and performance. Biol Lett 11:20141051
Halfwerk W, Page RA, Taylor RC, Wilson PS, Ryan MJ (2014) Crossmodal comparisons of signal components allow for relative-distance assessment. Curr Biol 24:1751–1755
Hebets EA, Papaj DR (2005) Complex signal function: developing a framework of testable hypotheses. Behav Ecol Sociobiol 57:197–214
Hebets EA, Barron AB, Balakrishnan CN, Hauber ME, Mason PH, Hoke KL (2016) A systems approach to animal communication. Proc R Soc B 283:20152889
Hews DK, Benard MF (2001) Negative association between conspicuous visual display and chemosensory behavior in two Phrynosomatid lizards. Ethology 107:839–850
Hews DK, Martins EP (2013) Visual and chemical signals of social communication: providing the link to habitat and Environment. In: Lutterschmidt W (ed) Reptiles in Research: Investigations of Ecology, Physiology and Behavior from Desert to Sea. Nova Publishers, Hauppauge, pp 111–141
Hews DK, Hara E, Anderson MC (2012) Sex and species differences in plasma testosterone and in counts of androgen receptor-positive cells in key brain regions of Sceloporus lizard species that differ in aggression. Gen Comp Endocrinol 176:493–499
Hews DK, Pruett JA, Campos SM, Zúñiga-Vega JJ, Vital García C, Martins EP (2015) Throat color morphs in male Sceloporus parvus lizards: morphology, mite loads and behavior. Integr Comp Biol 55:E78 (abstract)
Hews DK, Seddon R, Zúñiga-Vega JJ, Vital García C, Martins EP (2016) Phylogenetic analyses of Sceloporus lizards reveal that species with abdominal blue patches have higher plasma testosterone levels. Integr Comp Biol 56:E90 (abstract)
Higham JP, Hebets EA (2013) An introduction to multimodal communication. Behav Ecol Sociobiol 67:1381–1388
Leaché AD (2010) Species trees for spiny lizards (genus Sceloporus): identifying points of concordance and conflict between nuclear and mitochondrial data. Mol Phylogenet Evol 54:162–171
Losos JB (2011) Lizards in an evolutionary tree: ecology and adaptive radiation of anoles. University of California Press, Berkeley
Martins EP (1993) Contextual use of the push-up display by the sagebrush lizard, Sceloporus graciosus. Anim Behav 45:25–36
Martins EP, Labra A, Halloy M, Thompson JT (2004) Large-scale patterns of signal evolution: an interspecific study of Liolaemus lizard headbob displays. Anim Behav 68:453–463
Martins EP, Ossip-Klein AG, Zúñiga-Vega JJ, Vital García C, Campos SM, Hews DK (2015) Evolving from static to dynamic signals: evolutionary compensation between two communicative signals. Anim Behav 102:223–229
Moore MC (1987) Circulating steroid hormones during rapid aggressive responses of territorial male mountain spiny lizards, Sceloporus jarrovi. Horm Behav 21:511–521
Ord TJ, Martins EP (2006) Tracing the origins of signal diversity in anole lizards: phylogenetic approaches to inferring the evolution of complex behaviour. Anim Behav 71:1411–1429
Ord TJ, Blumstein DT, Evans CS (2001) Intrasexual selection predicts the evolution of signal complexity in lizards. Proc R Soc Lond B 268:737–744
Ossip-Drahos AG, Oyola Morales JR, Vital-García C, Zúñiga-Vega JJ, Hews DK, Martins EP (2016) Shaping communicative colour signals over evolutionary time. R Soc Open Sci 3:160728
Ossip-Klein AG, Fuentes JA, Hews DK, Martins EP (2013) Information content is more important than sensory system or physical distance in guiding the long-term evolutionary relationships between signaling modalities in Sceloporus lizards. Behav Ecol Sociobiol 67:1513–1522
Partan SR (2013) Ten unanswered questions in multimodal communication. Behav Ecol Sociobiol 67:1523–1539
Partan SR, Marler P (1999) Communication goes multimodal. Science 283:1272–1273
Preininger D, Boeckle M, Freudmann A, Starnberger I, Sztatecsny M, Hödl W (2013) Multimodal signaling in the small torrent frog (Micrixalus saxicola) in a complex acoustic environment. Behav Ecol Sociobiol 67:1449–1456
Pruett JA (2017) Chemical ecology of male Sceloporus lizards: an integrative approach to the study of multimodal signals, hormones, and behavior. PhD dissertation, Department of Biology, Indiana State University, Terre Haute, IN
Pruett JA, Zúñiga-Vega JJ, Campos SM, Soini H, Novotny M, Vital García C, Martins EP, Hews DK (2016) Evolutionary interactions between visual and chemical signals: chemosignals compensate for the loss of a visual signal in male Sceloporus lizards. J Chem Ecol 42:1164–1174
R Development Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna http://www.R-project.org
Robinson CD, Patton MS, Andre BM, Johnson MA (2015) Convergent evolution of brain morphology and communication modalities in lizards. Curr Zool 61:281–291
Ronald KL, Fernández-Juricic E, Lucas JR (2012) Taking the sensory approach: how individual differences in sensory perception can influence mate choice. Anim Behav 84:1283–1294
Ronald KL, Fernández-Juricic E, Lucas JR (2018) Mate choice in the eye and ear of the beholder? Female multimodal sensory configuration influences her preferences. Proc R Soc B 285:20180713. https://doi.org/10.1098/rspb.2018.0713
Ruby DE (1978) Seasonal changes in the territorial behavior of the Iguanid lizard Sceloporus jarrovi. Copeia 1978:430–438
Ruppli CA, Dreiss AN, Roulin A (2013) Efficiency and significance of multiple vocal signals in sibling competition. Evol Biol 40:579–588
Stafstrom JA, Hebets EA (2013) Female mate choice for multimodal courtship and the importance of the signaling background for selection on male ornamentation. Curr Zool 59:200–209
Stamps JA, Barlow GW (1973) Variation and stereotypy in the displays of Anolis aeneus (Sauria: Iguanidae). Behaviour 47:67–93
Suriyampola PS, Cacéres J, Martins EP (2018) Effects of short-term turbidity on sensory preference and behaviour of adult fish. Anim Behav 146:105–111
Thompson JT, Bissell AN, Martins EP (2008) Inhibitory interactions between multimodal behavioural responses may influence the evolution of complex signals. Anim Behav 76:113–121
Uetz GW, Roberts JA, Taylor PW (2009) Multimodal communication and mate choice in wolf spiders: female response to multimodal versus unimodal signals. Anim Behav 78:299–305
Uetz GW, Roberts JA, Clark DL, Gibson JS, Gordon SD (2013) Multimodal signals increase active space of communication by wolf spiders in a complex litter environment. Behav Ecol Sociobiol 67:1471–1482
Uy JAC, Safran RJ (2013) Variation in the temporal and spatial use of signals and its implications for multimodal communication. Behav Ecol Sociobiol 67:1499–1511
Wade J (2011) Relationships among hormones, brain and motivated behaviors in lizards. Horm Behav 59:637–644
Ward JL, Love EK, Vélez A, Buerkle NP, O'Bryan LR, Bee MA (2013) Multitasking males and multiplicative females: dynamic signalling and receiver preferences in Cope’s grey treefrog. Anim Behav 86:231–243
Wenderoth N (2015) Changing the brain with multimodal mirrors: combining visual and somatosensory stimulation to enhance motor plasticity. Clin Neurophysiol 126:1065–1066
Wiens JJ, Kuczynski CA, Arif S, Reeder TW (2010) Phylogenetic relationships of phrynosomatid lizards based on nuclear and mitochondrial data, and a revised phylogeny for Sceloporus. Mol Phylogenet Evol 54:150–161
Wilson A, Dean M, Higham J (2013) A game theoretic approach to multimodal communication. Behav Ecol Sociobiol 67:1399–1415
Zanollo V, Griggio M, Robertson J, Kleindorfer S (2013) Males with a faster courtship display have more white spots and higher pairing success in the Diamond Firetail, Stagonopleura guttata. Ethology 119:344–352
Acknowledgments
We are indebted to Sharon Downes, Thomas Madsen, and four reviewers of the manuscript, all of whom offered numerous insightful comments. We thank Jesualdo Fuentes-G., Jay Goldberg, Delia Shelton, Cristina Romero-Diaz, Julio Rivera, Piyumika Suriyampola, and Delawrence Sykes for many helpful discussions, as well as Jake Pruett, José Oyola-Morales, and Patrick Cain for help in the field. We also thank the staff and volunteers at the Parque Nacional Huatulco for logistical support. We thank Big Bend Ranch State Park and El Parque Nacional Huatulco for granting access to public lands, and we thank Carolyn Ohl-Johnson for granting access to private land.
Funding
This material is based upon work supported by the National Science Foundation under grant numbers IOS-1050274 to EPM and IOS-1052247 to DKH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
We followed all applicable international, national, and institutional guidelines for the care and use of animals. All procedures were in accordance with the ethical standards of the institutions at which we conducted the research. The work was approved as part of IACUC protocols 10-013 and 13-009. Permission to conduct this work was also granted by the Texas Parks and Wildlife Department (S. merriami: SPR-0511-129) and the Secretaría de Medio Ambiente y Recursos Naturales of México (SEMARNAT; S. siniferus: 09/O1-0557/12/13; S. cozumelae and S. parvus: 09/k50904/01/13).
Additional information
Communicated by S. J. Downes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Martins, E.P., Ossip-Drahos, A.G., Vital García, C. et al. Trade-offs between visual and chemical behavioral responses. Behav Ecol Sociobiol 72, 189 (2018). https://doi.org/10.1007/s00265-018-2617-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-018-2617-0