Abstract
Asian honeybees (Apis cerana) have evolved a thermal collective defense against the sympatric giant hornet (Vespa mandarinia) in which workers surround the predator en masse and produce heat up to 46 °C to kill it. This characteristic behavior is called “hot defensive bee ball formation.” Many studies have described the uniqueness and efficiency of this behavior; however, little attention has been paid to the potential cost to the honeybee. In this study, we focused on potential effects to bee ball-participating honeybees. We compared life expectancy of same-age ball-participating honeybees and nonparticipating honeybees and demonstrated that the life expectancy of the bee ball-participating honeybees was dramatically shortened. The 46 °C exposure also shortened the life expectancy of honeybees with induced expression of the heat shock protein gene, strongly implicating the increased temperature inside the bee ball in the deleterious effect on participating honeybees. We additionally found that bee ball-participating and then short-lived worker honeybees had a tendency to join a subsequent bee ball more aggressively. This tendency could mitigate accumulation of the short-lived worker honeybees within the colony, which otherwise would cause a severe reduction of honeybee colony activity.
Significance statement
Social insects have evolved unique anti-predator altruistic behaviors for colony defense. Evaluation of the potential cost of these behaviors provides valuable insight into their evolution. Asian honeybees (Apis cerana) exhibit a sophisticated collective defense (bee ball formation) against the sympatric giant hornet (Vespa mandarinia) whose rigid exoskeleton resists the common stinging attack of the honeybees, utilizing heat. We found that the high temperature inside the ball itself dramatically reduced honeybee longevity. Furthermore, bee ball-experienced honeybees were found to be more likely to engage in subsequent bee ball formation. Our results pointed out unavoidable cost for the honeybee colony associated with the use of heat and a “division of risk” strategy in the bee colony for minimizing the cost.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animals have evolved a variety of adaptive traits to escape from or actively defend themselves against predators (Kavaliers and Choleris 2001). Some animals have evolved sophisticated anti-predator traits against their natural enemies under strong selective pressure to avoid predation, maximizing the benefit while minimizing the cost. Investigation of the benefit and cost of these behaviors provides valuable insight into the evolutionary processes of such unique traits (Hoso 2012).
Asian honeybees (Apis cerana) exhibit a unique collective behavior to defend their colony against the sympatric giant hornet (Vespa mandarinia) in summer and autumn. V. mandarinia is the largest predatory hornet species and the most formidable natural enemy of the honeybee, potentially causing severe damage to the honeybee colony (Matsuura 1988). The rigid exoskeleton of the hornet resists the common stinging attack of the honeybees in their counterattack on predators. Instead, A. cerana is able to detect a foraging-site marking pheromone of the giant hornet (Ono et al. 1995), and upon attack by a giant hornet worker, nearly 400 worker honeybees surround the hornet en masse and vibrate their flight muscles to produce heat in a process called “hot defensive bee ball formation” (Ono et al. 1995). The ball formation is usually maintained for over 30 min, and the heat produced by the mass kills the hornet.
Many studies have focused on the uniqueness and effectiveness of bee ball formation (Ono et al. 1987, 1995; Tan et al. 2005, 2010, 2013; Abrol 2006; Sugahara and Sakamoto 2009; Sugahara et al. 2012; Wang et al. 2016). The potential cost of this behavior, however, has been almost completely ignored. Although it has recently been reported that approximately a quarter of participating honeybees were killed in a bee ball by the attacking hornet (Tan et al. 2016), the effect of the bee ball formation on the survivors (the majority of the bee ball participants) remains totally unknown.
The temperature inside the bee ball reaches almost 46 °C (maintained for over 10 min) (Ono et al. 1995; Sugahara and Sakamoto 2009). Exceptionally high thermotolerance of honeybees compared with other insects has been well documented (Heinrich 1980; Roberts and Harrison 1999; Elekonich 2009). The high-temperature inside the bee ball is believed to be lethal to only the hornet not to the honeybees, based on findings that the thermal lethal limit for the honeybee is approximately 50 °C, while that for the hornet is approximately 45 °C (Ono et al. 1995; Tan et al. 2005; Sugahara and Sakamoto 2009; Sugahara et al. 2012). Importantly, however, these findings only indicate that temperatures greater than 45 °C but less than 50 °C are not acutely lethal for honeybees. Because the beehive temperature is maintained at only around 32 °C, the high-temperature condition within the bee ball could affect the physiological processes in the relic participating honeybees. In this study, we examined delayed adverse effects of hot defensive bee ball formation on participating honeybees to evaluate the potential cost to the bee colony of this characteristic anti-predator behavior.
Materials and methods
Animals
All the experiments were performed in the summer and autumn of 2014–2017. We used the Japanese honeybee (A. cerana japonica), a subspecies of the Asian honeybee that is native to Japan. Honeybee colonies from Nagano City (Nagano, Japan) were maintained at Tamagawa University (Tokyo, Japan) using an observation beehive kept at 25 °C and placed in a mostly dark room. The hive consisted of two frames with a nest entrance covered on a transparent chamber for the bee ball experiment (Fig. 1a). In total, we prepared eight colonies. The nest entrance was connected by a corridor to an orifice through which the bees were able to leave the hive for foraging. Color marking was performed on the thoraces of the bees using colored paint markers (PX-21; Mitsubishi Pencil, Japan), with bees gently restrained by a soft mesh sheet. It was not possible to record data blind because an experienced person (YY) was needed to accurately identify and collect the color-marked bees from a crowded nest or bee ball without accidental stinging. Giant hornet workers used in this study were captured on the campus of Tamagawa University.
Experimental setup. a An observation beehive with two frames is constructed. The white and black arrows indicate the nest entrance and orifice, respectively. The arrowhead indicates the corridor. b Outside view of the orifice. Only the worker honeybees but neither the queen bee nor the hornets can pass through the protector. c A wire-hung hornet. d A formed bee ball
Bee ball formation
A colony protector was always placed at the orifice of the beehive to prevent intrusion by hornets and to keep the colony bee ball-naïve (Fig. 1b). A worker hornet was hung by a wire around its petiole (Fig. 1c) and presented to the beehive. As soon as a bee ball was formed (Fig. 1d), we sequestered the mass away from the colony until bee collection. Gentle stimulation of the bee ball allowed removal of the bees on the periphery of the ball (Online resource: Supplementary Movie). The spherical assemblage remaining after removal of these bees on the periphery was called the center.
High temperature exposure to guard bees
Workers quickly responding to forceps that were presented at the nest entrance were collected as guard bees. Five to eight guard bees were collected in transparent plastic cups (9.8 cm in diameter × 9.8 cm high) that were then simultaneously incubated into either an air-conditioned room (NP-13-18-2; NKsystem, Japan) set at 32 °C or into incubators (LP-300-D; NKsystem) set to 44 and 46 °C for 30 min (Online resource: Table S1) under dark conditions.
Survival analysis
To discriminate 15–20-day-old worker honeybees, a frame containing many brood cells was incubated at 32 °C. Newly emerging adult workers were marked with different colors denoting the days of emergence for five consecutive days, and then the marked bees were kept in the bee ball-naïve colony. Fifteen days after the last marking, a bee ball experiment was performed, and 40 min after the beginning of the bee ball formation, we collected the color-marked bees from the center of the ball. An identical number of nonparticipating color-marked bees to the bee ball participants were also collected from within the colony. All of the collected bees were further color-marked and returned to their hive for observation. The number of surviving bees was investigated every day. It was almost impossible to locate corpses of color-marked bees once they were removed from the hive; therefore, death was assumed when bees disappeared. We counted the number of color-marked bees in the hive twice per day (between 08:00–10:00 and again between 15:00–17:00) and used the result from the afternoon observation as the number of surviving bees on that day. The number of surviving bees observed in the afternoon was never lower than that observed on the morning of the next day, indicating that missing bees did not simply leave the hive (e.g., for foraging) but instead the suggestion that they died. We carried out three trials using two honeybee colonies. Approximately 100 honeybees out of a total of 368 color-marked bees were alive at the days of experiments. In total, 5, 3, 4, 2, and 2 individuals of 15-, 17-, 18-, 19-, and 20-day-old bees, respectively, were analyzed.
The high temperature-exposed guard bees were also returned to their hive, and survival rates were checked daily. We performed three trials using two colonies. In total, 19 individuals in each temperature condition were analyzed.
Quantification of the heat shock protein 70 transcript
In order to detect early induction of heat shock protein 70 (hsp70) expression immediately following the 30 min exposure to high temperature, we measured the amount of de novo transcripts (i.e., pre-mRNAs) (Guzowski et al. 2005; Ugajin et al. 2018). The high temperature-exposed guard bees (six individuals, in each temperature condition) were anesthetized in ice-cold water, and their brains, fat bodies, and flight muscles were dissected. Total RNA samples were isolated using the RNeasy Mini (for brain and fat body) or Fibrous Tissue Mini (for flight muscle) Kits (Qiagen, the Netherlands). The total RNAs (approximately 70 ng) were then reverse transcribed using the PrimeScript RT Reagent Kit with gDNA Eraser (Takara, Japan), which allowed elimination of potentially contaminated genomic DNA and reverse transcription including pre-mRNAs. For selective amplification of hsp70 pre-mRNA (LOC107996313), gene-specific primers (5′-CGATGCTAATGGTATGTGCATT-3′ and 5′-TTGCGGATACATTCAGAATACCT-3′) were designed within the intron region based on the genome database of the Asian honeybee (Park et al. 2015). We used ribosomal protein L32 (rpl32) gene (LOC107997702) as an internal control (5′-AAAGAGAAACTGGCGTAAACC-3′ and 5′-TGGCAACATGTGACGAGTTT-3′). Quantitative RT-PCR was performed using SYBR Premix Ex Taq II (Tli RNase H Plus) (Takara) and the Eco Real-Time PCR System (Illumina, CA, USA), according to the manufacturer’s protocol with six biological replicates in each temperature condition. Standard curves were prepared using five points with progressive quantities of PCR amplicons (1 × 10−2 to 1 × 10−6 pg/μl for the quantification of hsp70 pre-mRNA; 1 × 10−1 to 1 × 10−5 pg/μl for rpl32).
Behavioral analysis upon subsequent hornet attack
We collected guard bees from a bee ball-naïve colony by repetitive presentation of the forceps at the nest entrance until no further workers rushed out in response. The collected guard bees were isolated from their colony and color-marked as “bee ball-naïve guard bees.” Immediately after the collection of the guard bees, we presented a hornet to the same colony. Thirty minutes after the beginning of bee ball formation, approximately the same number of workers as the collected guard bees was separately sampled from the center and periphery of the bee ball. We color-marked the collected bees and returned them to their hive with the bee ball-naïve guard bees. The other bees in the bee ball formation were not returned to the hive. In Asian honeybee colonies, foraging is suppressed temporarily in response to attack by a hornet (Tan et al. 2005). Therefore, 1 h after the end of the initial bee ball formation, we confirmed the resumption of the foraging flight and presented another hornet to the colony. As soon as a new bee ball was formed, participants were again separately collected from both the periphery and center of the mass. The participation rates of color-marked ball-naïve guard bees and ball-experienced bees in the new ball were calculated. We carried out three trials using three honeybee colonies. A total of approximately 40 individuals were analyzed in each experimental group.
High temperature-exposed guard bees were also returned to their hive, and 1 h later, a hornet was presented to the colony. The number of high temperature-exposed bees participating in the bee ball was then calculated. We carried out two trials using two honeybee colonies. In total, 13 individuals from each temperature condition were analyzed.
Statistical analysis
For survival analysis, we generated Kaplan–Meier curves and employed the generalized Wilcoxon test to compare survival rates. The expression levels of hsp70 in high temperature-exposed bees compared with expression levels in 32 °C-incubated bees were evaluated by Dunnett’s test. The differences in participation tendency toward the subsequent bee ball were tested using Fisher’s exact test by comparing the distribution rates of bees between the center and the rest (periphery and nonparticipation). All the statistical analyses were conducted using JMP13 (SAS, USA).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Results
Life expectancy of bee ball participants
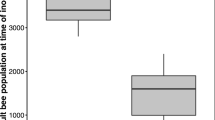
We first compared the life expectancy of bee ball participants with that of nonparticipants of identical age. In the honeybee colony, nest defense is mainly carried out by 2–3-week-old workers (Nouvian et al. 2016). An increase in flight muscle metabolism which enables bees to generate heat also occurs during this period (Roberts and Elekonich 2005). In support of this, our preliminary experiments revealed the tendency for younger honeybees less than 15 days old to rarely participate in bee ball formation (Online resource: Table S2). Therefore, we focused on 15–20-day-old workers. The captured hornet was typically dead within 30 min after the beginning of the bee ball formation (Ono et al. 1995; Sugahara and Sakamoto 2009), when, in addition, the number of participating honeybees gradually decreased (Online resource: Fig. S1). To analyze the bees who participated throughout the bee ball formation, we waited 40 min before selectively collecting the 15–20-day-old bees from the center of the ball. The bee ball participants died much earlier than nonparticipants (Fig. 2). The position of participants (center or periphery of the bee ball) 40 min after the bee ball formation seemed to not have a significant effect on bee life expectancy (Online resource: Fig. S2).
The impact of high temperature exposure
Although nest guarding is mainly performed by 2–3-week-old workers as mentioned above (Nouvian et al. 2016), some studies have reported that the behavioral responses and physiological states of these “middle-aged workers” are considerably diverse (Johnson 2003, 2010). Therefore, to confirm that the shortened life expectancy of the middle-aged bee ball participants was not derived from innate differences between participants and nonparticipants, but instead was caused by high temperature conditions inside of the ball, we performed further survival analysis using guard bees. We collected guard bees based on behavioral response and exposed them to high temperature (44 or 46 °C) for 30 min. Compared with bees incubated at 32 °C, the 46 °C-exposed bees were significantly shorter-lived; however, interestingly, no significant difference in lifespan was detected for the 44 °C-exposed bees (Fig. 3a). Correspondingly, the quantification of the de novo transcript of hsp70 gene, which is widely used as a biomarker of cellular stress responses (Elekonich 2009; King and MacRae 2015; Koo et al. 2015), revealed significantly induced expression in the brains and fat bodies of 46 °C-exposed bees but not in those of 44 °C-exposed bees (Fig. 3b). These findings provide a strong evidence of the negative impact of high temperature inside a bee ball on the life expectancy of the participants.
Effect of high-temperature exposure to guard bees. a Kaplan–Meier survival curves for high temperature-exposed bees. To compare the survival curves, generalized Wilcoxon tests were conducted (32 vs. 44 °C; P = 0.4722, 32C vs. 46 °C; P = 0.0058). The results from three trials were combined. b The expression level of hsp70 normalized by rpl32. The relative expression values were calculated by taking the value of 32 °C samples as 1. Asterisks indicate significant differences compared with the bees incubated at 32 °C (P < 0.01; Dunnett’s test). All data are shown as the means ± SEM
Behavioral response upon subsequent hornet attack
In autumn, giant hornets visit honeybee colonies very frequently: Matsuura (1988) reported that over 30 individuals were trapped in an apiary each day at its peak. The frequent visiting of giant hornets during the daytime was also observed in our apiary (YY and AU, personal observation). We investigated the behavioral tendency of bee ball-participating workers in subsequent attack by a hornet 1 h after initial bee ball formation. Compared with the bee ball-naïve guard bees, which appeared to be the most aggressive individuals in the colony, the bee ball-experienced honeybees showed a significantly higher participation rate toward the center of the new bee ball (Fig. 4). The zone of participation during the initial bee ball formation had no significant effect on the participation rate during the subsequent bee ball (Fig. 4). Similarly, 46 °C-exposed guard bees were also more frequently observed in the center of the subsequent bee ball than were 32 °C-incubated bees (Online resource: Fig. S3).
Participation rate in a subsequent bee ball. Small pie graphs show detailed results of bee ball-experienced bees. The distribution rates between the center and the rest (periphery and nonparticipation) were compared using Fisher’s exact tests (bee ball-naïve vs. bee ball-experienced; P = 0.0002, center-experienced vs. periphery-experienced; P = 0.6346). The results from three trials were combined
Discussion
In this study, we examined the effects of bee ball formation on worker honeybees that survive the bee ball. Specifically, we focused on the potential effect of participation and elevated temperature on honeybee life expectancy. Our experiments revealed that bee ball participants died significantly earlier than nonparticipants of identical age (Fig. 2). We also found that 46 °C-exposed guard bees were significantly shorter-lived and exhibited induced hsp70 expression (Fig. 3). These findings strongly suggested that the high temperature created inside of the bee ball had a physiological effect on participating workers and resulted in reduced life expectancy.
Due to the increasing nutritional demands in hornet colony to rear their broods, giant hornets attack honeybee colonies intensely in autumn (Matsuura 1988). For honeybees, vigorous foraging in autumn is essential for stockpiling large amounts of food for use during overwintering (Döke et al. 2015). Accumulation of short-lived workers causes a severe reduction of honeybee colony activity, thereby increasing the possibility of failure to overwinter successfully. Despite the potential cost of the extensive bee ball formation, A. cerana adopts this strategy, reaffirming that serious predation from giant hornets is an important selection pressure during their evolution. Intriguingly, with regard to effects of increased temperature on the bees, in contrast to the 46 °C exposure, the 44 °C exposure apparently had no negative effect (Fig. 3). As the acute upper lethal temperature for the hornet is approximately 45 °C (Ono et al. 1995; Sugahara and Sakamoto 2009), honeybees cannot kill a hornet by heat without deleterious impacts on the participating workers. There might be evolutionary constraints preventing the development of an efficient physiological mechanism in honeybees to overcome the harmful effects of exposure to 46 °C.
We also found that there was a tendency for the bee ball-experienced (then short-lived) honeybees to join the center of a subsequent bee ball more aggressively at the beginning of bee ball formation (Fig. 4). It takes approximately 5 min to raise the temperature inside the bee ball to over 45 °C (Sugahara and Sakamoto 2009), and the captured hornet can counterattack the honeybees in the center of the bee ball during that time. Aggregation of the bee ball-experienced workers at the dangerous central zone in the bee ball may mitigate an accelerated increase in short-lived worker honeybees, at least partially. In the European honeybee, Woyciechowski and Moroń (2009) reported that short-lived workers caused by anesthesia with CO2 or infection with Nosema apis began riskier foraging earlier in life. The behavioral changes of the bee ball-experienced Asian honeybee workers might be a further example of division of risk in the honeybee colony. The tendency of bees to occupy the center of the subsequent ball was commonly observed in the bee ball-experienced workers, regardless of the zone of participation in the initial bee ball (Fig. 4). Also, there was no significant difference in life expectancies between the periphery- and center-experienced workers (Fig. S2). These findings may imply the existence of the changes of bees between the periphery and center inside the bee ball. Further detailed investigation is needed to clarify the behavior of individual workers during a bee ball formation.
When the Asian honeybee colonies are attacked by hornets, worker honeybees produce vibrational signals called a “stop signal,” leading to a temporary (at most 20 min) inhibition of foraging (Tan et al. 2005, 2016). We performed the second bee ball experiment only after the foraging inhibition signal had expired; therefore, it was the internal states of the bee ball-experienced workers rather than the overall alert level in the bee colony that mainly contributed to the tendency of the bee ball-experienced workers to aggressively join the center of the subsequent bee balls. Both bee ball formation and exposure to a temperature of 46 °C made bees short-lived and more aggressive (Figs. 2, 3a, and 4; Fig. S3). Induced expression of hsp70 in the brains and fat bodies of 46 °C-exposed bees (Fig. 3b) gave further evidence for the activation of stress response systems in these tissues under the high-temperature conditions. It is widely known in animals that stressors not only affect lifespan but also often induce behavioral changes (Reeder and Kramer 2005; Epel and Lithgow 2014; Johnson 2016). In insects, the brain and fat bodies are considered to be key components of the stress response system via the secretion of biogenic amines, which are the main regulators of aggression in honeybees (Nouvian et al. 2016), the release of metabolically active hormones, and an increase in carbohydrate metabolism (Even et al. 2012; Johnson 2016). A recent study demonstrated that metabolic changes in the brain affect the aggression level of the European honeybee (A. mellifera) (Li-Byarlay et al. 2014). Other extreme environmental conditions inside of the bee ball, such as high humidity and high CO2 concentration (almost 90 and 4%, respectively) (Sugahara and Sakamoto 2009; Sugahara et al. 2012), could also act as stressors. Taken together, it is plausible that endocrine systems activated by the environmental stressors mediate the observed aggression of bee ball-experienced workers.
In a previous study, we examined the brains of bee ball participants and those of 46 °C-exposed workers, and we found upregulation of an immediate early gene in a specific neuronal subpopulation of the mushroom body (Ugajin et al. 2012), which is the higher order center of the insect brain involved in the integration of sensory inputs (Fahrbach 2006). In the nervous system, immediate early genes are rapidly and transiently expressed in response to neural activation (Clayton 2000) and are thought to play key roles in neural plasticity (Guzowski et al. 2005; Loebrich and Nedivi 2009; Lutz and Robinson 2013; Ugajin et al. 2013, 2018). More recently, a study involving the European honeybee reported that workers having responded aggressively to an intruder from another colony would remain in an enhanced aggression state for 1–2 h and, interestingly, with an increased expression of the immediate early genes in the mushroom body as well as hsps (Shpigler et al. 2017). For the release of the bee ball formation, a combination of olfactory inputs including odor from the hornet and the alarm pheromone of the honeybees is necessary (Ono et al. 1995; Wang et al. 2016). In addition to the enhancement of aggression regulated by the endocrine systems, it is also possible that increased sensitivity to such odors in the brain olfactory circuit contributes to the tendency of bees to occupy the center of the subsequent ball.
Our study sheds light on the adverse effects and subsequent behavioral changes associated with the hot defensive bee ball formation. Future research will uncover the detailed physiological mechanisms underlying the shortened life expectancy and the behavioral changes of the participants in this unique anti-predator behavior.
References
Abrol DP (2006) Defensive behaviour of Apis cerana F. against predatory wasps. J Apicult Sci 50:39–46
Clayton DF (2000) The genomic action potential. Neurobiol Learn Mem 74:185–216
Döke MA, Frazier M, Grozinger CM (2015) Overwintering honey bees: biology and management. Curr Opin Insect Sci 10:185–193. https://doi.org/10.1016/j.cois.2015.05.014
Elekonich MM (2009) Extreme thermotolerance and behavioral induction of 70-kDa heat shock proteins and their encoding genes in honey bees. Cell Stress Chaperones 14:219–226. https://doi.org/10.1007/s12192-008-0063-z
Epel ES, Lithgow GJ (2014) Stress biology and aging mechanisms: toward understanding the deep connection between adaptation to stress and longevity. J Gerontol A Biol Sci Med Sci 69:S10–S16. https://doi.org/10.1093/gerona/glu055
Even N, Devaud JM, Barron AB (2012) General stress responses in the honey bee. Insects 3:1271–1298. https://doi.org/10.3390/insects3041271
Fahrbach SE (2006) Structure of the mushroom bodies of the insect brain. Annu Rev Entomol 51:209–232. https://doi.org/10.1146/annurev.ento.51.110104.150954
Guzowski JF, Timlin JA, Roysam B, McNaughton BL, Worley PF, Barnes CA (2005) Mapping behaviorally relevant neural circuits with immediate-early gene expression. Curr Opin Neurobiol 15:599–606. https://doi.org/10.1016/j.conb.2005.08.018
Heinrich B (1980) Mechanisms of body-temperature regulation in honeybees, Apis mellifera. J Exp Biol 85:61–72
Hoso M (2012) Cost of autotomy drives ontogenetic switching of anti-predator mechanisms under developmental constraints in a land snail. Proc Biol Sci 279:4811–4816. https://doi.org/10.1098/rspb.2012.1943
Johnson BR (2003) Organization of work in the honeybee: a compromise between division of labour and behavioural flexibility. Proc R Soc Lond B 270:147–152. https://doi.org/10.1098/rspb.2002.2207
Johnson BR (2010) Division of labor in honeybees: form, function, and proximate mechanisms. Behav Ecol Sociobiol 64:305–316. https://doi.org/10.1007/s00265-009-0874-7
Johnson EC (2016) Stressed-out insects II. Physiology, behavior, and neuroendocrine circuits mediating stress responses. In: Pfaff DW, Joëls M (eds) Hormones, brain and behavior. Academic Press, San Diego, pp 465–481
Kavaliers M, Choleris E (2001) Antipredator responses and defensive behavior: ecological and ethological approaches for the neurosciences. Neurosci Biobehav Rev 25:577–586. https://doi.org/10.1016/S0149-7634(01)00042-2
King AM, MacRae TH (2015) Insect heat shock proteins during stress and diapause. Annu Rev Entomol 60:59–75. https://doi.org/10.1146/annurev-ento-011613-162107
Koo J, Son TG, Kim SY, Lee KY (2015) Differential responses of Apis mellifera heat shock protein genes to heat shock, flower-thinning formulations, and imidacloprid. J Asia-Pacific Entomol 18:583–589. https://doi.org/10.1016/j.aspen.2015.06.011
Li-Byarlay H, Rittschof CC, Massey JH, Pittendrigh BR, Robinson GE (2014) Socially responsive effects of brain oxidative metabolism on aggression. Proc Natl Acad Sci U S A 111:12533–12537. https://doi.org/10.1073/pnas.1412306111
Loebrich S, Nedivi E (2009) The function of activity-regulated genes in the nervous system. Physiol Rev 89:1079–1103. https://doi.org/10.1152/physrev.00013.2009
Lutz CC, Robinson GE (2013) Activity-dependent gene expression in honey bee mushroom bodies in response to orientation flight. J Exp Biol 216:2031–2038. https://doi.org/10.1242/jeb.084905
Matsuura M (1988) Ecological study on vespine wasps (Hymenoptera: Vespidae) attacking honeybee colonies. I. Seasonal changes in the frequency of visits to apiaries by vespine wasps and damage inflicted, especially in the absence of artificial protection. Appl Entomol Zool 23:428–440
Nouvian M, Reinhard J, Giurfa M (2016) The defensive response of the honeybee Apis mellifera. J Exp Biol 219:3505–3517. https://doi.org/10.1242/jeb.143016
Ono M, Okada I, Sasaki M (1987) Heat production by balling in the Japanese honeybee, Apis cerana japonica as a defensive behavior against the hornet, Vespa simillima xanthoptera (Hymenoptera: Vespidae). Experientia 43:1031–1032
Ono M, Igarashi T, Ohno E, Sasaki M (1995) Unusual thermal defense by a honeybee against mass attack by hornets. Nature 377:334–336. https://doi.org/10.1038/377334a0
Park D, Jung JW, Choi B-S, Jayakodi M, Lee J, Lim J, Yu Y, Choi YS, Lee ML, Park Y, Choi IY, Yang TJ, Edwards OR, Nah G, Kwon HW (2015) Uncovering the novel characteristics of Asian honeybee, Apis cerana, by whole genome sequencing. BMC Genomics 16(1). https://doi.org/10.1186/1471-2164-16-1
Reeder DM, Kramer KM (2005) Stress in free-ranging mammals: integrating physiology, ecology, and natural history. J Mammal 86:225–235. https://doi.org/10.1644/BHE-003.1
Roberts SP, Harrison JF (1999) Mechanisms of thermal stability during flight in the honeybee Apis mellifera. J Exp Biol 202:1523–1533
Roberts SP, Elekonich MM (2005) Muscle biochemistry and the ontogeny of flight capacity during behavioral development in the honey bee, Apis mellifera. J Exp Biol 208:4193–4198. https://doi.org/10.1242/jeb.01862
Shpigler HY, Saul MC, Murdoch EE, Cash-Ahmed AC, Seward CH, Sloofman L, Chandrasekaran S, Sinha S, Stubbs LJ, Robinson GE (2017) Behavioral, transcriptomic and epigenetic responses to social challenge in honey bees. Genes Brain Behav 16:579–591. https://doi.org/10.1111/gbb.12379
Sugahara M, Sakamoto F (2009) Heat and carbon dioxide generated by honeybees jointly act to kill hornets. Naturwissenschaften 96:1133–1136. https://doi.org/10.1007/s00114-009-0575-0
Sugahara M, Nishimura Y, Sakamoto F (2012) Differences in heat sensitivity between Japanese honeybees and hornets under high carbon dioxide and humidity conditions inside bee balls. Zool Sci 29:30–36. https://doi.org/10.2108/zsj.29.30
Tan K, Hepburn HR, Radloff SE, Yusheng Y, Yiqiu L, Danyin Z, Neumann P (2005) Heat-balling wasps by honeybees. Naturwissenschaften 92:492–495. https://doi.org/10.1007/s00114-005-0026-5
Tan K, Li H, Yang MX, Hepburn HR, Radloff SE (2010) Wasp hawking induces endothermic heat production in guard bees. J Insect Sci 10(142):1–6. https://doi.org/10.1673/031.010.14102
Tan K, Wang Z, Chen W, Hu Z, Oldroyd BP (2013) The ‘I see you’ prey-predator signal of Apis cerana is innate. Naturwissenschaften 100:245–248. https://doi.org/10.1007/s00114-013-1019-4
Tan K, Dong S, Li X, Liu X, Wang C, Li J, Nieh JC (2016) Honey bee inhibitory signaling is tuned to threat severity and can act as a colony alarm signal. PLoS Biol 14:e1002423. https://doi.org/10.1371/journal.pbio.1002423
Ugajin A, Kiya T, Kunieda T, Ono M, Yoshida T, Kubo T (2012) Detection of neural activity in the brains of Japanese honeybee workers during the formation of a “hot defensive bee ball”. PLoS One 7:e32902. https://doi.org/10.1371/journal.pone.0032902
Ugajin A, Kunieda T, Kubo T (2013) Identification and characterization of an Egr ortholog as a neural immediate early gene in the European honeybee (Apis mellifera L.). FEBS Lett 587:3224–3230. https://doi.org/10.1016/j.febslet.2013.08.014
Ugajin A, Uchiyama H, Miyata T, Sasaki T, Yajima S, Ono M (2018) Identification and initial characterization of novel neural immediate early genes possibly differentially contributing to foraging-related learning and memory processes in the honeybee. Insect Mol Biol 27:154–165. https://doi.org/10.1111/imb.12355
Wang Z, Qu Y, Dong S, Wen P, Li J, Tan K, Menzel R (2016) Honey bees modulate their olfactory learning in the presence of hornet predators and alarm component. PLoS One 11:e0150399. https://doi.org/10.1371/journal.pone.0150399
Woyciechowski M, Moroń D (2009) Life expectancy and onset of foraging in the honeybee (Apis mellifera). Insect Soc 56:193–201. https://doi.org/10.1007/s00040-009-0012-6
Acknowledgements
We are grateful to Mr. Tomio Yamaguchi for providing the honeybee colonies. We would like to thank Mr. Masaki Maruyama for his assistance in the bee ball experiments. Drs. Satoshi Miyazaki and Ryohei Kubo provided constructive comments on our study. We also thank Dr. James FA Traniello and two anonymous reviewers for their valuable comments on the manuscript.
Funding
This work was supported in part by Japan Society for the Promotion of Science (JSPS) KAKENHI grant number JP14J12036 (Grant-in-Aid for JSPS Research Fellow, for AU).
Author information
Authors and Affiliations
Contributions
YY, AU, SU, and MO conceived and designed the study. YY, SU, and MN carried out the behavioral experiments. AU performed the molecular lab work. YY and AU analyzed the data. AU and MH wrote the manuscript. YY and AU contributed equally to this work. All authors gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by O. Rueppell
Rights and permissions
About this article
Cite this article
Yamaguchi, Y., Ugajin, A., Utagawa, S. et al. Double-edged heat: honeybee participation in a hot defensive bee ball reduces life expectancy with an increased likelihood of engaging in future defense. Behav Ecol Sociobiol 72, 123 (2018). https://doi.org/10.1007/s00265-018-2545-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-018-2545-z