Abstract
Singing to create and defend territory boundaries is common among birds but rare in mammals. The African heart-nosed bat, Cardioderma cor, is hypothesized to use loud, low-frequency songs to reestablish foraging territories nightly. Territoriality can be defined ecologically, whereby an individual exclusively uses an area repeatedly, and behaviorally, through defense of an area. C. cor males sing on tightly abutting, exclusive areas nightly, which they return to throughout the season and sometimes across seasons. C. cor meets the ecological determinants of territoriality, but assessments of the use of song to maintain territories are lacking. We explore the singing behavior in this species by conducting song playback experiments within the borders of singing areas of 10 target individuals, with echolocation playbacks conducted as a control. In addition, we further explore the influence of song metrics on the behavioral response. Song playbacks prompted investigative and aggressive behavior, including passing by, approaching, and in one case, attacking the speaker, whereas echolocation did not. Additional post hoc analyses suggested that major song parameters, including song length, syllable frequency, intersyllable interval, and the number of double syllables comprising song stimuli influenced the level of response. For five bats we assessed whether their songs changed in response to the playback, and found that they sang faster, lower-frequency songs. These results are consistent with observations in other territorial animals including birds and gibbons, and provide a basis for further exploration of the territory defense hypothesis in the heart-nosed bat. We conclude that C. cor song features likely play an important role in mediating behavioral interactions within signaling networks of foraging bats.
Significance statement
It has been hypothesized that birdsong first evolved in support of territorial defense because it offered a cost-effective alternative to patrolling large spaces by flight. Singing-like behaviors have also been documented in several species of bats but never as a tool for maintaining foraging territories. However, evidence of foraging territoriality is scarce for bats, likely due to technical challenges associated with documenting such behaviors for a small, flying animal that may travel large distances at night. Here, we show for the first time that a bat responds to conspecific songs in a manner strikingly similar to many songbirds, providing support from outside songbirds for the hypothesis that territorial defense is a key selective pressure for singing in small, flying animals. This work provides the important basis for continuing to explore the role of singing, including song variability, in natural bat behavior outside of the roost.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Defending and maintaining an exclusive area is an important behavior of many animals to protect mates and resources. Territoriality can be established based upon multiple criteria, including behavioral responses (i.e., the defense of an area) and spatial ecology (i.e., exclusivity of an area and maintenance of spatial boundaries) (Maher and Lott 1995). Singing can support territoriality as a mechanism to advertise territory tenure, maintain spacing between individuals, and defend exclusivity of the territory (Tinbergen 1957; Bradbury and Vehrencamp 2011). Songbirds are the best-known examples of an animal that sings to defend an area for reproduction, roosting, and foraging (Hinde 1956), but there are a few reports of territorial singing in mammals, mostly from gibbon research (Mitani 1984; Brockelman 2009). Singing by bats has so far only been described within and around day roosts where it plays a role in the attraction and defense of mates (Behr and von Helversen 2004; Behr et al. 2006; Bohn et al. 2008). Singing has been observed in five bat families, but the role of this behavior outside of the roost is largely unclear due to historical constraints in following, observing, and recording fast-flying bats at night. However, evidence suggests that some bat species might also sing to establish and defend a preferred foraging area (Smotherman et al. 2016). We investigated the singing behavior of the heart-nosed bat (Cardioderma cor), a species that sings on private foraging areas.
Cardioderma cor is endemic to savannah areas of Eastern Africa. This species roosts in mixed-sex and age groups in the hollows of baobab trees. In the evening individuals disperse to separate areas and begin foraging (Vaughan 1976; Smarsh and Smotherman 2015a) (Fig. 1). Like other species of the small Megadermatidae family, C. cor uses short, ultrasonic, broadband echolocation pulses to navigate in flight but forages through passive gleaning, relying on prey-generated noises to passively localize terrestrial prey (e.g., arthropods, frogs) while listening from perches in Acacia trees and bushes (Ryan and Tuttle 1987; Denzinger and Schnitzler 2013; Kaňuch et al. 2015; Smarsh and Smotherman 2015b). Previous observations, recapture data, and tracking data suggest that both male and female C. cor individuals display spatial fidelity for preferred foraging areas both within and across seasons (Vaughan 1976; McWilliam 1987; Smarsh and Smotherman 2015a). Tracking data for one female and field observations suggest that females may overlap somewhat in their foraging areas with nearby males, and join the male at their perches at certain times of the year (McWilliam 1987; Smarsh and Smotherman 2015a). During the later period of the night, individuals move from perch to perch on these preferred areas (Vaughan 1976). Previous studies suggest that C. cor singing is a male behavior (McWilliam 1987; Smarsh and Smotherman 2015a). While perched, they broadcast loud, low-frequency songs, which they emit in bouts as they vocalize back and forth with nearby neighbors (Vaughan 1976; McWilliam 1987; Smarsh and Smotherman 2015a; Online Resource 1). This species emits echolocation pulses nasally, but produces social vocalizations orally as they slowly rotate back and forth on their perch, quickly orienting their head and ears towards the direction of sounds nearby (Vaughan 1976). Their songs consist of multiple syllable types including introductory syllables, end syllables, and various “hook” syllables with accompanying accessory notes of various bandwidths. The type, number, and order of these compound syllables vary within and across individuals, creating variability of songs within a bout and creating distinct hierarchically complex patterns (Smarsh and Smotherman 2015a). C. cor’s song characteristics meet the definition of Catchpole and Slater, whereby a “song” is a complex multisyllabic vocalization often produced during the breeding season, and broadcast spontaneously with characteristic patterns of the day. A “call” is a more stereotyped, simpler vocalization produced in specific social contexts such as alarm, food, or flight (Catchpole and Slater 2008). C. cor singing is produced seasonally, geared towards the long dry season when prey availability is low between May and October (McWilliam 1987; Smarsh and Smotherman 2015a). Singing behavior breaks down at the start of the November–December rainy season, at which time males may disperse from the area (Vaughan 1976). More simplistic contact calls are produced while foraging by both sexes at all times of the year (Vaughan 1976; Smarsh and Smotherman 2015a, Online Resource 2).
C. cor males meet a major criterion of territoriality—spatial fidelity of an area that is exclusively used by the individual. However, assessments of territorial behavior on these areas are lacking. We hypothesize, as initially proposed by Vaughan, that the male singing behavior is used to advertise and maintain territories (Vaughan 1976). This function was assumed in songbirds with similar behavior until detailed, difficult-to-carry out experiments were conducted in species such as great tits, white-throated sparrows, and song sparrows whereby the singer was removed and the encroachment of neighbors was observed (Catchpole and Slater 2008). More recently, a combination of observation of singing behavior and aggressive context, observation of singing predicting conflict, and assessment of response of the receiver to songs has been determined to be satisfactory (Searcy and Beecher 2009). We begin to explore the use of singing by C. cor by playing back songs obtained from distant individuals and measuring the movement and singing responses of individuals targeted on their singing areas. Movement towards the speaker, aggressive responses, and singing are suggestive of territorial advertisement and defense, whereas moving away from the sound source suggests mutual avoidance of singers to maintain interindividual spacing (Kinzey and Robinson 1983; Catchpole and Slater 2008; Fichtel and Hilgartner 2013). An alternative hypothesis is that movement towards the speaker is indicative of recruitment of foraging individuals through singing. A related species of singing bat, Megaderma lyra, produces calls with similar syllables for recruitment at roosts (Schmidt 2013). We compare the behavioral response of C. cor individuals to song playbacks to that of a control: echolocation pulses played back in the same manner on the individual foraging areas. We predicted that song playbacks would elicit strong movement and singing behavioral responses, whereas echolocation would not due to acoustic detection limitations and serve as a negative control.
Singing can effectively mediate social interactions when songs vary within and across individuals by providing information on the identity or motivation of the signaler (Temeles 1994; Tibbetts and Dale 2007; Wiley 2013). Territory theory predicts that in social contexts sustained by repeated interactions, individuals benefit from recognizing competitors by conserving energy and minimizing conflicts, termed the “dear-enemy” effect (Temeles 1994; Tibbetts and Dale 2007; Wiley 2013). Songbirds and gibbons both rely upon song metrics to discriminate neighbors from strangers, and some songbirds discriminate among individuals (Mitani 1987; Catchpole and Slater 2008; Ham et al. 2016). When used as an aggressive signal, song metrics can be graded to express heightened motivation to prevent unnecessary escalation to physical conflict (Searcy and Beecher 2009). Singing rate, intersyllable rate, and song duration have each been shown to signal motivation in birds and mammals (Bradbury and Vehrencamp 2011), as has song type matching and amplitude changes (Akçay et al. 2011, 2013). Spectral parameters such as fundamental frequency or formant dispersion can also cue the receiver to quality, size, or identity of the signaler (Tibbetts and Dale 2007; Koren et al. 2008; Fan et al. 2009). Multisyllabic and multiphrasic songs offer additional parameters to express identity, quality, or motivation of an individual (Catchpole et al. 1986). Some of these vocal behaviors have been demonstrated in both bat communication and echolocation vocalizations, but not in the context of foraging territoriality (Fitch and Hauser 2002; Behr et al. 2006; Puechmaille et al. 2014). Our preliminary analyses of C. cor song composition revealed that songs displayed enough variability within and across individuals to support a territorial network function (Smarsh and Smotherman 2015a). We hypothesized that C. cor song variability influences social interactions between bats. We measured temporal and spectral parameters of our playback stimuli and assessed how they may have influenced the behavioral response levels.
Methods
Field site
We conducted this project in the open areas of the Kikavuchini and Mkalama villages in the Hai District of northern Tanzania (3° 27′ 18.324″ S, 37° 16′ 51.312″ E) (Fig. 1). This rocky, dry habitat is characterized by Acacia-Commiphora scrub vegetation (Acacia tortilis and Commiphora africana) scattered with baobab trees (Adansonia digitata) and fragmented by fields of maize and beans. We targeted three C. cor baobab roosts in the area.
Stimulus collection
Cardioderma cor individuals have been noted to return to the same foraging areas nightly (Vaughan 1976; McWilliam 1987; Smarsh and Smotherman 2015a). C. cor’s nightly behavior combined with the loud, low-frequency attributes of C. cor songs allowed us to locate other singing individuals with discrete foraging areas for recording (Vaughan 1976; Smarsh and Smotherman 2015a). We collected song stimuli in June–July 2013 and March 2014 during which we recorded 20 min of songs per individual from within 5 m using the SM2+ ultrasonic recorder from Wildlife Acoustics (96 kHz sample rate, 48-dB gain). We collected echolocation for playbacks on March 25 and April 22, 2014, from 17 individuals that we captured at the main Kikavuchini baobab roost using a single high mistnet (Avinet, Inc., Dryden, New York) (Smarsh and Smotherman 2015b).
Stimulus design
We selected 10 singers for playback experiments. To ensure that we knew that our focal bat was responding rather than a different individual, we avoided conducting these experiments to bats near the main roost where the density of individuals was higher. The sparseness of the habitat also eased our ability to target separate singers for playback. Eight of the 10 of these individuals were netted throughout the field season and confirmed to be adult males. No bats were caught right before the trials. For each target bat, we selected one representative song with high signal-to-noise ratio from another bat located at least two foraging areas away in the field site to use as the stimulus (Kroodsma 1989; Catchpole and Slater 2008). With one exception, we used a song from a different bat for the playlist for each target individual to avoid pseudoreplication, and thus had nine different playlists (Kroodsma 1989). Song duration varied between 1.31 and 2.12 s (\( \overline{\mathrm{x}} \) ± SD = 1.85 ± 0.35 s). We constructed the playlists using BatSound and Avisoft-SASLab Pro. We normalized the nine songs to 50% amplitude. The song playlist consisted of 5 min of precontrol silence (PreC), Set 1 of the selected song repeated 20 times, 1 min of silence, Set 2 of the same selected song repeated 20 times, and lastly 5 min of postcontrol silence (PostC) (Online Resource 3, Online Resource 4). We used the intersong interval of 9.4 s for all playlists, determined by calculating the mean of the mean of intersong intervals measured from sets of 20 songs from 10 individuals recorded in 2013. We tapered the amplitude at the end of each song set to transition into silent intervals in the file.
Echolocation passes were normalized to 75% amplitude. Echolocation playlists also included 5 min of PreC and PostC silent periods, with two sets of echolocation pulses separated by 1 min of silence. Each echolocation set consisted of 20 echolocation passes recorded from 10 individuals (males and females).
Experimental design
Data from these experiments were not recorded blind because this study involved following and observing focal individuals in the field. We conducted the majority of playback experiments in April 2014, between 22:00 and 3:00. We placed our custom-made loudspeaker (Vifa XT25TG30-04, 1″dual ring radiator tweeter powered by an 18-W amplifier) in a tree or bush within the bat’s singing area, approximately 20 m away from the individual’s perch facing in the direction of the bat (\( \overline{\mathrm{x}} \) ± SD = 24.26 ± 9.1 m). We used a 96-kHz sample rate digital/analog converter (uDAC-2, 24-bit; NuForce, Milpitas, CA) connected to a laptop with a USB-2 output. Amplitude was held constant for each playback. System output was calibrated in the lab using a Brüel and Kjær type 4139 microphone in an anechoic chamber. By adjusting the peak output voltage of the DAC to ≈ 1 V and subsequently amplifying the analog signal by 10 dB, the tweeter produced an on-axis signal of approximately 94 ± 6 dB re 20 μPa from 5 to 48 kHz at 1 m. The tweeter’s beam projection pattern at 20 kHz (at the top of the 1st harmonic and 2nd harmonic of the song playback stimuli) exhibited a − 6-dB drop-off at ± 30°. Based on this, we conservatively estimate that at the start of each experiment the typical song stimulus level at the target bat would have varied from approximately 64 to 76 dB. We videotaped the speaker bush during the playback using a Sony Nightshot Camcorder with two infrared lights for illumination and also recorded songs from the target bat during the trial. One field assistant was responsible for following the bat with an SM2+ ultrasonic recorder to collect the songs. We required that the perched bat sing at least 10 consecutive songs before initiating song Set 1 followed by the rest of the playlist. If the bat moved or fell silent before Set 1 was about to start, we restarted the trial. This ensured that we knew where the bat was before the stimulus started, which was important to make sure the bat was still in hearing distance of our speaker and to prevent the possibility of extra variability in the data set due to highly variable starting conditions. Importantly, these criteria allowed us to repeat starting conditions for both song and echolocation playback trials for each bat. From previous tracking of male singers, video recording of individuals, and preliminary playback trials with mistnets, we have found that the only singer to respond to a playback on a foraging area is the male who occupies the area, which he uses largely exclusively. From tracking, we found that we could largely discriminate individuals by their song, so on the occasion when another male intruded on the territory, this was obvious by perch location and song differences (Smarsh and Smotherman 2015a). While females may occasionally enter onto a male foraging area and produce contact calls, we have not observed them to sing in response to playback, nor have we caught a female in a mistnet near the speaker bush during a test playback trial. In addition, the majority of playbacks were conducted in April when pairing behavior seems to be minimal (McWilliam 1987). Thus, with confidence we noted passes by the speaker, attacks to the speaker (direct flight to the speaker and away), approach or retreat, and singing times of our focal bat during the trial. Passes were categorized as flights past the speaker bush, whereas an approach was noted when the bat vocalized in a perch closer to the speaker bush than the bat’s starting point. Movements to different perches during the trial were marked with a Magellan Triton GPS unit, and we measured the distance from the speaker tree to each of these perches. We conducted echolocation playbacks from the same positions following the same methods, on the same or a subsequent night.
Data analysis
We calculated set length for each stimulus file and then determined the equivalent length of PreC from which to analyze data. We calculated the number of passes, songs, and attacks during the PreC, Set 1, and Set 2 time periods of each trial. Singing Rate was the average number of songs per minute in each period of the trial. We used interval coding of approach and away movements to make these variables comparable across individuals. The movement data were coded to match the distance from the speaker to which the bat moved. Approach varied from 6 (moved to the speaker bush) to 0 (no movement). Away varied from 0 (no movement) to 6 (moved more than 25 m away). Only each individual’s largest approach and away movements during each time period were later used in the analysis. We analyzed the responses to playbacks using the Fathom toolbox in MATLAB (v. R2015a) (Jones 2015). We used a Canonical Analysis of Principal Coordinates (CAP) procedure to analyze the response variables across PreC, Set 1, and Set 2 periods of the trial. We used a nonparametric permutation MANOVA, repeated measures univariate analyses, and post hoc matched-pair two-tailed tests using JMP (v. 12) and the Real Statistics Resource Pack.
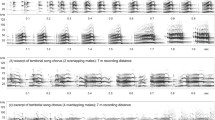
To assess whether the target bats’ songs changed during the trial, or whether the stimulus songs influenced the response to playback, we analyzed all of the song stimuli as well as the target bat songs. We analyzed 10–15 songs from the PreC period for 10 individuals and an additional 10–15 songs during Set 1 and Set 2 for a subset of five individuals. Here, a “note” is the basic unit of C. cor song. “Syllables” are clustered notes or single notes that are clearly temporally separated. C. cor songs consist of introductory syllables, end syllables, and main hook syllables that may be coupled with accessory notes (Fig. 2). For all of the bats’ songs, we randomly selected one to three main hook notes (M notes) per song for the analysis. In BatSound, we measured the start and end times of each note using the oscillogram. In MATLAB, we bandpass-filtered each wave file. Using the pwelch function, we generated the power spectral density (PSD) of each note, using four equal-sized Hanning windows with 50% overlap, zero-padded to 1024 FFT for short notes (frequency resolution = 93.8 Hz). We extracted the frequency at the highest amplitude (Fpeak), and minimum and maximum frequencies defined as the frequencies at − 20 dB on either side of the peak. We used the following song and syllable metrics for analysis: Length (song length), Sylls (number of syllables), Doubles (number of “double” syllables), ISIB (average intersyllable interval of the body of the song, excluding introductory and end syllables), DurB (duration of the M note), FPeakM (peak frequency of the M note), FMinM (minimum frequency of the M note), LowN (number of low-frequency accessory notes), and HighN (number of high-frequency accessory notes). HighN accessory notes had fundamental frequencies of at least 3 kHz greater than the M note fundamental frequency. We tested the influence of these song metrics on the behavioral response to playback with partial least squares regression with Monte Carlo permutation using the PopTools plugin (v. 3.2) in Excel.
a–c Example spectrograms of three song stimuli. a Examples of syllables are marked with “Syll.” Songs consist of main hook notes (M) that are sometimes coupled with one or more accessory notes (N). An M note and N note coupling are termed “double” syllables. Songs are frequently accompanied by varying numbers of introductory (I) and end (E) syllables. b N notes were classified as HighN if the fundamental frequency was at least 3 kHz greater than the fundamental frequency of the M note; otherwise, they were termed LowN
Results
Behavioral response to playback
Individuals reacted to the song playbacks initially by reducing their singing rate as they began moving about by passing by and approaching the speaker. We observed only one individual attack the speaker in this data set, and thus, this variable was not included in the rest of the analyses. The first canonical axis of the CAP analysis is strongly correlated with Singing Rate and Songs, as well as Passes and Approach in the opposite direction. The second axis is largely correlated with movements Away from the speaker (Table 1, Fig. 3). We used the first three Principal Coordinates for the Canonical Discriminant Analysis to test for significant difference in response between the PreC, Set 1, and Set 2 trial periods. The three PCOs explained 93.7% of the variability of the response matrix. Leave-one-out testing resulted in a 63.3% correct classification rate of time periods and was significant (1000 permutations, p = 0.001).
These results were supported by a nonparametric perMANOVA of the five response variables (F 2,27 = 5.2629, n = 10, p = 0.001, 1000 permutations). Post hoc tests showed that PreC differed significantly from both Set 1 and Set 2 (PreC vs Set 1: T = 3.46, n = 10, p = 0.003; PreC vs set 2: T = 2.294, n = 10, p = 0.006; set 1 vs set 2: T = 1.268, n = 10, p = 0.56, Bonferroni-corrected). Songs and Singing Rate varied significantly across periods (ANOVA, songs: F 2,18 = 9.847, p = 0.001; rate: F 2,18 = 14.512, p = 0.0002), whereby singing behavior dropped significantly in Set 1, but increased again in Set 2 for most bats (Table 2, Fig. 4). No passes were observed during the PreC period of the experiment, but this behavior increased rapidly during Set 1 of the trial where all but one individual passed by the speaker (Kruskal-Wallis, Passes: χ 2 = 13.043, df = 2, p = 0.002) (Table 2, Fig. 4). Approach varied significantly across time period, but Away did not (Friedman’s test, Approach: H = 6.95, df = 2, p = 0.031; Away: H = 1.8, df = 2, p = 0.41; Table 2, Fig. 4). Similar to the singing and passing behaviors, approaching was most frequently observed during Set 1 of the experiment with 8 out of 10 individuals approaching; this variable significantly differed between PreC and Set 1 time periods (Table 2, Fig. 4).
Behavioral response of 10 individuals to song playbacks by time period of the trial. Each line represents the matched behavior of an individual across the three periods. Black diamonds represent either mean for parametric data (Song Rate and Song plots) or median for nonparametric data. Asterisk denotes p values less than 0.05; double asterisks denotes p values less than 0.01
Unlike the song playbacks, the echolocation playbacks did not elicit significant behavioral responses. Passes and approaches did not occur in any of the trials after the start of the stimulus, and attacks were not observed at all. The top two canonical axes of the CAP analysis thus correlated strongly with Songs, Singing rate, and Away (Table 1). With three principal coordinate axes accounting for 88.1% of variability in the response retained for the CDA, classification across time periods of the trial was not significant (33.3% correct classification, p = 0.58, 1000 permutations). The nonparametric perMANOVA was not significant (F 2,27 = 0.878, p = 0.53, 1000 permutations). Repeated measures ANOVA of Songs was significant, but matched-pair post hoc tests with Bonferroni-corrected p values were not (\( \overline{\mathrm{x}} \) PreC ± SD = 7.3 ± 3.4, \( \overline{\mathrm{x}} \) Set1 ± SD = 7.4 ± 3.7, \( \overline{\mathrm{x}} \) Set2 ± SD = 6.5 ± 4.5, F 2,18 = 3.742, p = 0.01). Singing Rate did not change significantly across time periods (\( \overline{\mathrm{x}} \) PreC ± SD = 3.7 ± 1.7, \( \overline{\mathrm{x}} \) Set1 ± SD = 3.7 ± 1.5, \( \overline{\mathrm{x}} \) Set2 ± SD = 3.5 ± 2.04, F 2,18 = 1.9, p = 0.13), nor did Away (\( \overline{\mathrm{x}} \) PreC ± SD = 0 ± 0, \( \overline{\mathrm{x}} \) Set1 = 1.8 ± 2.9, \( \overline{\mathrm{x}} \) Set2 ± SD = 1.0 ± 2.2, Friedman’s test, H = 1.05, df = 2, p = 0.59), Passes (\( \overline{\mathrm{x}} \) PreC ± SD = 0.1 ± 0.3, \( \overline{\mathrm{x}} \) Set1 ± SD = 0 ± 0, \( \overline{\mathrm{x}} \) Set2 ± SD = 0 ± 0, ANOVA, F 2,18 = 1, p = 0.4), and Approach (\( \overline{\mathrm{x}} \) PreC ± SD = 0.2 ± 0.6, \( \overline{\mathrm{x}} \) Set1 = 0 ± 0, \( \overline{\mathrm{x}} \) Set2 ± SD = 0 ± 0, Friedman’s test, H = 0.15, df = 2, p = 0.92).
The influence of song metrics on behavioral response
We explored the relationship between C. cor songs and the level of response to song playback using partial least squares (PLS) regression with permutation. We used the following song metrics for the predictor matrix: Length, Sylls, Doubles, ISIB, DurB, FPeakM, FMinM, LowN, and HighN. The response matrix consisted of the absolute values of the difference in Passes, Songs, Rate, Approach, and Away between the PreC and Set 1 periods of the trial. The variables of the predictor and response matrix were scaled and centered prior to computing the cross-variance matrix of the PLS regression. In PLS regression, the predictor and response matrices are simultaneously decomposed to find components (vectors) that explain the greatest covariance between the two matrices. The resulting vectors cannot be interpreted alone, and are transposed with the initial variables to create singular value scores. These singular value scores of predictor and response matrices are regressed to further look at the relationship between them (Abdi 2003). We have also correlated the singular value scores with the initial variables (resulting in singular coefficients) to interpret the results in Table 3. Larger coefficients indicate greater weight in the results of the analysis (Table 3).
The regression of the first singular scores of the stimulus song metrics and the response variables had an R 2 of 0.49 (p = 0.575, 1000 permutations). The singular coefficients indicated that fewer doubles and longer intervals relate to smaller changes in singing and passing behavior. The second singular axis regression had an R 2 of 0.63, and suggests that lower-frequency, longer songs predict stronger movement behaviors (Table 3). The difference between the stimulus metrics and mean centroids of the target bats’ songs (n = 10 to 15 songs per bat) had the strongest relationship with song playback response, with an R 2 value of 0.69, but was still not significant (p = 0.48, 1000 permutations). Greater similarity in frequency of the song, but greater divergence in intersyllable interval and the number of doubles, related to greater passing, singing, and approaching behavior (Table 3, Fig. 5). The second singular vectors had an R 2 value of 0.241 (Table 3). The mean centroids of the nine metrics of the target bats’ songs had little relationship with the behavioral response variables with an R 2 of 0.39 (p = 0.894, 1000 permutations).
Partial least squares regression of the singular value scores of the response and predictors. The predictor matrix was the difference between the song stimulus metrics and the centroid of the target bat’s songs metrics, and the response matrix was the behavioral response to playback variables. a Regression of the first singular value scores. b Regression of the second singular value scores
Song changes in response to playback
For five bats with at least 10 clear songs recorded during Set 1 and Set 2 periods of the trial, we assessed whether the songs changed acoustically and temporally during the trial. We compared the mean song metrics of the songs of each bat during control (PreC) and trial (Set 1 and Set 2) periods using matched-pair t tests. The changes for six of these parameters are illustrated in Fig. 6. ISIB significantly decreased between the periods (\( \overline{\mathrm{x}} \) control ± SD = 121.74 ± 22.03 ms, \( \overline{\mathrm{x}} \) trial ± SD = 115.91 ± 20.4 ms, T = 3.16, df = 4, p = 0.03; Fig. 6, Table 4). FMinM tended to decrease (\( \overline{\mathrm{x}} \) control ± SD = 8.03 ± 0.91 kHz, \( \overline{\mathrm{x}} \) trial ± SD = 7.79 ± 0.69 kHz, T = 2.32, df = 4, p = 0.08; Fig. 6, Table 4).
Discussion
Behavioral response to acoustic playbacks
Evidence of both singing and territorial behaviors displayed by foraging bats is poorly documented in the literature, but likely a common and key behavior in many bat species (Smotherman et al. 2016). C. cor’s robust passing and approaching behavior is similar to that of many territorial songbird species, in which territory maintenance and defense have been well-established (Catchpole et al. 1986). Bornean, white-bearded, and Javan gibbons have also been shown to move quickly towards a playback source positioned within the singing area (Mitani 1984, 1985a, b; Raemaekers and Raemaekers 1985; Ham et al. 2016). There were some instances where bats moved away during the playback trials, but escape behavior by less aggressive individuals is a common behavior in contests between competitors (Bradbury and Vehrencamp 2011). Alternatively, the movement responses of the receiver towards the song playbacks could be explained by investigation or recruitment of individuals, as has been observed in other bat species to recruit individuals to roosts, to coordinate group foraging, or to contact other individuals when in isolation (Fenton et al. 1976; Wilkinson and Boughman 1998; Chaverri et al. 2010; Carter and Wilkinson 2016). However, along with the exclusivity and fidelity of foraging areas, two key observations point to behavior in line with territoriality: (1) Preliminary trials of playbacks to tracked individuals at various locations on their singing areas in 2013 showed that C. cor did not respond to songs played beyond their outermost singing perches, consistent with the behavior of animals with foraging territories (Dhondt 1966; Falls 1978; Mitani 1985a, b). (2) We observed that the death of a tracked singing individual due to a snake in 2013 was soon followed by the encroachment of a singing neighbor onto the deceased bat’s former singing area. This follows the removal experiments of songbirds that clearly illustrated the role of singing to advertise and maintain territories when other individuals encroached upon the areas (Catchpole and Slater 2008). These observations, radiotracking data, and behavioral assays support the conclusion that C. cor maintains exclusivity and fidelity of tightly abutting foraging areas outside of the roost within seasons and sometimes across seasons, and support the ecological criterion of territoriality (Vaughan 1976; McWilliam 1987; Maher and Lott 1995; Smarsh and Smotherman 2015a). We thus maintain that although singing alone and in response to neighbors is a common criterion of the interindividual spacing hypothesis of singing (Marler 1969), the territory defense hypothesis is a more appropriate working hypothesis of the role of singing in the species, and should be further tested in future experiments.
How singing behavior changes in response to playback varies across species (Bradbury and Vehrencamp 2011). In many songbirds, individuals heighten their singing rate in response to playback to display aggressive intent before initiating a more aggressive response such as chasing or an attack (Searcy and Beecher 2009). During C. cor trials, we observed that singing was often reduced at the start of the playback. C. cor individuals sing while perched rather than while flying, so this initial drop in singing may be attributable to a combination of listening and then investigating the “intruder” by flying towards the song source. The most extreme response observed during these experiments was silence preceding attack on the speaker, a behavior reminiscent of territorial song sparrows that attack in silence without vocal warning (Beecher et al. 1998). Similarly, Carolina wrens sing and move about in response to degraded songs but also silently attack in response to undegraded songs indicative of a nearby conspecific (Richards 1981). As playback trials progressed from the later part of Set 1 and into Set 2, some C. cor individuals increased their singing rate again at a perch close to the speaker, even singing in the same tree as the speaker. While tracking individuals, we observed territory holders singing in response to another bat intruding onto the edge of the territory, sometimes moving towards the intruder, and sometimes moving to a more interior perch and singing. Thus, much like songbirds, singing seems to play an important role in motivational signaling in C. cor.
C. cor individuals did not respond to the echolocation playbacks. This may be due to several reasons: lack of interest or importance of hearing conspecific echolocation pulses on the target bats’ foraging areas, minimal detection ability of the high-frequency echolocation pulses from the speaker (since high-frequency sounds attenuate faster than low-frequency sounds (Lawrence and Simmons 1982)), or, if loud enough to be detected, poor quality of the stimulus due to the short, broadband structure of calls that may be difficult to mimic at loud amplitudes naturally in these “whispering bats” (Neuweiler 1990; Waters and Jones 1995; Denzinger and Schnitzler 2013). We cannot be conclusive about the role of echolocation in facilitating communication in this species from this single study, although it has been demonstrated in two other singing bats (Knӧrnschild et al. 2012; Bohn et al. 2013). However, the negative results from the echolocation trials importantly support the observed effect of the songs on the behavior of our target individuals.
Song metric influence on the response to song playback
Song variability mediates social interactions in a variety of animals by signaling quality, motivation, identity, or a combination of the three (Fitch and Hauser 2002; Rendall et al. 2009; Taylor and Reby 2010; Byers et al. 2016; Terleph et al. 2016). Our results indicate that for C. cor, stimulus songs that were faster, more complex, or lower in frequency relative to the focal bat’s songs evoked stronger responses, including more singing, passing, and approaching or retreating responses. These song parameters could thus be indicative of index cues (i.e., size of the animal) or motivational state cues (such as level of aggression) in the song that can evoke greater behavioral response when a receiver detects them. Generally in animals, fundamental frequency correlates inversely with body size, as larger body size often correlates with larynx size and vocal tract length, such that fundamental frequency can be an honest signal of quality (Hall et al. 2013). Motivational state can be expressed by temporal (i.e., duration, syllable rate, singing rate) or spectral (i.e., bandwidth, fundamental frequency) metrics (Taylor and Reby 2010; Linhart et al. 2013; Cardoso 2014; Funghi et al. 2015). Motivational state signaling has been heavily studied in birds, where frequently faster, longer signals can predict heightened aggression in some songbirds (Linhart et al. 2013; Cardoso 2014). “Vocal deviation,” the trade-off between rapidly produced trill phrases with large bandwidth, and a similar metric termed “vocal gap deviation” are established measures of physically challenging vocal performance in songbirds that correlate with territoriality (Podos 1997; DuBois et al. 2009; Geberzahn and Aubin 2014). Vocal deviation represents a trade-off that is constrained by beak movements, however, so when considering C. cor results a question that comes to mind is what sort of physical constraints are involved with bat vocalization production that can tie into performance in social contexts (Podos 1997). Acoustically similar to trills, energetically costly “buzz phrases” are produced by superfast laryngeal muscles in echolocating bats that are used during the approach to prey items when hunting (Ratcliffe 2015). If used in songs or calls, buzzes can also provide honest signals of individual quality (Behr et al. 2006; Ratcliffe 2015). For male sac-winged bats (Saccopteryx bilineata), for example, lower-frequency buzzes in their territory songs have been correlated with reproductive success, and additionally, males also respond more strongly to “buzzy” territory songs of other males that are lower in frequency (Behr et al. 2006, 2009). C. cor, like other megadermatids, have not been noted to use buzzes in echolocation sequences or their songs (Kulzer et al. 1984; Guppy et al. 1985; Schmidt 2000; Leippert et al. 2002; Schmidt 2013; Kaňuch et al. 2015; Smarsh and Smotherman 2015b). However, they are able to produce faster, longer songs that are presumably more energetically expensive by decreasing intersyllable intervals and including more double syllables, and our preliminary results support the conclusion that these song parameters modulate the receiver’s behavioral responses (passing, approaching, and singing) to experimental playbacks. An alternative motivational state cue may stem from the hierarchical characteristics of C. cor syllables with the arrangement of syllable types and the different numbers of accessory notes within songs (such as more double syllables). This possible relationship between hierarchical arrangement of vocalizations and emotional affect has been proposed for the calls of the related singing bat, M. lyra (Schmidt 2013). Janßen and Schmidt manipulated the frequency, rhythm, and single calls of call series stimuli, and found that individuals discriminated rhythmic differences of call series as well as frequency (Janßen and Schmidt 2009). We did not find evidence of influence of number of high- or low-frequency accessory notes in the PLS regression analysis, but double syllables, which also contain accessory notes, held greater weight. Syllable arrangement could thus be a useful subject of a future target study.
Similarity of songs between territory holders has been observed in songbirds to mediate aggressive interactions. Song matching and repertoire matching, or responding to intruders with similar songs, have been observed in many song birds when signaling heightened aggression (Burt et al. 2002; Searcy and Beecher 2009; Bradbury and Vehrencamp 2011; Akçay et al. 2013). We found that differences in intersyllable interval, number of doubles, and to a lesser extent, frequency of the dominant main hook notes between the stimulus and target bat songs related to the level of response to the playback. We did not observe song matching, but if these frequency or temporal metrics express quality or motivation, then to a territory holder, an intruder of similar quality or motivation may be a greater threat resulting in a stronger response to their song. The songs metrics we have targeted in this analysis can be further investigated experimentally with C. cor.
Song changes in response to song playback
Not only did faster, lower-frequency songs seem to influence behavioral response to playbacks, but our exploratory analysis indicated that C. cor songs also changed in response to playbacks most often by producing faster, lower-frequency songs. These changes are consistent with elevation of aggressive intent seen in many songbird species (DuBois et al. 2009; Bradbury and Vehrencamp 2011; Linhart et al. 2013; Geberzahn and Aubin 2014). Modifications of vocalizations in response to conspecific signals have been demonstrated in other singing bat species as well. M. lyra produces female-directed flight songs in the roost, as well as a variety of calls mediating social interaction. During high-intensity interactions, individuals produce higher duration noisy, “chevron” syllables in their multisyllabic aggressive calls (Bastian and Schmidt 2008). During interactions with females, aroused male M. lyra modify the “strophe” phrase of the flightsong by decreasing the intersyllable interval, and producing more syllables that are higher in frequency but are shorter in duration (Bastian and Schmidt 2009; Schmidt 2013). T. brasiliensis respond to the echolocation of passing conspecifics in the roost with directed songs that are shorter and more likely to contain buzz phrases (Bohn et al. 2013). Songbirds exhibit a variety of ways of changing their songs in response to an intruder, such as switching song types, changing song composition, or increasing song performance via vocal deviation (Searcy and Beecher 2009). Further assessment of song changes in C. cor as well as other singing bats will continue to elucidate the flexibility of bat song repertoires (Smotherman et al. 2016).
Why have territories?
Emergence of territorial behaviors is expected to be dependent on resource distribution and quantity, including the defensibility of the resource. Resources can include food, roosts, and mates (Pereira et al. 2003). C. cor singing behavior occurs on areas whereby individuals forage during the long dry season, and it has been suggested that terrestrial arthropod prey decrease during that time period (Vaughan 1976; McWilliam 1987). However, this begs the question—if territories are solely to protect food resources, why does singing appear to be a male behavior when females also appear to have spatial fidelity to particular foraging areas? In songbirds, a territory is often defended by the male to protect his mate(s) and the additional food and nesting resources on the territory; however, in some songbird species, females do engage in singing and territory defense seasonally, throughout the year, or in response to heightened female competition or lack of a mate (Langmore 2000). Our observations suggest that females do not have foraging areas as exclusive as males (Smarsh and Smotherman 2015a). In addition, McWilliam’s careful observations as well as our opportunistic ones of females joining male singers during part of the dry season is suggestive of a courtship function of the territories (McWilliam 1987; Smarsh and Smotherman 2015a), and leads us to tentatively hypothesize that females benefit from foraging partially on nearby male territories, whereas males benefit by having greater access to mates. For the sympatric, monogamous, and territorial yellow-winged bat, Lavia frons, males seem to be the sole sex to actively defend their multipurpose territories via vocalizing, patrolling, and chasing (Vaughan and Vaughan 1986). The behavior of C. cor can be clarified with focused studies on C. cor females.
Conclusion
Playbacks of conspecific songs elicited strong positive phonotaxis and singing changes when presented within the singing areas of C. cor males. These results are suggestive of a role of singing in territory advertisement and maintenance outside of the roost. Observations of responsiveness to playbacks solely within the singing area and encroachment of a neighbor after the death of an individual further support this idea. Song variability within and across individuals can be useful for assessing and recognizing neighbors as well as preventing habituation of the receiver. From exploratory analyses in this study, we provide evidence that song temporal and frequency metrics are predictive of level of response to song playback, and that individuals change their songs in response to playbacks. C. cor songs likely function as a graded signal of intent, or index cues of the quality of the singer. Similar to songbirds, we conclude that the territorial defense hypothesis is a key working hypothesis for this system and should be investigated further.
References
Abdi H (2003) Partial least squares (PLS) regression. In: Lewis-Beck M, Bryman A, Futing T (eds) Encylopedia of social sciences research methods. SAGE Publications, Thousand Oaks, CA, pp 792–795
Akçay C, Tom ME, Holmes D, Campbell SE, Beecher MD (2011) Sing softly and carry a big stick: signals of aggressive intent in the song sparrow. Anim Behav 82:377–382
Akçay C, Tom ME, Campbell SE, Beecher MD (2013) Song type matching is an honest early threat signal in a hierarchical animal communication system. Proc R Soc B 280:20122517
Bastian A, Schmidt S (2008) Affect cues in vocalizations of the bat, Megaderma lyra, during agonistic interactions. J Acoust Soc Am 124:598–608
Bastian A, Schmidt S (2009) Individual communication affects ritualized courtship: a case study in the bat (Megaderma lyra). In: Abstracts 31th International Ethological Conference, Rennes, France, p 140
Beecher MD, Campbell ES, Nordby JC (1998) The cognitive ecology of song communication and song learning in the song sparrow. In: Dukas R (ed) Cognitive ecology: the evolutionary ecology of information processing and decision making. University of Chicago Press, Chicago, pp 175–196
Behr O, von Helversen O (2004) Bat serenades–complex courtship songs of the sac-winged bat (Saccopteryx bilineata). Behav Ecol Sociobiol 56:106–115
Behr O, von Helversen O, Heckel G, Nagy M, Voigt CC, Mayer F (2006) Territorial songs indicate male quality in the sac-winged bat Saccopteryx bilineata (Chiroptera, Emballonuridae). Behav Ecol 17:810–817
Behr O, Knörnschild M, von Helversen O (2009) Territorial counter-singing in male sac-winged bats (Saccoperyx bilineata): low-frequency songs trigger a stronger response. Behav Ecol Sociobiol 63:433–442
Bohn KM, Schmidt-French B, Ma ST, Pollak GD (2008) Syllable acoustics, temporal patterns, and call composition vary with behavioral context in Mexican free-tailed bats. J Acoust Soc Am 124:1838–1848
Bohn KM, Smarsh GC, Smotherman M (2013) Social context evokes rapid changes in bat song syntax. Anim Behav 85:1485–1491
Bradbury JW, Vehrencamp SL (2011) Principles of animal communication, 2nd edn. Sinauer Associates Inc., Massachusetts, pp 397–462
Brockelman WY (2009) Ecology and the social systems of gibbons. In: Lappan S, Whittaker D (eds) The gibbons: new perspectives on small ape socioecology and population biology. Springer, New York, pp 217–223
Burt JM, Bard SC, Campbell ES, Beecher MD (2002) Alternative forms of song matching in song sparrows. Anim Behav 63:1143–1151
Byers BE, Akresh ME, King DI (2016) Song and male quality in prairie warblers. Ethology 122:1–11
Cardoso GC (2014) Studying the silent side of birdsong. BMC Biol 12:62
Carter GG, Wilkinson GS (2016) Common vampire bat contact calls attract past food-sharing partners. Anim Behav 116:45–51
Catchpole CK, Slater PJB (2008) Bird song: biological themes and variations, 2nd edn. Cambridge University Press, Cambridge
Catchpole C, Leisler B, Dittami J (1986) Sexual differences in the responses of captive great reed warblers (Acrocephalus arundinaceus) to variation in song structure and repertoire size. Ethology 73:69–77
Chaverri G, Gillam EH, Vonhof MJ (2010) Social calls used by a leaf-roosting bat to signal location. Biol Lett 64:441–444
Denzinger A, Schnitzler H-U (2013) Bat guilds, a concept to classify the highly diverse foraging and echolocation behaviors of Microchiropteran bats. Front Physiol 4:164
Dhondt A (1966) A method to establish boundaries of bird territories. Gerfaut-Giervalk 56:404–408
DuBois AL, Nowicki S, Searcy WA (2009) Swamp sparrows modulate vocal performance in an aggressive context. Biol Lett 5:163–165
Falls JB (1978) Bird song and territorial behavior. In: Kramer L, Pliner P, Alloway T (eds) Aggression, dominance, and individual spacing. Academic Press, New York, pp 61–89
Fan PF, Xiao W, Huo S, Jiang XL (2009) Singing behavior and singing functions of black-crested gibbons (Nomascus concolor jingdongensis) at Mt. Wuliang, Central Yunnan, China. Am J Primatol 71:539–547
Fenton MB, Belwood JJ, Fullard JH, Kunz TH (1976) Responses of Myotis lucifigus (Chiroptera: Vespertilionidae) to calls of conspecifics and to other sounds. Can J Zool 54:1443–1448
Fichtel C, Hilgartner R (2013) Noises in the dark: vocal communication in Lepilemur ruficaudatus and other nocturnal pair-living primates. In: Masters J, Gamba M, Genin F (eds) Leaping ahead: advances in prosimian biology. Springer-Verlag, New York, pp 297–304
Fitch WT, Hauser MD (2002) Unpacking ‘honest’: vertebrate vocal production and the evolution of acoustic signals. In: Simmons AM, Fay RR, Popper AN (eds) Acoustic communication. Springer, New York, pp 65–137
Funghi C, Cardoso GC, Mota PG (2015) Increased syllable rate during aggressive singing in a bird with complex and fast song. J Avian Biol 46:283–288
Geberzahn N, Aubin T (2014) Assessing vocal performance in complex birdsong: a novel approach. BMC Biol 12:58
Guppy A, Coles RB, Pettigrew JD (1985) Echolocation and acoustic communication in the Australian ghost bat, Macroderma gigas (Microchiroptera: Megadermatidae). Aust Mammal 8:299–308
Hall ML, Kingma SA, Peters A (2013) Male songbird indicates body size with low-pitched advertising songs. PLoS One 8:e56717
Ham S, Hedwig D, Lappan S, Choe JC (2016) Song functions in nonduetting gibbons: evidence from playback experiments on Javan gibbons (Hylobates moloch). Int J Primatol 37:225–240
Hinde RA (1956) The biological significance of the territories of birds. Ibis 98:340–369
Janßen S, Schmidt S (2009) Evidence for a perception of prosodic cues in bat communication: contact call classification by Megaderma lyra. J Comp Physiol A 195:663–672
Jones DL (2015) Fathom toolbox for Matlab: software for multivariate ecological and oceanographic data analysis. College of Marine Science, University of Florida, St. Petersburg, FL, USA
Kaňuch P, Aghová T, Meheret Y, Šumbera R, Bryja J (2015) New discoveries on the ecology and echolocation of the heart-nosed bat Cardioderma cor with a contribution to the phylogeny of Megadermatidae. Afr Zool 50:53–57
Kinzey WG, Robinson JG (1983) Intergroup loud calls, range size, and spacing in Callicebus torquatus. Am J Phys Anthropol 60:539–544
Knӧrnschild M, Jung K, Nagy M, Metz M, Kalko E (2012) Bat echolocation calls facilitate social communication. Proc R Soc Lond B 279:4827–4835
Koren L, Mokady O, Geffen E (2008) Social status and cortisol levels in singing rock hyraxes. Horm Behav 54:212–216
Kroodsma DE (1989) Suggested experimental designs for song playbacks. Auk 37:600–609
Kulzer E, Nelson JE, McKean JL, Mohres FP (1984) Prey-catching behaviour and echolocation in the Australian ghost bat, Macroderma gigas (Microchiroptera: Megadermatidae). Austr Mammal 7:37–50
Langmore NE (2000) Why female birds sing. In: Espmark Y, Amundsen T, Rosenqvist G (eds) Animal signals: signalling and signal design in animal communication. Tapir Academic Press, Trondheim, pp 317–327
Lawrence BD, Simmons JA (1982) Measurements of atmospheric attenuation at ultrasonic frequencies and the significance for echolocation by bats. J Acoust Soc Am 71:585–590
Leippert D, Frank E, Gabriel P, Kutter S, Scheiderman KD, von Stillfried N, Weller F (2002) Prey-correlated spectral changes in echolocation sounds of the Indian false vampire Megaderma lyra. Ethology 108:139–156
Linhart P, Jaska P, Petruskova T, Petrusek A, Fuchs R (2013) Being angry, singing fast? Signalling of aggressive motivation by syllable rate in a songbird with slow song. Behav Process 100:139–145
Maher CR, Lott DF (1995) Definitions of territoriality in the study of variation in vertebrate spacing systems. Anim Behav 49:1581–1597
Marler P (1969) Colobus guereza: territoriality and group composition. Science 163:93–95
McWilliam A (1987) Territoriality and pair behavior of the African false vampire bat, Cardioderma cor (Chiroptera: Megadermatidae), in coastal Kenya. J Zool 213:243–252
Mitani JC (1984) The behavioral regulation of monogamy in gibbons (Hylobates muelleri). Behav Ecol Sociobiol 15:225–229
Mitani JC (1985a) Gibbon song duets and inter-group spacing. Behaviour 92:59–95
Mitani JC (1985b) Location-specific responses of gibbons (Hylobates muelleri) to male songs. Z Tierpsychol 70:219–224
Mitani JC (1987) Territoriality and monogamy among agile gibbons (Hylobates agilis). Behav Ecol Sociobiol 20:265–269
Neuweiler G (1990) Auditory adaptations for prey capture in echolocating bats. Physiol Rev 70:615–641
Pereira HM, Bergman A, Roughgarden J (2003) Socially stable territories: the negotiation of space by interacting foragers. Am Nat 161:143–152
Podos J (1997) A performance constraint on the evolution of trilled vocalizations in a songbird family (Passeriformes: Emberizidae). Evolution 51:537–551
Puechmaille SJ, Borissov IM, Zsebok S, Allegrini B, Hizem M, Kuenzel S, Schuchmann M, Teeling EC, Siemers BM (2014) Female mate choice can drive the evolution of high frequency echolocation in bats: a case study with Rhinolophus mehelyi. PLoS One 9:e103452
Raemaekers JJ, Raemaekers PM (1985) Field playback of loud calls to gibbons (Hylobates lar): territorial, sex-specifi, and species-specific responses. Anim Behav 33:481–493
Ratcliffe J (2015) Ultrasonic and superfast: design constraints on echolocation in bats. J Acoust Soc Am 138:1931
Rendall D, Owren MJ, Ryan MJ (2009) What do animal signals mean? Anim Behav 78:233–240
Richards DG (1981) Estimation of distance of singing conspecifics by the Carolina wren. Auk 98:127–133
Ryan MJ, Tuttle MD (1987) The role of prey-generated sound, vision, and echolocation in prey localization by the African bat Cardioderma cor (Megadermatidae). J Comp Physiol A 161:59–66
Schmidt S (2000) The role of echolocation in the hunting of terrestrial prey—new evidence for an underestimated strategy in the gleaning bat, Megaderma lyra. J Comp Physiol A 186:975–988
Schmidt S (2013) Beyond echolocation: emotional acoustic communication in bats. In: Altenmüller E, Schmidt S, Zimmerman E (eds) Evolution of emotional communication: from sounds in nonhuman mammals to speech and music and man. Oxford University Press, Oxford, pp 92–104
Searcy WA, Beecher MD (2009) Song as an aggressive signal in songbirds. Anim Behav 78:1281–1292
Smarsh GC, Smotherman M (2015a) Singing away from home: songs are used on foraging territories in the heart-nosed bat, Cardioderma cor. In: Proceedings of Meetings on Acoustics, vol. 25 010002. Acoustical Society of America, Jacksonville
Smarsh GC, Smotherman M (2015b) Intra- and interspecific variability of echolocation pulse acoustics in the African megadermatid bats. Acta Chiropterol 17:429–443
Smotherman M, Knörnschild M, Smarsh G, Bohn K (2016) The origins and diversity of bat songs. J Comp Physiol A 202:535–554
Taylor AM, Reby D (2010) The contribution of source-filter theory to mammal vocal communication research. J Zool 280:221–236
Temeles EJ (1994) The role of neighbours in territorial systems: when are they ‘dear enemies’? Anim Behav 47:339–350
Terleph TA, Malaivijitnond S, Reichard UH (2016) Age related decline in female lar gibbon great call performance suggest that call features correlate with physical condition. BMC Biol 16:4
Tibbetts EA, Dale J (2007) Individual recognition: it is good to be different. Trends Ecol Evol 22:529–537
Tinbergen N (1957) The functions of territory. Bird Study 4:14–27
Vaughan T (1976) Nocturnal behavior of the African false vampire bat (Cardioderma cor). J Mammal 57:227–248
Vaughan TA, Vaughan RP (1986) Seasonality and the behavior of the African yellow-winged bat. J Mammal 67:91–102
Waters DA, Jones G (1995) Echolocation call structure and intensity in five species of insectivorous bats. J Exp Biol 198:475–489
Wiley RH (2013) Specificity and multiplicity in the recognition of individuals: implications for the evolution of social behaviour. Biol Rev 88:179–195
Wilkinson GS, Boughman JW (1998) Social calls coordinate foraging in greater spear-nosed bats. Anim Behav 55:337–350
Acknowledgements
We thank the reviewers for their critical assessments of this work. We feel that their suggestions have greatly improved this manuscript. We thank Felix Mpelembwa, Nuhu Bahaty Mhapa, Alfred Absolem Mollel, and Nickodemasy Obeid for their assistance in field work. We thank the officers of Kikavuchini, Mkalama, and Longoi Villages, the Machame Weru Weru Ward, and the Hai District for their cooperation. Dassa Nkini of the Tanzania Conservation Resource Centre assisted with permit acquisition. We thank Brian Pierce, Thomas DeWitt, David Jones, Mirjam Knörnschild, and Kirsten Bohn for advice and discussion on statistics and experimental design. This research was funded by the National Science Foundation Graduate Research Fellowship Program, Bat Conservation International, and Wildlife Acoustics.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All applicable international, national, and institutional guidelines for the use of animals were followed. We acquired all necessary permits and permissions to work with this species and in these regions: Institutional Animal Care and Use Committee, AUP 2012-087; Tanzania Commission for Science and Technology, 2014-53-ER-2012-58, 2013-65-NA-2012-58, and NA-2012-58.
Conflict of interest
The authors declare that they have no conflict of interest.
Data availability
The datasets analyzed from the current study are available from the corresponding author on reasonable request.
Additional information
Communicated by M. Knoernschild
Electronic supplementary material
Online Resource 1
Video of a typical Cardioderma cor male singing in a perch. Individuals rotate while perched, broadcasting songs in bouts orally throughout the night (WMV 711 kb)
Online Resource 2
Spectrogram of an example contact call of C. cor, produced by both males and females while foraging. Contact calls consist of varying numbers of “upsweep” syllables (GIF 34 kb)
Online Resource 3
Song playlist for playback experiments. Each playlist consisted of a song repeated 20 times to create a set, which was repeated twice (Set 1 and Set 2) with 1 min of silence in between. Precontrol (PreC) and Postcontrol (PostC) time periods consisted of 5 min of silence at the beginning and the end of the playlist (GIF 8 kb)
Online Resource 4
Audio file of an example of a song playlist for playback. The silent PreC and PostC time periods on the ends of this playlist are five minutes in duration each (MP2 22126 kb)
Rights and permissions
About this article
Cite this article
Smarsh, G.C., Smotherman, M. Behavioral response to conspecific songs on foraging territories of the heart-nosed bat. Behav Ecol Sociobiol 71, 142 (2017). https://doi.org/10.1007/s00265-017-2370-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-017-2370-9