Abstract
When the range of similar species begins to overlap, niche separation may develop to maintain species boundaries. Reproductive traits are often subject to these adjustments, particularly when there is selection against hybrids. Shifts in range overlap can result in call interference, increased hybridization, and reduced mating success. Previous research has shown that these conditions can drive reproductive character displacement (RCD). Consistent with RCD, green tree frogs (Hyla cinerea) call at higher frequencies and perch higher in syntopy with their sister species (barking tree frogs, Hyla gratiosa), relative to allotopy. However, the time needed for these changes to occur and the corresponding effects on H. gratiosa remain unclear. We investigated if RCD is detectable in populations of these two species after 8 years or less of syntopy. We found an elevated high-frequency peak in calls of syntopic H. cinerea, while an increase in call duration was detected in syntopic H. gratiosa. Our results suggest that RCD can occur rapidly, in a manner consistent with the plasticity-first model, and that traits improving signal detection may be the first to respond to the pressures of syntopy and selection against hybrids.
Significance statement
Related species are expected to change their mating signals when breeding together to avoid mating with the wrong species. Green tree frogs change their mating calls when they breed in the same ponds as barking tree frogs, but it remains unclear how fast these changes can take place, or if there are any changes in barking tree frogs. We analyzed calls and perch locations within areas of recent (<8 years) range overlap in ponds where these species breed together and ponds where only one breeds. We found that when the two frogs co-occur, green tree frogs call at higher frequencies and barking tree frogs increase their calling duration. These changes were in the same direction, but diminished relative to ponds where they have lived together for long periods. Our results demonstrate that behavioral changes to avoid hybridization can occur in as few as 8 years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As climate change and other anthropogenic factors continually alter the geographic ranges of terrestrial animals, the interactions of formerly partitioned species are anticipated to increase. These range shifts can reorganize ecosystems, causing variation in species abundance, feedback processes, and community composition (Van der Putten 2012). Examples of such shifts include the Comma butterfly and purple finch (Parmesan and Yohe 2003), both of which have shown a significant northward range shift in response to rising temperatures associated with global warming. In species such as cutthroat and rainbow trout, climate change-induced range shifts in the Western USA have brought the two species into contact, resulting in frequent hybridization (Muhlfeld et al. 2014). Hybridization among flying squirrels has similarly occurred in Canada as the southern species expands northward (Garroway et al. 2010). When newly interacting species share similar ecological roles, niche separation will likely require species to adjust their behaviors. When overlap involves reproductive systems, mating behaviors are likely to be adjusted to maintain reproductive efficiency and reduce hybridization (Lemmon 2009). This interference selects against individuals that spend time, energy, and gametes on heterospecific mates, and can be reinforced in subsequent generations by strong preference against hybrids. This process, known as reproductive character displacement (RCD), is expected to result in a divergence of communication traits in sympatric populations relative to allopatric conspecifics (Brown and Wilson 1956; Blair 1974).
The causes of RCD are not widely established, and its role as a mechanism in divergent evolution and sympatric speciation is debated (Höbel and Gerhardt 2003; Lemmon et al. 2004; Pfennig and Pfennig 2009). RCD, in theory, can be determined by the strength of selection against hybridization (through a pre-zygotic barrier), the degree of sympatry, and the relative density of each species (Gerhardt 1999; Goldberg and Lande 2006). Okamoto and Grether (2013) concluded that RCD is crucial in determining the rate of evolution, and that strong RCD increases the rate while weak RCD slows divergence. Research on this topic not only provides insight into the speed and progression of divergence but can also reveal the plasticity of certain characters. Evolutionary theory has long predicted that phenotypic plasticity could precede and promote adaptation, though the application of this hypothesis to character displacement remains under-explored (Waddington 1953; Pfennig and Pfennig 2010). In this “plasticity-first” model, displacement is expected to produce consistent patterns of evolution across multiple independent populations (Pfennig and Pfennig 2012). Moreover, plasticity is expected to drive divergence at a faster rate than genetic canalization (but see Sætre et al. (2017)).
The energetic cost and selective pressure on individual characteristics of the behavior should predict which behaviors undergo the most displacement. A behavior such as perch site selection, for example, is likely to be more plastic than a largely genetically controlled character such as call frequency, and would be expected to undergo displacement sooner (Höbel and Gerhardt 2003). The importance of RCD studies, then, are their application to predicting how other species may adjust to future novel interactions.
Communication systems offer an opportunity for studying sympatric shifts in reproductive characters. Mating calls are crucial to reproduction in many species, and are often subject to RCD in the maintenance of isolation (Blair 1958). Therefore, mating signal interference between two similar species can rapidly affect the rate of divergent evolution to sustain reproductive isolation. Anurans are good candidates for the study of RCD, because there are many closely related species with overlapping ranges competing for a single parameter, acoustic space, which must be partitioned temporally or spectrally (Blair 1974; Leary 2001; Gerhardt and Huber 2002). In frogs, displacement can occur in features of the male’s call (call rate, call duration, and frequency of the spectral peaks), or other aspects of behavior (e.g., calling perch height).
Given that two interacting species are genetically compatible enough to reproduce, reduced survival and/or reproductive disadvantages of the hybrid offspring can result in increased selective pressure against mismating. For example, females of both barking tree frogs (Hyla gratiosa) and green tree frogs (Hyla cinerea) show a strong preference against the intermediate calls produced by hybrid males, which reinforces selection against mismating (Gerhardt 1974; Höbel and Gerhardt 2003). Hybrids of these two species also display reduced fitness (Schlefer et al. 1986). Direct selective pressure is more effective as a mechanism of niche separation in cases of RCD (Albert and Schluter 2004; Goldberg and Lande 2006), and the selective disadvantage of hybrid males that drives selection against hybridization has been shown to create divergence in female preference and male acoustic traits (Higgie and Blows 2008).
The speed of character divergence due to RCD remains an open question. Theoretical modeling addresses population changes in characters over simulated generations (Lemmon et al. 2004; Pfennig and Ryan 2006; Okamoto and Grether 2013). Genetic models of character displacement predict slow evolutionary rates during the earliest phases of trait shifts (Slatkin 1980; Taper and Case 1985; Doebeli 1996). While simulated displacement is often slow, recent empirical studies of living sympatric populations show surprisingly accelerated rates. For example, displacement was detected in sympatric species of mosquitoes after only 20 years of range overlap (Bargielowski et al. 2013), and studies using lab-reared populations of species in the Drosophila serrata complex have demonstrated that displacement can be detected after only 9–14 generations (Higgie et al. 2000; Higgie and Blows 2008). Vertebrates have also shown relatively rapid displacement with changes consistent with character displacement in sympatry detected following 22 years in finches (Grant and Grant 2006), 27 years in spadefoot toads (Pfennig 2003), and after 20 generation in Anolis lizards (Stuart et al. 2014). However, many prior studies of character displacement in sympatric species have been conducted in regions where the duration of overlap is unknown, and as a result, they do not address evolutionary rates (e.g., Höbel and Gerhardt 2003; Albert and Schluter 2004; Lemmon 2009).
We identified a potential case of RCD between the green tree frog, H. cinerea, and its sister species the barking tree frog, H. gratiosa, in a multi-county region of range overlap in western Kentucky where the duration of sympatry is either known or limited to a few years. Additionally, the general migration pattern of H. cinerea into formerly allopatric regions of H. gratiosa is known in this region (Lodato et al. 2015). These species are known to hybridize (Mecham 1960; Gerhardt 1974; Gerhardt et al. 1980a), and, at least in H. cinerea, have shown displacement of male call and female preference strength in syntopy consistent with RCD and reinforcement (Höbel and Gerhardt 2003). In western Kentucky, H. cinerea were known to have initially invaded native populations of H. gratiosa 8 years prior to our data collection (Kentucky Department of Fish and Wildlife Resources 2014; Lodato et al. 2015; J. MacGregor and B. Palmer-Ball, personal communication), and evidence suggests a continued migration since then, presenting even more recent invasions along a northwest-southeast sympatric transect.
Previous work has investigated RCD in sympatric H. cinerea and H. gratiosa (Höbel and Gerhardt 2003); however, call characters in H. gratiosa were not examined, so the possibility that RCD occurred in this species was tested. Our analysis of H. cinerea acts as a measure of the repeatability of RCD in this species. We analyzed syntopic and allotopic populations for four characteristics of male calls (high-frequency peak, low-frequency peak, call rate, and call duration) and advertising call perch height. We expected that RCD would have occurred within some of these characters and used these data to test the hypothesis that an 8-year period of overlap is sufficient for the emergence of detectable displacement of male characters.
Methods
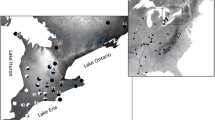
Allotopic and syntopic choruses of both H. gratiosa and H. cinerea were studied during their breeding seasons (May–July, 2014). Data were collected from choruses within Crittenden, Livingston, Lyon, and Trigg counties in Kentucky where syntopic calling sites have previously been reported (Fig. 1) (Kentucky Department of Fish and Wildlife Resources 2014; J. MacGregor and B. Palmer-Ball, personal communication). Choruses were located by road cruising and listening for active calling sites after dusk, 5–6 days a week, and mapping coordinates of sites using a GPS. It was not possible to record data blind, because our study involved focal animals in the field.
We defined syntopic sites as ponds where both H. gratiosa and H. cinerea were actively calling together, while allotopic sites had only one of either of the two species calling. Status of each site (syntopic/allotopic), air temperature, weather (wind, cloud cover, precipitation), and the presence of all calling species including H. gratiosa and H. cinerea were recorded upon arrival at each site. An approximately 2-min acoustic recording of the entire chorus was also taken upon arrival. All acoustic data were recorded on a Zoom H4n digital recorder using both a Sennheiser ME 62 microphone and MZW64 Windscreen with an SME PR-1000 parabolic reflector and a Sennheiser ME66 microphone with a KP-6 power module in two separate recorder channels for redundancy. Individuals in a chorus were located using acoustic cues and a headlamp before each recording. Once a calling male had been located, we advanced to within a few meters of the male, turned off all headlamps, and waited at least 3 min to allow the male to acclimate to our presence and resume calling regularly before recording with both the parabolic and directional microphones. The recording of each individual encompassed at least 20 consecutive calls. We recorded 27 H. cinerea and 17 H. gratiosa at seven syntopic sites. We also recorded 25 H. cinerea and 20 H. gratiosa at five and four allotopic sites, respectively. On average, each pond yielded usable data for four males of each species (range: one to nine males of a species per pond). One site did not clearly fit into our dichotomous variable of allotopy/syntopy. Though previously documented as being syntopic in 2006 (Kentucky Department of Fish and Wildlife Resources 2014; J. MacGregor and B. Palmer-Ball, personal communication), our data collection yielded only H. cinerea. Because we could not account for any character displacement occurring prior to the loss of H. gratiosa at this site, it was excluded from subsequent analyses. After recording, each individual was hand captured to take physical measurements. Mass was determined by placing an individual in a plastic bag and suspending the bag from a Pesola spring scale with a 50 g/2.5 N capacity. Snout/vent length (SVL) and tibia/fibula length (TFL) were measured using a clear, plastic ruler pressed firmly against the frog. Perch temperature was determined by placing a quick reading thermometer on the surface on which the frog was sitting immediately after capture. Perch height was recorded relative to the surface of the water. For example, if an individual was captured while floating on the surface of the water, its perch height was recorded as 0 cm.
The advertisement calls of H. gratiosa and H. cinerea are distinguishable by the rate, length, and spectral composition of each species’ call (Höbel and Gerhardt 2003). The mean call duration of H. cinerea is shorter than H. gratiosa (Oldham and Gerhardt 1975; Höbel and Gerhardt 2003). The spectral composition of both species’ calls is characterized by two frequency peaks: a low-frequency peak (LFP) and a high-frequency peak (HFP). Both spectral peaks of H. gratiosa are significantly lower than those of H. cinerea (Gerhardt 1974; Oldham and Gerhardt 1975). Hybrid crosses between H. cinerea and H. gratiosa are discernable, because the frequencies of the two spectral peaks of hybrids are intermediate to those of the two parent species (Gerhardt 1974; Gerhardt et al. 1980b).
To determine if the calls of H. gratiosa were affected by the presence of H. cinerea and vice versa, the calls of H. gratiosa and H. cinerea were analyzed for temporal and spectral variation using Audacity software (http://audacity.sourceforge.net/). The calls were evaluated to determine HFP, LFP, call rate, and call duration. For each individual recorded, the same 20 consecutive calls were used in the analysis of all call characteristics to ensure consistency. Consecutive calls were typically in groups divided by long pauses. Call duration was defined as the time period from the start to the end of a single call. Call rate was the number of calls that was emitted per minute within the grouping of 20 consecutive calls. If the calls of two individuals overlapped, the owner of each call was discerned by ear and by spectrographic characteristics (one individual would have a slightly higher or lower frequency peak than the other throughout their calls, aiding in their identification) and then analyzed separately.
Call parameters may be affected by fluctuations in air temperature (Gerhardt and Mudry 1980). Consequently, we performed linear regression analyses to test if temperature significantly correlated with both frequency peaks, call duration, and call rate. Datasets found to be affected by ambient temperature (perch height and call rate in H. gratiosa; low frequency and call rate in H. cinerea) were then adjusted to a common 25 °C prior to further analysis. Though body size may also affect advertisement calls, we did not correct acoustic data for size differences in our initial analyses, because call variation due to body size would be experienced by selecting females, and these differences should be maintained in analysis. In addition, there is evidence that in some anurans, call divergence is not a result of pleiotropic size effects (Loftus-Hills and Littlejohn 1992). We did, however, analyze size differences between syntopic and allotopic populations using the scaled mass index (SMI) as a measure of body size (Peig and Green 2009), because there may be additional selective pressures on body size as a result of syntopy that are independent of female choice. The SMI adjusts for males that might be especially thin or fat for a given mass-length relationship and hence is a preferable metric for amphibians.
ANOVA was performed on call duration to determine if shifts had occurred between allotopic and syntopic populations of conspecifics. Due to normality issues (skewedness), a Kruskal-Wallis rank sum test was used to test for effects of allotopy/syntopy on all of the remaining acoustic parameters. Because data on perch height similarly failed to meet normality requirements, a Kruskal-Wallis rank sum test was used to determine if syntopic populations showed detectable perching differences when compared to allotopic populations. We used data from the Kentucky Department of Fish and Wildlife herpetological records, field notes of Kentucky state herpetologist John MacGregor, and personal observations to estimate county-level year of invasion for H. cinerea into our study ponds. Given a likely general northwest to southeast migration pattern, ANOVA and regression analysis were performed by comparing call data across years of observed invasion and by linear distance from our northwestern-most site.
Results
Among H. cinerea, body size (SMI) was found to correlate with both high- (F 1, 47 = 7.879, P = 0.0073) and low- (F 1, 48 = 7.698, P = 0.0079) frequency peaks, while in H. gratiosa, only perch height correlated with SMI (F 1, 32 = 6.842, P = 0.0135). Mean SMI also differed between topic populations in both species, with syntopic males being significantly smaller than allotopic males (H. cinerea; F 1, 47 = 4.905, P = 0.0317, H. gratiosa; F 1, 32 = 5.345, P = 0.0274).
Among invading H. cinerea, evidence of a significant difference in syntopic conditions was detected only for its high-frequency peak (χ 2 1 = 11.05, P = 0.0009). This consisted of an average upward frequency shift of 199 Hz (an 8.3% change). Because high frequency peaks are affected by body size in this species, we confirmed that the observed topic difference was not an artifact of size difference by performing a Kruskal-Wallis rank sum test using frequency data corrected for SMI (χ 2 1 = 4.889, P = 0.027). There was no difference between syntopic and allotopic populations of H. cinerea in low-frequency peak, call duration, call rate, or perch height (Table 1).
H. gratiosa displayed evidence of syntopic character displacement in its average call duration (F 1, 37 = 5.26, P = 0.028). This shift resulted in a call duration increase of 12 ms (a 6.7% change). Allotopic and syntopic H. gratiosa did not differ in high-frequency peak, low-frequency peak, call rate, or perch height (Table 1).
In our analysis of potential changes in behavioral traits over time across an assumed northwest to southeast migration corridor (the most likely direction of migration based on observation records and geography), no statistically significant migration trends were found (data not shown). Minimum duration of syntopy by county ranged from 0 to 8 years. Distances from the northwestern-most collection site ranged from 0 to 79 km (Fig. 1). There was no effect of distance from the assumed first site of syntopy or of the minimum number of years in syntopy for either H. cinerea or H. gratiosa on any call or behavioral characters (data not shown).
We suspected that natural variability in vegetation around chorus ponds would set stricter limits on perching behavior in some sites while allowing greater range of perching options in others. Only the dataset of perching heights for H. cinerea varied significantly by site (Kruskal-Wallis rank sum test, H. cinerea (χ 2 1 = 28.35, P = 0.0029), H. gratiosa (χ 2 1 = 22.29, P = 0.014)). To correct for the effect of variation in perch height options, a bivariate rank sum test of H. cinerea perch height by pond type was performed; sites were classified as either low (flooded fields with no brush or trees available) or high (presence of brush and/or trees). The difference between the means (62 cm) was added to all data points in the “low” category, and a final population-type comparison of H. cinerea perch heights was performed using the corrected data. Syntopy and allotopy did not differ significantly (χ 2 1 = 0.59, P = 0.44). H. gratiosa was not corrected for perch height, because there was no difference in means between “high” and low pond types (F 1, 37 = 0.0, P = 1.0). This was expected, since H. gratiosa typically float on the surface of the water while calling, so the presence of high or low vegetation around a chorus pond was unlikely to have an effect on their perching habits.
Discussion
We have shown that 8 years is a sufficient amount of time for shifts in traits associated with RCD to occur in both H. gratiosa and H. cinerea. Additionally, our results support the hypothesis that the risk of heterospecific hybridization in syntopic populations causes a shift in reproductive characteristics associated with communication relative to allotopic populations.
The effect of syntopy between these two species has been examined previously; however, the duration of syntopy was unknown and data were only presented on how the interaction influenced H. cinerea (Höbel and Gerhardt 2003). Höbel and Gerhardt (2003) found that H. cinerea had both a significantly higher LFP and calling perch height in syntopy relative to allotopy, whereas the H. cinerea included in our study had no significant change in LFP or perch height. It is possible that the 8 years of syntopy at our study sites are insufficient to cause a shift in LFP or perch height—though shifts in LFP have been documented in H. cinerea after more than 20 years of syntopy (Schlefer et al. 1986). Additionally, this may also suggest that these characters are not as plastic as we suspected or there is not enough selective pressure on these traits in our populations. Höbel and Gerhardt did not specifically present data on the effect of syntopy on H. cinerea HFP, but HFP was one of three spectral properties loaded within a principal component (PC1) for PCA. The frequency components of PC1 were found to be significantly higher in syntopic H. cinerea populations relative to allotopic populations. This is in agreement with the upward shift in syntopic HFP relative to allotopic HFP that we observed. In both our study and Höbel and Gerhardt’s, no significant difference in call duration was observed between allotopic and syntopic H. cinerea populations.
Our study had the advantage of comparing the changes observed in both H. cinerea and H. gratiosa as a result of syntopy within a known time frame (8 years). Both species exhibited a significant shift in only one acoustic characteristic during these 8 years; however, both traits shifted in a manner that increases character differences between species as predicted by RCD. H. cinerea exhibited significantly higher HFPs in syntopy relative to allotopy, whereas no significant difference in LFP was observed. H. gratiosa exhibited no change in either HFP or LFP. Low-frequency peak plays an important role in mate selection in H. cinerea, and presumably plays a similar role in H. gratiosa. Gerhardt (1976) suggests that LFPs attract female conspecifics from greater distances than HFPs, which function in more proximal species recognition. Altering HFP rather than LFP may be a means for females to distinguish conspecific males from heterospecifics while still maintaining a LFP that is able to attract females from a distance (Gerhardt 1976).
Because body size is known to be negatively correlated with call frequencies (Oldham and Gerhardt 1975), the reduced body size among syntopic H. cinerea males may be related to the observed upward HFP shift. Considering that differences in HFP between allotopic and syntopic populations exist both with and without corrections for body size, our results may indicate indirect selection for smaller males in addition to direct selection for higher HFP.
Call duration of syntopic H. gratiosa males was significantly longer than both allotopic conspecifics and syntopic H. cinerea calls, whereas H. cinerea had no significant change in call duration between allotopy and syntopy. Lengthening the duration of a call increases the likelihood that a calling male will be heard by a female (Brumm and Slabbekoorn 2005), thereby improving that individual’s chances of mating. Female preference for longer calls is common in anurans (reviewed in Gerhardt and Huber (2002)). Longer calls may provide females with a greater opportunity to assess the attractiveness of conspecifics, as well as differentiate between conspecifics and heterospecifics at syntopic sites to improve mating efficiency. Selective pressure against hybridization and sexual selection via female preference may have both contributed to the lengthening of syntopic H. gratiosa call duration.
Our data for H. gratiosa support the hypothesis that displacement in call characteristics tends to occur in a direction that increases energetic costs (Lemmon 2009). An explanation for this trend is that males with higher fitness are able to produce more attractive and more energetically costly sexual signals than less fit males. Changes in call frequency in anurans are largely dependent on the size of the larynx and not energy use (Walkowiak 2007; Gingras et al. 2013). Hence, the changes we observed in H. cinerea do not support the argument that displacement is increasing energetic costs in this species.
Call effort represents the inverse relationship between call rate and call duration (Lemmon 2009). We would expect that the increase in call duration that we observed in syntopic H. gratiosa would result in a decrease in call rate; however, no significant change in call rate was observed. Since females prefer faster call rates, it would be beneficial for males to increase call rate, though it is energetically costly to do so without concurrently decreasing call duration (Gerhardt 1994; Lemmon 2009). Considering that in our study call duration increased, it is suggestive that selective pressures on syntopic H. gratiosa in this study were great enough for the males to expend more energy by increasing call duration without decreasing call rate. Call rate may also be maintained in order to prevent auditory masking by call overlap between males (Grafe 1996), or because of the leader advantage phenomenon observed in many anurans including H. cinerea (Höbel and Gerhardt 2007; Richardson et al. 2008). Male H. cinerea have been shown to adjust the timing of their calls (and hence, their instantaneous call rate) to avoid call overlap with both conspecifics (Höbel and Gerhardt 2007), and H. gratiosa (Höbel 2015) and sympatric males are less likely to overlap their calls than allopatric males. Issues of signal timing remain unexplored in H. gratiosa.
Though we did not measure population abundance, the difficulty with which we found native H. gratiosa choruses in western Kentucky supports the suspicion that this species may be outcompeted before isolation is reached (Mecham 1960; Schlefer et al. 1986). Additionally, we did encounter a previously syntopic pond that contained only H. cinerea, representing a loss or supplanting of native frogs. Change in characters due to RCD can be asymmetric, with greater displacement found among the rarer populations (Lemmon et al. 2004). Consequently, we anticipate that in the future, relatively greater displacement will be observed in H. gratiosa in the populations we observed. Further research may clarify the capacity for RCD to prevent the elimination of behaviorally disadvantaged species.
Conclusion
Our data show that changes in behavioral characters consistent with RCD occur in syntopic populations of both H. cinerea and H. gratiosa within 8 years of initial species range overlap: an upward shift in HFP in H. cinerea, and an increased call duration in H. gratiosa. One likely reason for this displacement is pre-zygotic selection against the production of sexually unattractive and less fit hybrids, though female preferences would need to be tested to confirm this mechanism.
While our data do support RCD as the mode of action for the changes observed in these acoustic characteristics, our data also are consistent with a mechanism of behavioral plasticity, especially in those characters that are not strictly restrained by body size, temperature, or perch availability. Considering that plasticity is hypothesized to mediate character displacement during early phases of sympatric divergence, RCD and plasticity are not mutually exclusive explanations (Pfennig and Pfennig 2012). Indeed, rapid RCD driven by phenotypic plasticity (the “plasticity first” hypothesis of Pfennig and Pfennig (2012)) seems plausible for two reasons. First, the plasticity first hypothesis is supported when the changes induced by invasion of a sister species occur simultaneously in individual members of a population, as seen here. Second, it is likely that the capacity to adjust behavior to novel heterospecifics existed prior to syntopic invasion, resulting from adaptations for within-species competition. Furthermore, given that the changes we observed between recently syntopic and allotopic populations were intermediate between those observed in long established allotopic and syntopic populations (Höbel and Gerhardt 2003), we argue that the observed patterns are consistent with both RCD and explanations based on plasticity (Pfennig and Pfennig 2012). Ultimately, however, a common garden experiment will be necessary to determine to what extent behavioral plasticity may alter acoustic characteristics.
References
Albert AY, Schluter D (2004) Reproductive character displacement of male stickleback mate preference: reinforcement or direct selection? Evolution 58:1099–1107
Bargielowski IE, Lounibos LP, Carrasquilla MC (2013) Evolution of resistance to satyrization through reproductive character displacement in populations of invasive dengue vectors. Proc Natl Acad Sci USA 110:2888–2892
Blair WF (1958) Mating call in the speciation of anuran amphibians. Am Nat 92:27
Blair WF (1974) Character displacement in frogs. Integr Comp Biol 14:1119–1125
Brown WL, Wilson EO (1956) Character displacement. Syst Zool 5:49–65
Brumm H, Slabbekoorn H (2005) Acoustic communication in noise. Adv Stud Behav 35:151–209
Doebeli M (1996) An explicit genetic model for ecological character displacement. Ecology 77:510–520
Garroway CJ, Bowman J, Cascaden TJ, Holloway GL, Mahan CG, Malcolm JR, Steele MA, Turner G, Wilson PJ (2010) Climate change induced hybridization in flying squirrels. Glob Chang Biol 16:113–121
Gerhardt HC (1974) The vocalizations of some hybrid treefrogs: acoustic and behavioral analyses. Behaviour 49:130–151
Gerhardt HC (1976) Significance of two frequency bands in long distance vocal communication in the green treefrog. Nature 261:692–693
Gerhardt HC (1994) Reproductive character displacement of female mate choice in the grey treefrog, Hyla chrysoscelis. Anim Behav 47:959–969
Gerhardt HC (1999) Reproductive character displacement and other sources of selection on acoustic communication systems. In: Hauser MD, Konishi M (eds) The design of animal communication. Massachusetts Institute of Technology Press, Boston, pp 515–534
Gerhardt HC, Huber F (2002) Acoustic communication in insects and anurans: common problems and diverse solutions. University of Chicago Press, Chicago
Gerhardt HC, Mudry KM (1980) Temperature effects on frequency preferences and mating call frequencies in the green treefrog, Hyla cinerea (Anura: Hylidae). J Comp Physiol 137:1–6
Gerhardt HC, Guttman SI, Karlin AA (1980a) Accuracy of sound localization in a miniature dendrobatid frog. Naturwissenschaften 67:362–363
Gerhardt HC, Guttman SI, Karlin AA (1980b) Natural hybrids between Hyla cinerea and Hyla gratiosa: morphology, vocalization and electrophoretic analysis. Copeia 1980:577–584
Gingras B, Boeckle M, Herbst CT, Fitch WT (2013) Call acoustics reflect body size across four clades of anurans. J Zool 289:143–150
Goldberg E, Lande R (2006) Ecological and reproductive character displacement of an environmental gradient. Evolution 60:1344–1357
Grafe TU (1996) The function of call alternation in the African reed frog (Hyperolius marmoratus): precise call timing prevents auditory masking. Behav Ecol Sociobiol 38:149–158
Grant PR, Grant BR (2006) Evolution of character displacement in Darwin’s finches. Science 313:224–226
Higgie M, Blows MW (2008) The evolution of reproductive character displacement conflicts with how sexual selection operates within a species. Evolution 62:1192–1203
Higgie M, Chenoweth S, Blows MW (2000) Natural selection and the reinforcement of mate recognition. Science 290:519–521
Höbel G (2015) Sexual differences in responses to cross-species call interference in the green treefrog (Hyla cinerea). Behav Ecol Sociobiol 69:695–705
Höbel G, Gerhardt HC (2003) Reproductive character displacement in the acoustic communication system of green tree frogs (Hyla cinerea). Evolution 57:894–904
Höbel G, Gerhardt HC (2007) Sources of selection on signal timing in a tree frog. Ethology 113:973–982
Kentucky Department of Fish and Wildlife Resources (2014) Species information. County observation(s) for Amphibia. Green treefrog (Hyla cinerea). Commonwealth of Kentucky, http://app.fw.ky.gov/speciesinfo/speciesListCounty.asp?strScientificName=Hyla+cinerea&strGroup=5
Leary CJ (2001) Investigating opposing patterns of character displacement in release and advertisement vocalizations of Bufo fowleri and Bufo americanus (Anura; Bufonidae). Can J Zool 79:1577–1585
Lemmon EM (2009) Diversification of conspecific signals in sympatry: geographic overlap drives multidimensional reproductive character displacement in frogs. Evolution 63:1155–1170
Lemmon AR, Smadja C, Kirkpatrick M (2004) Reproductive character displacement is not the only possible outcome of reinforcement. J Evol Biol 17:177–183
Lodato MJ, Engbrecht NJ, Klueh-Mundy S, Walker Z (2015) The green treefrog, Hyla cinerea in Indiana. Proc Indiana Acad Sci 123:179–195
Loftus-Hills JJ, Littlejohn MJ (1992) Reinforcement and reproductive character displacement in Gastrophryne carolinensis and G. olivacea (Anura: Microhylidae): a reexamination. Evolution 46:896–906
Mecham JS (1960) Introgressive hybridization between two southeastern treefrogs. Evolution 14:445–457
Muhlfeld CC, Kovach RP, Jones LA, Al-Chokhachy R, Boyer MC, Leary RF, Lowe WH, Luikart G, Allendorf FW (2014) Invasive hybridization in a threatened species is accelerated by climate change. Nat Clim Chang 4:620–624
Okamoto KW, Grether GF (2013) The evolution of species recognition in competitive and mating contexts: the relative efficacy of alternative mechanisms of character displacement. Ecol Lett 16:670–678
Oldham RS, Gerhardt HC (1975) Behavioral isolating mechanisms of the treefrogs Hyla cinerea and H. gratiosa. Copeia 1975:223–231
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42
Peig J, Green AJ (2009) New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118:1883–1891
Pfennig KS (2003) A test of alternative hypotheses for the evolution of reproductive isolation between spadefoot toads: support for the reinforcement hypothesis. Evolution 57:2842–2851
Pfennig KS, Pfennig DW (2009) Character displacement: ecological and reproductive responses to a common evolutionary problem. Q Rev Biol 84:253–276
Pfennig DW, Pfennig KS (2010) Character displacement and the origins of diversity. Am Nat 176:S26–S44
Pfennig DW, Pfennig KS (2012) Development and evolution of character displacement. Ann N Y Acad Sci 1256:89–107
Pfennig KS, Ryan MJ (2006) Reproductive character displacement generates reproductive isolation among conspecific populations: an artificial neural network study. Proc R Soc Lond B 273:1361–1368
Richardson C, Léna JP, Joly P, Lengagne T (2008) Are leaders good mates? A study of call timing and male quality in a chorus situation. Anim Behav 76:1487–1495
Sætre GP, Cuevas A, Hermansen JS, Elgvin TO, Fernández LP, Sæther SA, Cascio Sætre CL, Eroukhmanoff F (2017) Rapid polygenic response to secondary contact in a hybrid species. Proc R Soc B 284:20170365
Schlefer EK, Romano MA, Guttman SI, Ruth SB (1986) Effects of twenty years of hybridization in a disturbed habitat on Hyla cinerea and Hyla gratiosa. J Herpetol 20:210–221
Slatkin M (1980) Ecological character displacement. Ecology 61:163–177
Stuart YE, Campbell TS, Hohenlohe PA, Reynolds RG, Revell LJ, Losos JB (2014) Rapid evolution of a native species following invasion by a congener. Science 346:463–466
Taper ML, Case TJ (1985) Quantitative genetic models for the coevolution of character displacement. Ecology 66:355–371
Van der Putten WH (2012) Climate change, aboveground-belowground interactions, and species' range shifts. Annu Rev Ecol Evol Syst 43:365–383
Waddington CH (1953) Genetic assimilation of an acquired character. Evolution 7:118–126
Walkowiak W (2007) Call production and neural basis of vocalization. In: Narins PM, Feng AS, Fay RR, Popper AN (eds) Hearing and sound communication in amphibians. Springer, New York, pp 87–112
Acknowledgments
We thank Mike Lodato, John MacGregor, and Gerlinde Hobel for discussions of field sites, sympatry, tree frogs, and experimental design; and Cris Hochwender for statistical advice. Comments by two anonymous reviewers substantially improved our manuscript. Mike Lodato and Daniel W. Mikesell provided assistance in the field.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Financial support was provided by a grant from the University of Evansville UExplore fund.
Conflict of interest
The authors declare they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of, and approved by, the University of Evansville Animal Care and Use Committee, approval no. 2014-01. All data were collected under the KY Dept. of Fish and Wildlife permit no. 52686.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Communicated by K. Summers
Rights and permissions
About this article
Cite this article
Gordon, N.M., Ralph, M.Z. & Stratman, K.D. Rapid character displacement of different call parameters in closely related treefrogs (Hyla cinerea and H. gratiosa). Behav Ecol Sociobiol 71, 112 (2017). https://doi.org/10.1007/s00265-017-2341-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-017-2341-1